Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases
Abstract
:1. Introduction
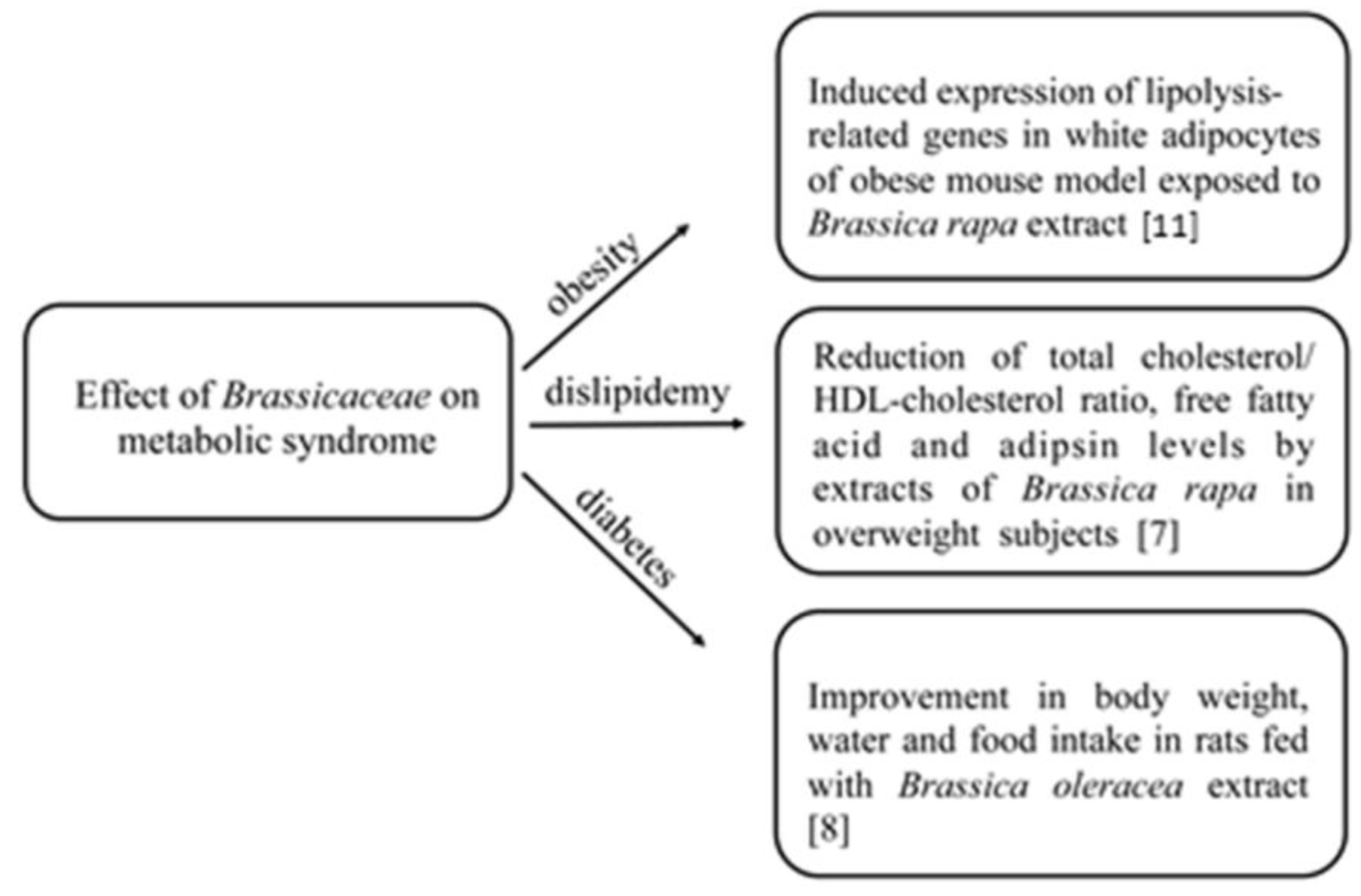
2. Bioactive Compounds in Brassicaceae and Their Effects on Chronic Diseases
3. Biofortification to Optimize the Content of Bioactive Compounds in Brassicaceae
4. Effect of Food Processing Techniques on Bioactive Compounds Content
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sasaki, K.; Takahashi, T. A flavonoid from Brassica rapa flower as UV-absorbing nectar guide. Phytochemistry 2002, 61, 339–343. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Comhaire, F. Nutriceutical approach to the metabolic syndrome. Endocrinol. Metab. Syndr. 2014, 3, 134. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Pernice, R.; Fogliano, V.; Barbieri, G. Sulphur fertilization may improve the nutritional value of Brassica rapa L. subsp. sylvestris. Eur. J. Agron. 2007, 26, 418–424. [Google Scholar] [CrossRef]
- Lippmann, D.; Lehmann, C.; Florian, S.; Barknowitz, G.; Haack, M.; Mewis, I.; Wiesner, M.; Schreiner, M.; Glatt, H.; Brigelius-Flohé, R. Glucosinolates from pakchoi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct. 2014, 5, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Sangha, M.H.; Banga, S.S.; Atwal, A.K.; Gupta, S. Heat stress tolerance in relation to oxidative stress and antioxidants in Brassica juncea. J. Environ. Biol. 2014, 35, 383–387. [Google Scholar] [PubMed]
- Jeon, S.M.; Kim, J.E.; Shin, S.K.; Kwon, E.Y.; Jung, U.J.; Baek, N.I.; Lee, K.T.; Jeong, T.S.; Chung, H.G.; Choi, M.S. Randomized double-blind placebo-controlled trial of powdered Brassica rapa ethanol extract on alteration of body composition and plasma lipid and adipocytokine profiles in overweight subjects. J. Med. Food 2013, 16, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Sarker, M.M.R.; Gousuddin, M. Antidiabetic potential of Brassica oleracea var. Italica in Type 2 diabetic Sprague dawley (sd) rats. Int. J. Phar. Phytochem. Res. 2016, 8, 462–469. [Google Scholar]
- Peluso, I.; Palmery, M. Is a flavonoid-rich diet with steamer cooking safe during calcineurin inhibitors therapy? J. Clin. Pharm. Therap. 2014, 39, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.H.; Kristal, A.R.; Stanford, J.L. Fruit and vegetable intakes and prostate cancer risk. J. Nat. Cancer Inst. 2000, 92, 61–68. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Han, J.I.; Kim, M.J.; Park, J.S.; Han, J.M.; Baek, N.I.; Chung, H.G.; Choi, M.S.; Lee, K.T.; Jeong, T.S. Ethanolic extracts of Brassica campestris spp. rapa roots prevent high-fat diet-induced obesity via beta(3)-adrenergic regulation of white adipocyte lipolytic activity. J. Med. Food. 2010, 13, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.A.; Nespereira, B.; Pérez-Ilzarbe, M.; Eguinoa, E.; Páramo, J.A. Vitamins C and E prevent endothelial VEGF and VEGFR-2 overexpression induced by porcine hypercholesterolemic LDL. Cardiovasc. Res. 2005, 65, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Esposito, G.; Ferracane, R.; Vinale, F.; Naviglio, D. Beneficial effects of Trichoderma genus microbes on qualitative parameters of Brassica rapa L. subsp. sylvestris L. Janch. var. esculenta Hort. Eur. Food Res. Technol. 2013, 236, 1063–1071. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Rose, P.; Huang, Q.; Ong, C.N.; Whiteman, M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2005, 209, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Tyagi, A.K.; Kaur, H. Cancer modulation by glucosinolates: A review. Current Sci. 2000, 79, 1665–1671. [Google Scholar]
- Yogeeta, S.K.; Hanumantra, R.B.R.; Gnanapragasam, A.; Senthilkumar, S.; Subhashini, R.; Devaki, T. Attenuation of abnormalities in the lipid metabolism during experimental myocardial infarction induced by isoproterenol in rats: beneficial effect of ferulic acid and ascorbic acid. Basic Clin. Pharmacol. Toxicol. 2006, 98, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Tomas-Barberan, F.A.; Garcia-Viguera, C. Effect of climatic and sulphur fertilisation conditions, on phenolic compounds and vitamin C, in the inflorescences of eight broccoli cultivars. Eur. Food Res. Technol. 2003, 216, 395–401. [Google Scholar] [CrossRef]
- Kaur, C.; Kumar, K.; Anil, D.; Kapoor, H.C. Variations in antioxidant activity in broccoli (Brassica oleracea L.) cultivars. J. Food Biochem. 2007, 31, 621–638. [Google Scholar] [CrossRef]
- Borowski, J.; Szajdek, A.; Borowska, E.J.; Ciska, E.; Zieliński, H. Content of selected bioactive components and antioxidant properties of broccoli (Brassica oleracea L.). Eur. Food Res. Technol. 2008, 226, 459–465. [Google Scholar] [CrossRef]
- Koh, E.; Wimalasiri, K.M.S.; Chassy, A.W.; Mitchell, A.E. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Compos. Anal. 2009, 22, 637–643. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Mena, P.; García-Viguera, C.; Moreno, D.A. Brassica foods as a dietary source of vitamin C: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1076–1091. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalána, E.; Beltrán-Debónc, R.; Menéndez, J.A.; Alonso-Villaverdee, C.; Segura-Carretero, A.; Jovenc, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis. Perspimpl. Phyt. 2012, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kang, M.; Xie, Q.; Xu, B.; Sun, C.; Chen, K.; Wu, Y. Anthocyanins from Chinese bayberry extract protect beta cells from oxidative stress-mediated injury via HO-1 upregulation. J. Agric. Food Chem. 2011, 59, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef]
- Tabatabaei-Malazy, O.; Larijani, B.; Abdollahi, M.A. Novel management of diabetes by means of strong antioxidants′ combination. J. Med. Hypotheses Ideas 2013, 7, 25–30. [Google Scholar] [CrossRef]
- Fard, M.H.; Naseh, G.; Lotfi, N.; Hosseini, S.M.; Hosseini, M. Effects of aqueous extract of turnip leaf (Brassica rapa) in alloxan-induced diabetic rats. Avicenna J. Phytomed. 2015, 5, 148–156. [Google Scholar]
- Büchert, A.M.; Lobato, M.E.G.; Villarreal, N.M.; Civello, P.M.; Martínez, G.A. Effect of visible light treatments on postharvest senescence of broccoli (Brassica oleracea L.). J. Sci. Food Agric. 2011, 91, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ito, Y.; Inoue, T.; Hamajima, N. Inverse association of serum carotenoids with prevalence of metabolic syndrome among Japanese. Clin. Nutr. 2011, 30, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, G.; Bottino, A.; Orsini, F.; De Pascale, S. Sulfur fertilization and light exposure during storage are critical determinants of the nutritional value of ready-to-eat friariello campano (Brassica rapa L. subsp. sylvestris). J. Agric. Food Chem. 2009, 89, 2261–2266. [Google Scholar] [CrossRef]
- Padilla, G.; Cartea, M.E.; Velasco, P.; de Haro, A.; Ordás, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2007, 68, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Gangopadhyay, H.; Das, D. Broccoli: A unique vegetable that protects mammalian hearts through the redox cycling of the thioredoxin superfamily. J. Agric. Food Chem. 2008, 56, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Sankhari, J.M.; Thounaojam, M.C.; Jadeja, R.N.; Devkar, R.V.; Ramachandran, A.V. Anthocyanin-rich red cabbage (Brassica oleracea L.) extract attenuates cardiac and hepatic oxidative stress in rats fed an atherogenic diet. J. Sci. Food Agric. 2012, 92, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Kural, B.V.; Küçük, N.; Yücesan, F.B.; Örem, A. Effects of kale (Brassica oleracea L. var. acephala DC) leaves extracts on the susceptibility of very low and low density lipoproteins to oxidation. Ind. J. Biochem. Biophys. 2011, 48, 361–364. [Google Scholar]
- Washida, K.; Miyata, M.; Koyama, T.; Yazawa, K.; Nomoto, K. Suppressive effect of yamato-mana (Brassica rapa L. oleifera group) constituent 3-Butenyl glucosinolate (gluconapin) on postprandial hypertriglyceridemia in mice. Biosci. Biotechnol. Biochem. 2010, 74, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Geremias, R.; Pedrosa, R.C.; Locatelli, C.; De Favere, V.T.; CouryPedrosa, R.; Laranjeira, M.C.M. Lipid lowering activity of hydrosoluble chitosan and association with Aloe vera L. and Brassica olearaceae L. Phytother. Res. 2006, 20, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trends Food Sci. Technol. 2014, 35, 114–128. [Google Scholar] [CrossRef]
- Thangam, R.; Suresh, V.; Rajkumar, M.; Vincent, J.D.; Gunasekaran, P.; Anbazhagan, C.; Kaver, K.; Kannan, S. Antioxidant and in vitro anticancer effect of 2-pyrrolidinone rich fraction of Brassica oleracea var. capitata through induction of apoptosis in human cancer cells. Phytother. Res. 2013, 27, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Galera, S.; Rojas, E.; Sudhakar, D.; Zhu, C.; Pelacho, A.M.; Capell, T.; Christou, P. Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Res. 2010, 19, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Farré, G.; Sanahuja, G.; Capell, T.; Zhu, C.; Christou, P. When more is better: Multigene engineering in plants. Trends Plant Sci. 2010, 15, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sanahuja, G.; Yuan, D.; Farré, G.; Arjó, G.; Berman, J.; Zorrilla-López, U.; Banakar, R.; Bai, C.; Pérez-Massot, E.; et al. Biofortification of plants with altered antioxidant content and composition: Genetic engineering strategies. Plant Biotechnol. J. 2013, 11, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.F.; Yousef, G.G.; Chebrolu, K.K.; Byrd, R.W.; Everhart, K.W.; Thomas, A.; Reid, R.W.; Parkin, I.A.; Sharpe, A.G.; Oliver, R.; et al. High-density single nucleotide polymorphism (SNP) array mapping in Brassica oleracea: Identification of QTL associated with carotenoid variation in broccoli florets. Theor. Appl. Genet. 2014, 127, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, T.; Soengas, P.; Velasco, P.; Rodríguez, V.M.; Cartea, M.E. Identification of metabolic QTLs and candidate genes for glucosinolate synthesis in Brassica oleracea leaves, seeds and flower buds. PLoS ONE 2014, 9, e91428. [Google Scholar] [CrossRef] [PubMed]
- Rahikainen, M.; Trotta, A.; Alegre, S.; Pascual, J.; Vuorinen, K.; Overmyer, K.; Moffatt, B.; Ravanel, S.; Glawischnig, E.; Kangasjärvi, S. PP2A-B′γ modulates foliar trans-methylation capacity and the formation of 4-methoxy-indol-3-yl-methyl glucosinolate in Arabidopsis leaves. Plant J. 2017, 89, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Velasco, P.; Rodríguez, V.M.; Francisco, M.; Cartea, M.E.; Soengas, P. Genetics and breeding of Brassica crops. In Glucosinolates; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–26. [Google Scholar]
- Zang, Y.X.; Kim, J.H.; Park, Y.D.; Kim, D.H.; Hong, S.B. Metabolic engineering of aliphatic glucosinolates in Chinese cabbage plants expressing Arabidopsis MAM1, CYP79F1, and CYP83A1. BMB Rep. 2008, 41, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Jin, M.; Chun, J.H.; Kim, S.J.; Park, B.S.; Shon, S.H.; Kim, J.S. Functional analysis of three BrMYB28 transcription factors controlling the biosynthesis of glucosinolates in Brassica rapa. Plant Mol. Biol. 2016, 90, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Veremeichik, G.N.; Grigorchuk, V.P.; Rybin, V.G.; Shkryl, Y.N. The rolB gene activates secondary metabolism in Arabidopsis calli via selective activation of genes encoding MYB and bHLH transcription factors. Plant Physiol. Biochem. 2016, 102, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Raclaru, M.; Gruber, J.; Kumar, R.; Sadre, R.; Lühs, W.; Zarhloul, M.K.; Friedt, W.; Frentzen, M.; Weier, D. Increase of the tocochromanol content in transgenic Brassica napus seeds by overexpression of key enzymes involved in prenylquinone biosynthesis. Mol. Breed. 2006, 18, 93–107. [Google Scholar] [CrossRef]
- Fujisawa, M.; Takita, E.; Harada, H.; Sakurai, N.; Suzuki, H.; Ohyama, K.; Shibata, D.; Misawa, N. Pathway engineering of Brassica napus seeds using multiple key enzyme genes involved in ketocarotenoid formation. J. Exp. Bot. 2009, 60, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, M.J.; Pan, H.Y.; Cui, D.J.; Gruber, M.Y. Purple canola: Arabidopsis PAP1 increases antioxidants and phenolics in Brassica napus leaves. J. Agric. Food Chem. 2010, 58, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of glucosinolates, phenolic compounds and associated gene expression profiles of hairy root cultures in turnip (Brassica rapa ssp. rapa). 3 Biotech 2016, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.X.; Lim, M.H.; Park, B.S.; Hong, S.B.; Kim, D.H. Metabolic engineering of indole glucosinolates in Chinese cabbage plants by expression of Arabidopsis CYP79B2, CYP79B3, and CYP83B1. Mol. Cells 2008, 30, 231–241. [Google Scholar]
- Zang, Y.X.; Kim, H.U.; Kim, J.A.; Lim, M.H.; Jin, M.; Lee, S.C.; Kwon, S.J.; Lee, S.I.; Hong, J.K.; Park, T.H.; et al. Genome-wide identification of glucosinolate synthesis genes in Brassica rapa. FEBS J. 2009, 276, 3559–3574. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pujante, P.J.; Borja-Martínez, M.; Pedreño, M.Á.; Almagro, L. Biosynthesis and bioactivity of glucosinolates and their production in plant in vitro cultures. Planta 2017, 246, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Tomás-Barberán, F.A.; García-Viguera, C. Phenolic compound contents in edible parts of broccoli inflorescences after domestic cooking. J. Sci. Food Agric. 2003, 83, 1511–1516. [Google Scholar] [CrossRef]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; Garcia-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. [Google Scholar] [CrossRef] [PubMed]
- Galgano, F.; Favati, F.; Caruso, M.; Pietrafesa, A.; Natella, S. The Influence of processing and preservation on the retention of health-promoting compounds in broccoli. J. Food Sci. 2007, 72, S130–S135. [Google Scholar] [CrossRef] [PubMed]
- Munkaya, A.W.; Mankule, E.E.; Oey, I.; Loey, A.V.; Hendrikx, M. Thermal stability of L-Ascorbic Acid and ascorbic acid oxidase in broccoli (Brassica oleracea var. italica). J. Food Sci. 2010, 75, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Thornalley, P.J. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem. Toxicol. 2007, 45, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Sun, B.; Yuan, J.; Wang, Q. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B. 2009, 10, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Jia, C.G.; Xu, C.J.; Wei, J.; Yuan, J.; Yuan, G.F.; Wang, B.L.; Wang, Q.M. Effect of modified atmosphere packaging on visual quality and glucosinolates of broccoli florets. Food Chem. 2009, 114, 28–37. [Google Scholar] [CrossRef]
- Dos Reis, L.C.R.; de Oliveira, V.R.; Hagen, M.E.K.; Jablonski, A.; Flores, S.H.; de Oliveira Rios, A. Carotenoids, flavonoids, chlorophylls, phenolic compounds and antioxidant activity in fresh and cooked broccoli (Brassica oleracea var. Avenger) and cauliflower (Brassica oleracea var. Alphina F1). LWT Food Sci. Technol. 2015, 63, 177–183. [Google Scholar] [CrossRef]
- Francisco, M.; Tortosa, M.; del Carmen Martínez-Ballesta, M.; Velasco, P.; García-Viguera, C.; Moreno, D.A. Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Ann. Appl. Biol. 2017, 170, 273–285. [Google Scholar] [CrossRef]
| Compound | Mechanism | Reference |
|---|---|---|
| Ascorbic acid | ROS reduction and neutralization | [12] |
| Protection against LDL oxidation | [13] | |
| Prevention of oxLDL-induced overexpression of Vascular Endothelial Growth Factor | ||
| Phenolics | ROS neutralization | [14] |
| Chelation of redox-active metal ions and inhibition of LDL-cholesterol oxidation | ||
| Carotenoids | Radical scavengers and quenches of singlet oxygen | [15] |
| Glucosinolates | Inhibition of the invasive potential of human cancer cell line in vitro | [16] |
| Regulation of the phase I and/or phase II detoxification enzymes activity | [17] |
| Treatment | Effect on Nutritional Quality | Reference |
|---|---|---|
| High pressure boiling | Degradation of hydroxycinnamic acids and flavonoids | [60] |
| Glucosinolates hydrolysis causing the formation of isothiocyanates | [62] | |
| Steaming cooking | Reduction of phenolic degradation | [59] |
| Inactivation of myrosinase and low loss of glucosinolates | [62] | |
| Microwaving- pressure cooking | Low loss of AsA and carotenoids | [60] |
| MAP treatment | Good preservation of glucosinolates | [65] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raiola, A.; Errico, A.; Petruk, G.; Monti, D.M.; Barone, A.; Rigano, M.M. Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases. Molecules 2018, 23, 15. https://doi.org/10.3390/molecules23010015
Raiola A, Errico A, Petruk G, Monti DM, Barone A, Rigano MM. Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases. Molecules. 2018; 23(1):15. https://doi.org/10.3390/molecules23010015
Chicago/Turabian StyleRaiola, Assunta, Angela Errico, Ganna Petruk, Daria Maria Monti, Amalia Barone, and Maria Manuela Rigano. 2018. "Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases" Molecules 23, no. 1: 15. https://doi.org/10.3390/molecules23010015
APA StyleRaiola, A., Errico, A., Petruk, G., Monti, D. M., Barone, A., & Rigano, M. M. (2018). Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases. Molecules, 23(1), 15. https://doi.org/10.3390/molecules23010015







