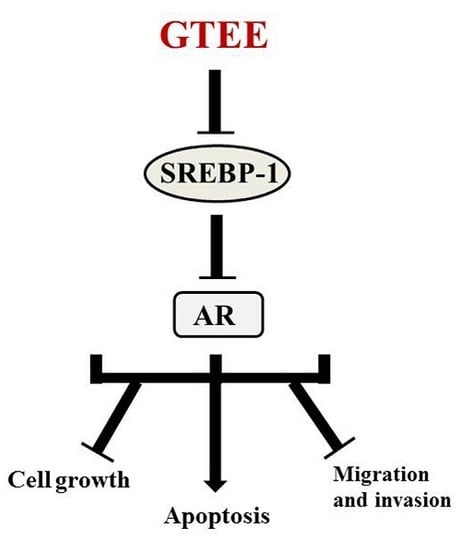
Ganoderma tsugae Inhibits the SREBP-1/AR Axis Leading to Suppression of Cell Growth and Activation of Apoptosis in Prostate Cancer Cells
Abstract
1. Introduction
2. Results
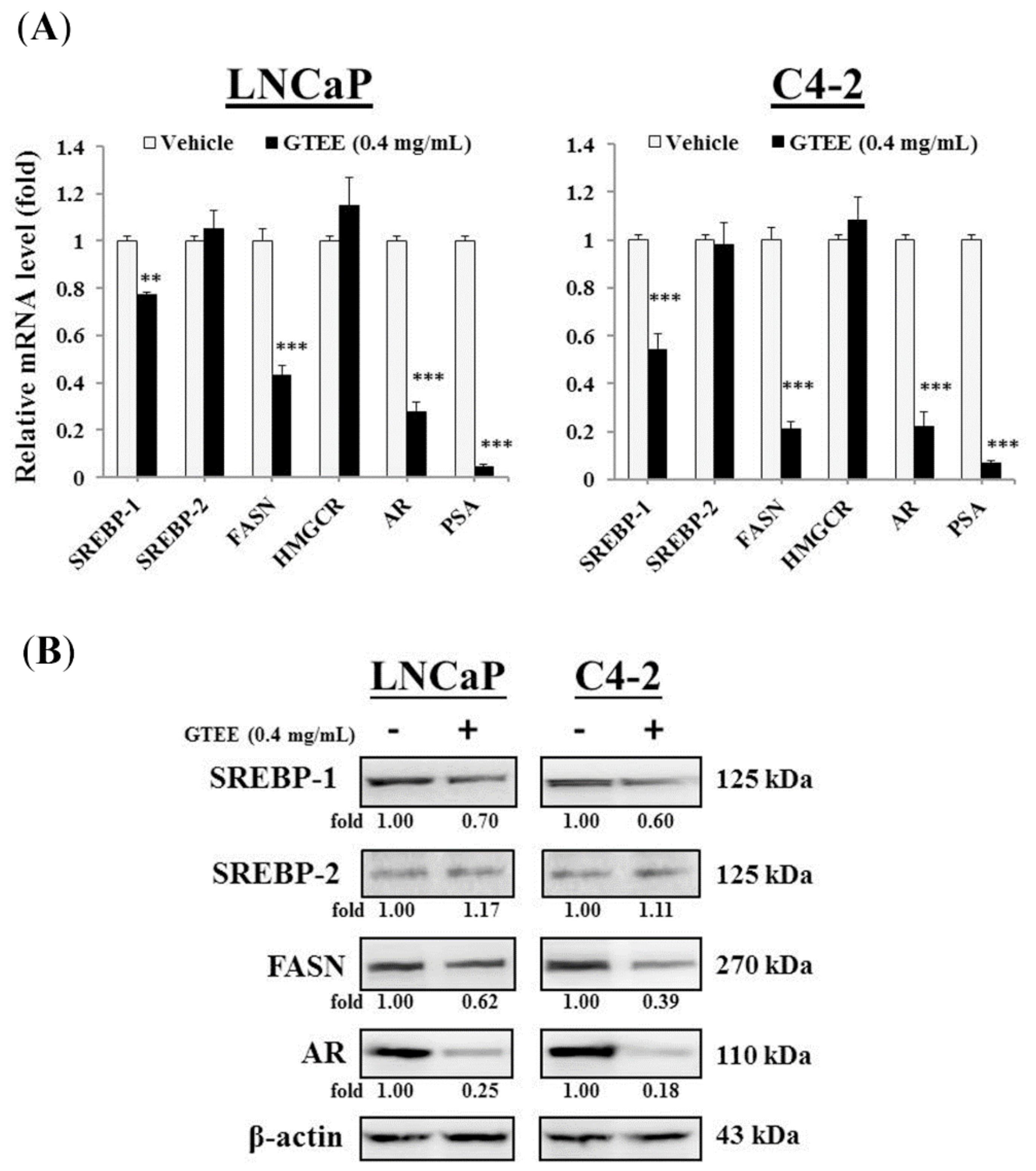
2.1. GTEE Inhibits the Expression of SREBP-1 and Its Downstream Associated Genes in PCa Cells
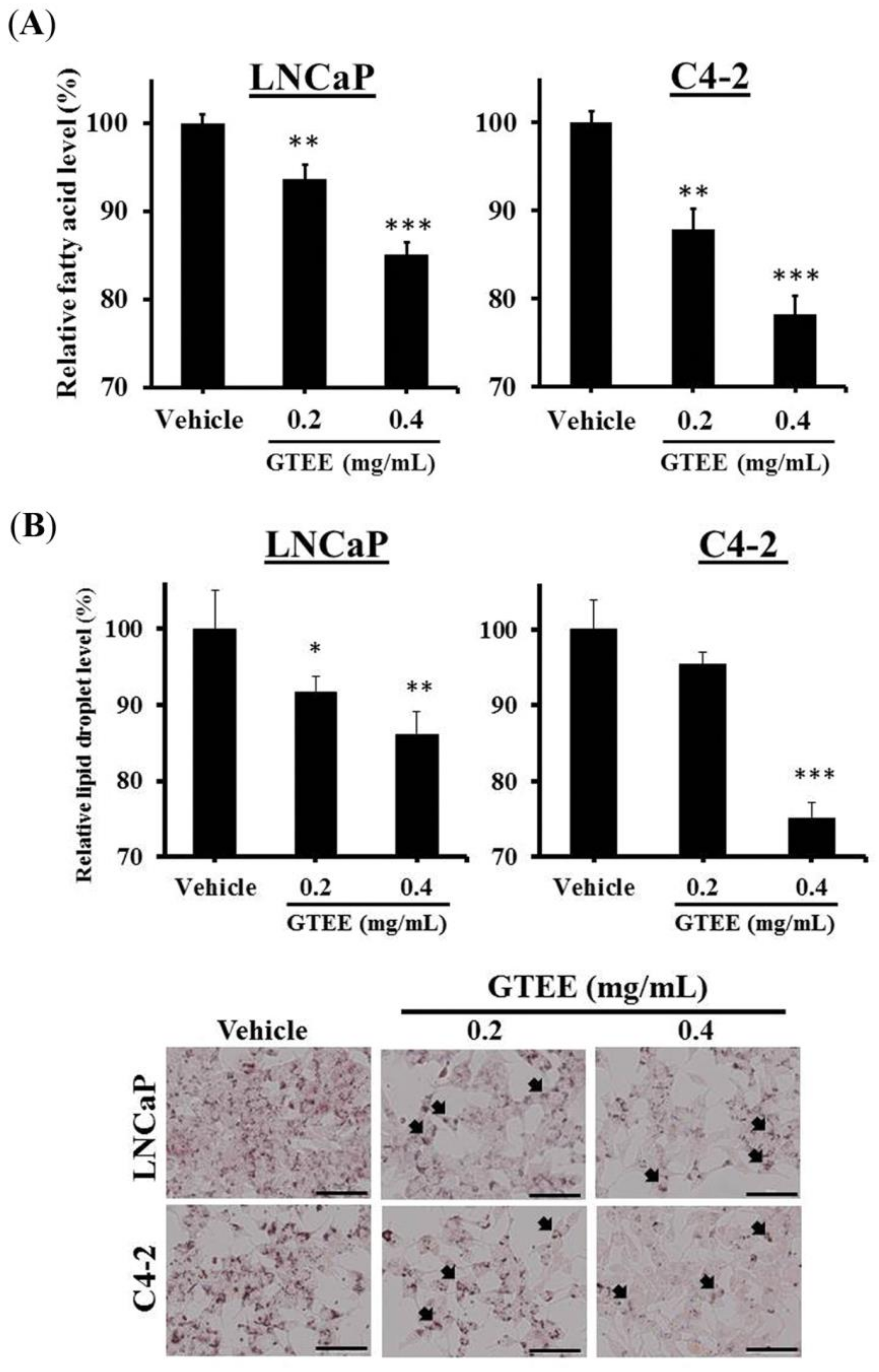
2.2. GTEE Reduces the Levels of Intracellular Fatty Acid and Lipid Accumulation in PCa Cells
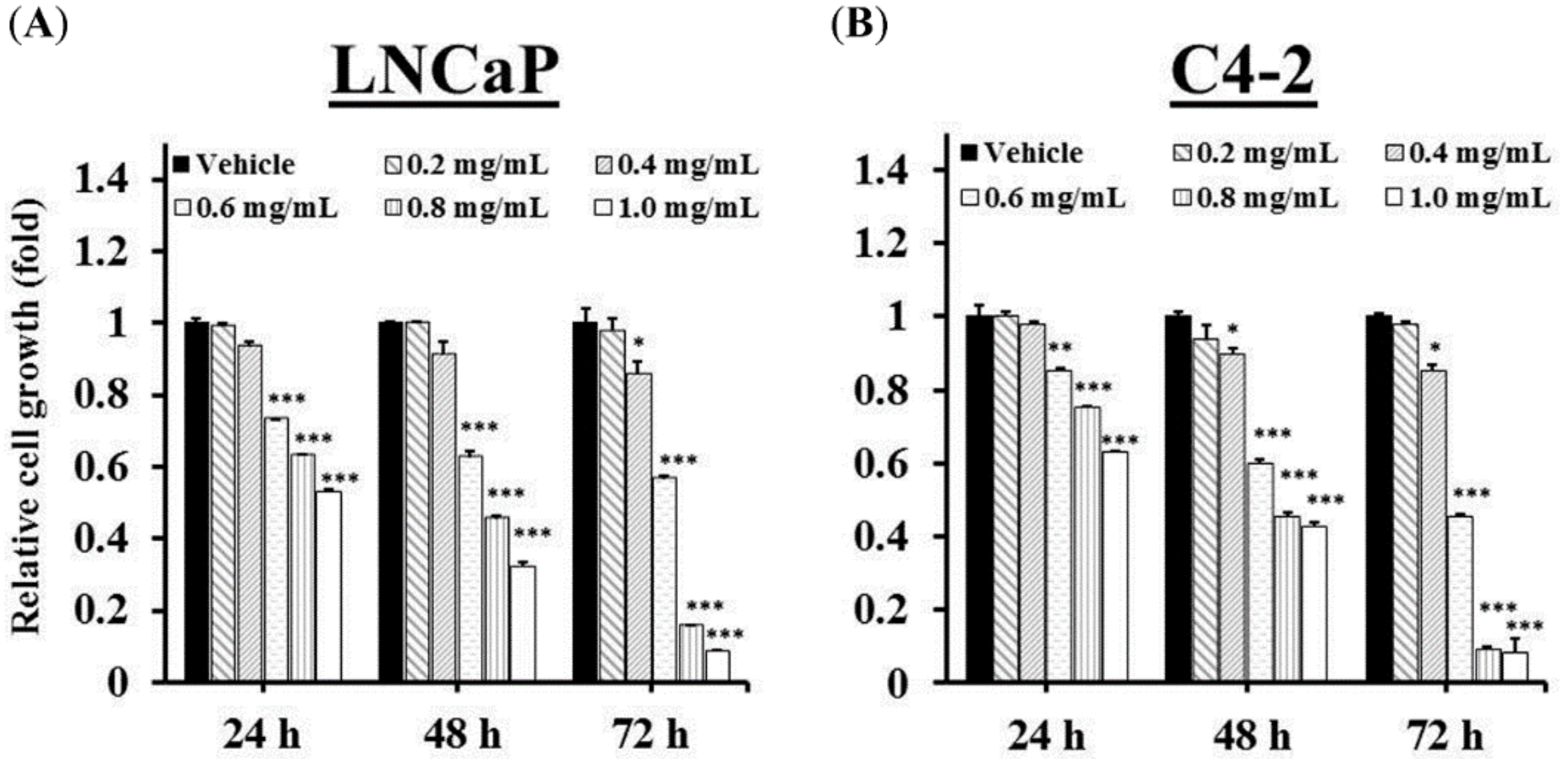
2.3. GTEE Suppresses the Cell Growth and In Vitro Progression of PCa Cells
2.4. GTEE Induces Apoptosis through the Caspase-Dependent Pathway in PCa Cells
3. Discussion
4. Materials and Methods
4.1. PCa Cell Lines and Cell Culture
4.2. Preparation of GTEE
4.3. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.4. Western Blot Analysis
4.5. The Fatty Acid Level and Lipid Droplet Accumulation Analyses
4.6. Cell Growth and In Vitro Progression Assays
4.7. Flow Cytometric Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lonergan, P.E.; Tindall, D.J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011, 10, 20. [Google Scholar] [PubMed]
- Heinlein, C.A.; Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H. Sterol regulatory element-binding proteins (SREBPs): Transcriptional regulators of lipid synthetic genes. Prog. Lipid Res. 2001, 40, 439–452. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997, 89, 331–340. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Heemers, H.; Deboel, L.; Foufelle, F.; Heyns, W.; Verhoeven, G. Stimulation of tumor-associated fatty acid synthase expression by growth factor activation of the sterol regulatory element-binding protein pathway. Oncogene 2000, 19, 5173–5181. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Shimomura, I.; Brown, M.S.; Hammer, R.E.; Goldstein, J.L.; Shimano, H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J. Clin. Invest. 1998, 101, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Li, X.; Liu, J.; Lin, J.T.; Chung, L.W. Activation of androgen receptor, lipogenesis and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol. Cancer Res. 2012, 10, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Zhau, H.E.; Chung, L.W. Androgen receptor survival signaling is blocked by anti-beta2-microglobulin monoclonal antibody via a MAPK/lipogenic pathway in human prostate cancer cells. J. Biol. Chem. 2010, 285, 7947–7956. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, S.L.; Sobel, R.; Whitmore, T.G.; Akbari, M.; Bradley, D.R.; Gleave, M.E.; Nelson, C.C. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004, 64, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Kuok, Q.Y.; Yeh, C.Y.; Su, B.C.; Hsu, P.L.; Ni, H.; Liu, M.Y.; Mo, F.E. The triterpenoids of Ganoderma tsugae prevent stress-induced myocardial injury in mice. Mol. Nutr. Food Res. 2013, 57, 1892–1896. [Google Scholar] [PubMed]
- Chen, M.L.; Hsieh, C.C.; Chiang, B.L.; Lin, B.F. Triterpenoids and Polysaccharide Fractions of Ganoderma tsugae Exert Different Effects on Antiallergic Activities. Evid. Based Complement. Alternat. Med. 2015, 2015, 754836. [Google Scholar] [PubMed]
- Kuo, H.P.; Hsu, S.C.; Ou, C.C.; Li, J.W.; Tseng, H.H.; Chuang, T.C.; Liu, J.Y.; Chen, S.J.; Su, M.H.; Cheng, Y.C.; et al. Ganoderma tsugae Extract Inhibits Growth of HER2-Overexpressing Cancer Cells via Modulation of HER2/PI3K/Akt Signaling Pathway. Evid. Based Complement. Alternat. Med. 2013, 2013, 219472. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Kuo, H.P.; Hsieh, H.H.; Li, J.W.; Hsu, W.H.; Chen, S.J.; Su, M.H.; Liu, S.H.; Cheng, Y.C.; Chen, C.Y.; et al. Ganoderma tsugae Induces S Phase Arrest and Apoptosis in Doxorubicin-Resistant Lung Adenocarcinoma H23/0.3 Cells via Modulation of the PI3K/Akt Signaling Pathway. Evid. Based Complement. Alternat. Med. 2012, 2012, 371286. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Ou, C.C.; Chuang, T.C.; Li, J.W.; Lee, Y.J.; Wang, V.; Liu, J.Y.; Chen, C.S.; Lin, S.C.; Kao, M.C. Ganoderma tsugae extract inhibits expression of epidermal growth factor receptor and angiogenesis in human epidermoid carcinoma cells: In vitro and in vivo. Cancer Lett. 2009, 281, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Ou, C.C.; Li, J.W.; Chuang, T.C.; Kuo, H.P.; Liu, J.Y.; Chen, C.S.; Lin, S.C.; Su, C.H.; Kao, M.C. Ganoderma tsugae extracts inhibit colorectal cancer cell growth via G(2)/M cell cycle arrest. J. Ethnopharmacol. 2008, 120, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.B.; Li, Q.; Shigemura, K.; Chung, L.W.; Huang, W.C. SREBP-2 promotes stem cell-like properties and metastasis by transcriptional activation of c-Myc in prostate cancer. Oncotarget 2016, 7, 12869–12884. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.T.; Josson, S.; Mukhopadhyay, N.K.; Kim, J.; Freeman, M.R.; Huang, W.C. MicroRNA-185 and 342 inhibit tumorigenicity and induce apoptosis through blockade of the SREBP metabolic pathway in prostate cancer cells. PLoS ONE 2013, 8, e70987. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Honda, M.; Takatori, H.; Nishino, R.; Minato, H.; Takamura, H.; Ohta, T.; Kaneko, S. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J. Hepatol. 2009, 50, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, J.V.; Brusselmans, K.; Verhoeven, G. Increased lipogenesis in cancer cells: New players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.R.; Cinar, B.; Lu, M.L. Membrane rafts as potential sites of nongenomic hormonal signaling in prostate cancer. Trends Endocrinol. Metab. 2005, 16, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Decker, J.P.; Lupu, R. In support of fatty acid synthase (FAS) as a metabolic oncogene: Extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J. Cell Biochem. 2005, 94, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, J.V.; Heemers, H.; van de Sande, T.; de Schrijver, E.; Brusselmans, K.; Heyns, W.; Verhoeven, G. Androgens, lipogenesis and prostate cancer. J. Steroid Biochem. Mol. Biol. 2004, 92, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.T.; Hu, P.; Huang, W.C. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol. Cancer Ther. 2014, 13, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zheng, X.; Qiu, C.; Dongol, S.; Lv, Q.; Jiang, J.; Kong, B.; Wang, C. SIRT1 promotes endometrial tumor growth by targeting SREBP1 and lipogenesis. Oncol. Rep. 2014, 32, 2831–2835. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.Y.; Lu, Q.T.; Li, W.H.; Yang, N.; Dongol, S.; Zhang, X.; Jiang, J. Sterol regulatory element-binding protein 1 is required for ovarian tumor growth. Oncol. Rep. 2013, 30, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.; Lewis, C.A.; Bensaad, K.; Ros, S.; Zhang, Q.; Ferber, E.C.; Konisti, S.; Peck, B.; Miess, H.; East, P.; et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer Metab. 2013, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Taplin, M.E. Androgen receptor: Role and novel therapeutic prospects in prostate cancer. Expert Rev. Anticancer Ther. 2008, 8, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; McDonnell, D.P. Androgen receptor-cofactor interactions as targets for new drug discovery. Trends Pharmacol. Sci. 2005, 26, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Boudadi, K.; Antonarakis, E.S. Resistance to Novel Antiandrogen Therapies in Metastatic Castration-Resistant Prostate Cancer. Clin. Med. Insights Oncol. 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Havel, J.J.; Zhau, H.E.; Qian, W.P.; Lue, H.W.; Chu, C.Y.; Nomura, T.; Chung, L.W. b2-Microglobulin Signaling Blockade Inhibited Androgen Receptor Axis and Caused Apoptosis in Human Prostate Cancer Cells. Clin. Cancer Res. 2008, 14, 5341–5347. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Yokomizo, A.; Fujimoto, N.; Naito, S. Androgen receptor cofactors in prostate cancer: Potential therapeutic targets of castration-resistant prostate cancer. Curr. Cancer Drug Targets. 2011, 11, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.H.; Brawer, M.K. Serum prostate-specific antigen: Its use in diagnosis and management of prostate cancer. Urology 1989, 33, 13–17. [Google Scholar] [CrossRef]
- Kim, J.; Coetzee, G.A. Prostate specific antigen gene regulation by androgen receptor. J. Cell Biochem. 2004, 93, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.B.; Chung, L.W.; Huang, W.C. Anti-cancer efficacy of SREBP inhibitor, alone or in combination with docetaxel, in prostate cancer harboring p53 mutations. Oncotarget 2015, 38, 41018–41032. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Li, S.; Zhuo, Y.; Chen, J.; Qin, X.; Guo, G. Anticancer effect of triterpenes from Ganoderma lucidum in human prostate cancer cells. Oncol. Lett. 2017, 14, 7467–7472. [Google Scholar] [PubMed]
- Kladar, N.V.; Gavaric, N.S.; Bozin, B.N. Ganoderma: Insights into anticancer effects. Eur. J. Cancer Prev. 2016, 25, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, G.N.; Sikes, R.A.; Wu, T.T.; Degeorges, A.; Chang, S.M.; Ozen, M.; Pathak, S.; Chung, L.W. LNCaP progression model of human prostate cancer: Androgen-independence and osseous metastasis. Prostate 2000, 44, 91–103. [Google Scholar] [CrossRef]
- Tilton, R.; Paiva, A.A.; Guan, J.Q.; Marathe, R.; Jiang, Z.; van Eyndhoven, W.; Bjoraker, J.; Prusoff, Z.; Wang, H.; Liu, S.H.; et al. A comprehensive platform for quality control of botanical drugs (PhytomicsQC): A case study of Huangqin Tang (HQT) and PHY906. Chin. Med. 2010, 5, 30. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-Y.; Huang, G.-J.; Wu, H.-C.; Kao, M.-C.; Huang, W.-C. Ganoderma tsugae Inhibits the SREBP-1/AR Axis Leading to Suppression of Cell Growth and Activation of Apoptosis in Prostate Cancer Cells. Molecules 2018, 23, 2539. https://doi.org/10.3390/molecules23102539
Huang S-Y, Huang G-J, Wu H-C, Kao M-C, Huang W-C. Ganoderma tsugae Inhibits the SREBP-1/AR Axis Leading to Suppression of Cell Growth and Activation of Apoptosis in Prostate Cancer Cells. Molecules. 2018; 23(10):2539. https://doi.org/10.3390/molecules23102539
Chicago/Turabian StyleHuang, Shih-Yin, Guan-Jhong Huang, Hsi-Chin Wu, Ming-Ching Kao, and Wen-Chin Huang. 2018. "Ganoderma tsugae Inhibits the SREBP-1/AR Axis Leading to Suppression of Cell Growth and Activation of Apoptosis in Prostate Cancer Cells" Molecules 23, no. 10: 2539. https://doi.org/10.3390/molecules23102539
APA StyleHuang, S.-Y., Huang, G.-J., Wu, H.-C., Kao, M.-C., & Huang, W.-C. (2018). Ganoderma tsugae Inhibits the SREBP-1/AR Axis Leading to Suppression of Cell Growth and Activation of Apoptosis in Prostate Cancer Cells. Molecules, 23(10), 2539. https://doi.org/10.3390/molecules23102539