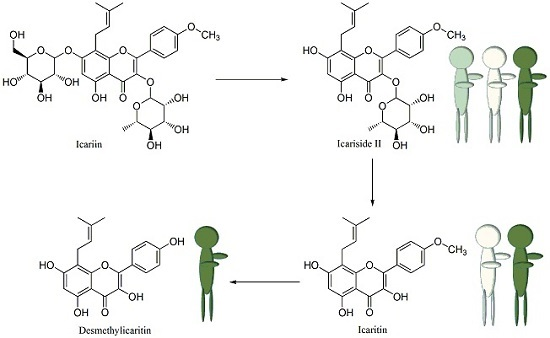
Icariin Metabolism by Human Intestinal Microflora
Abstract
:1. Introduction
2. Results
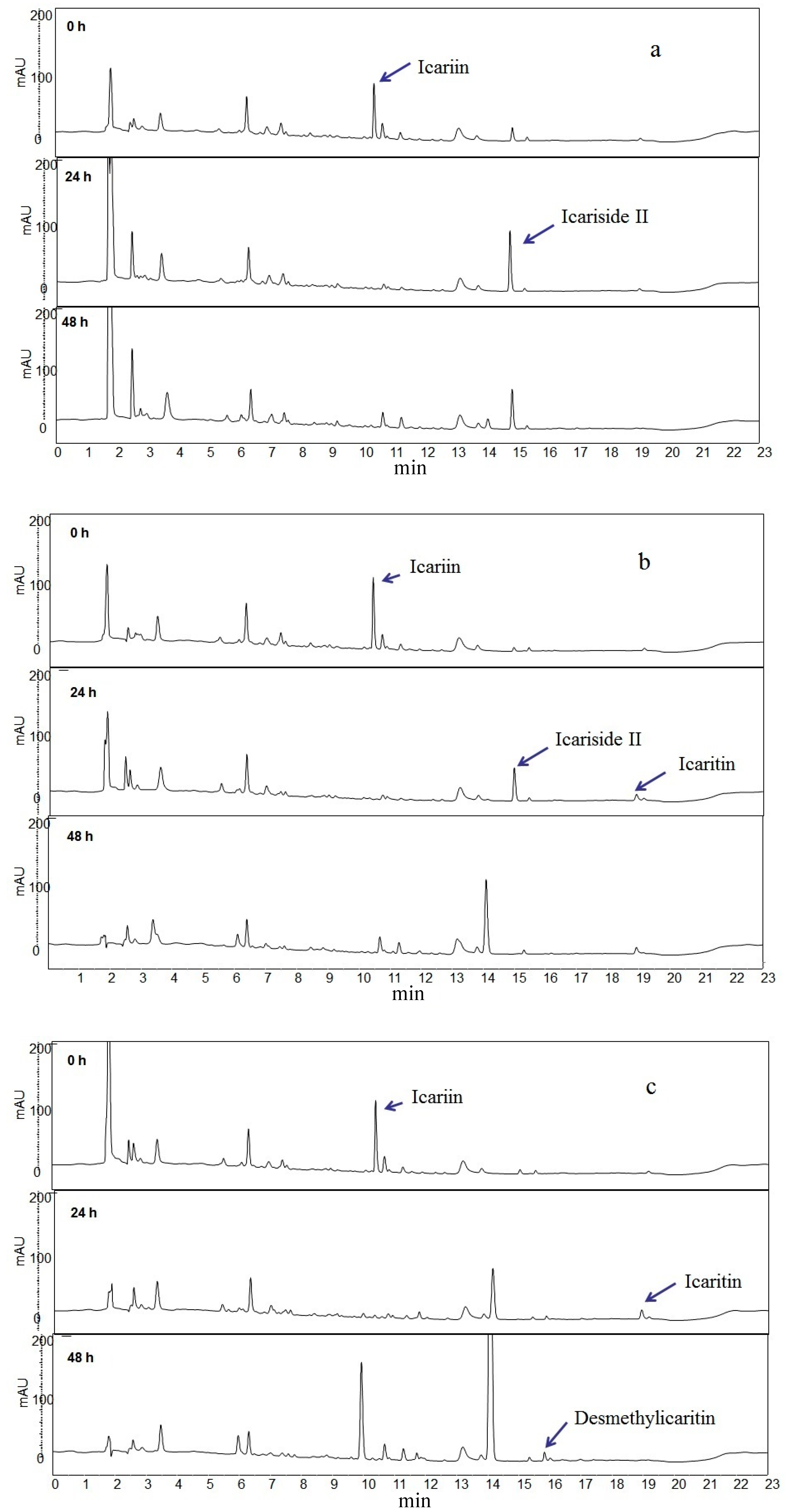
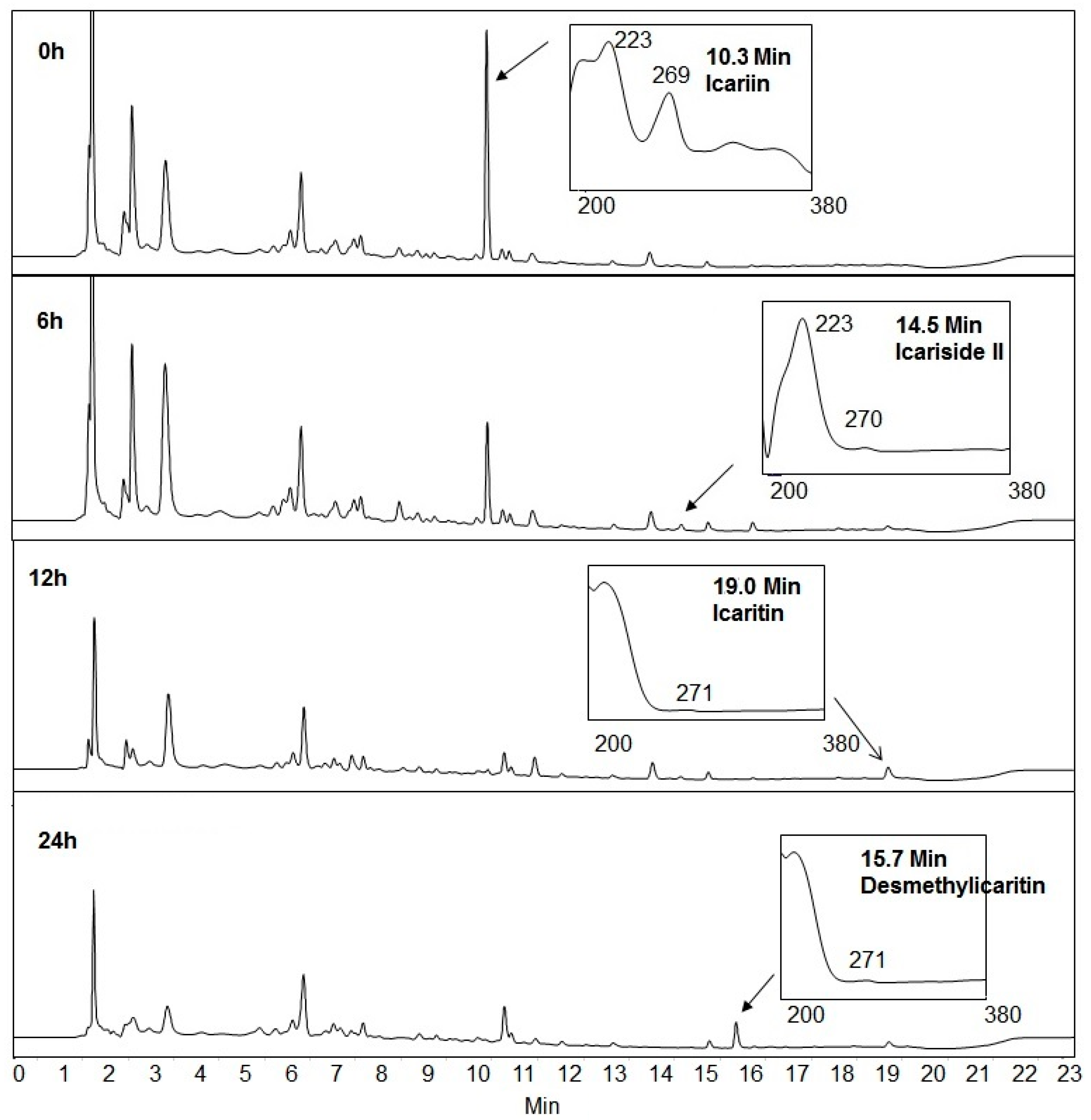
2.1. Icariin Metabolism by Human Intestinal Microflora
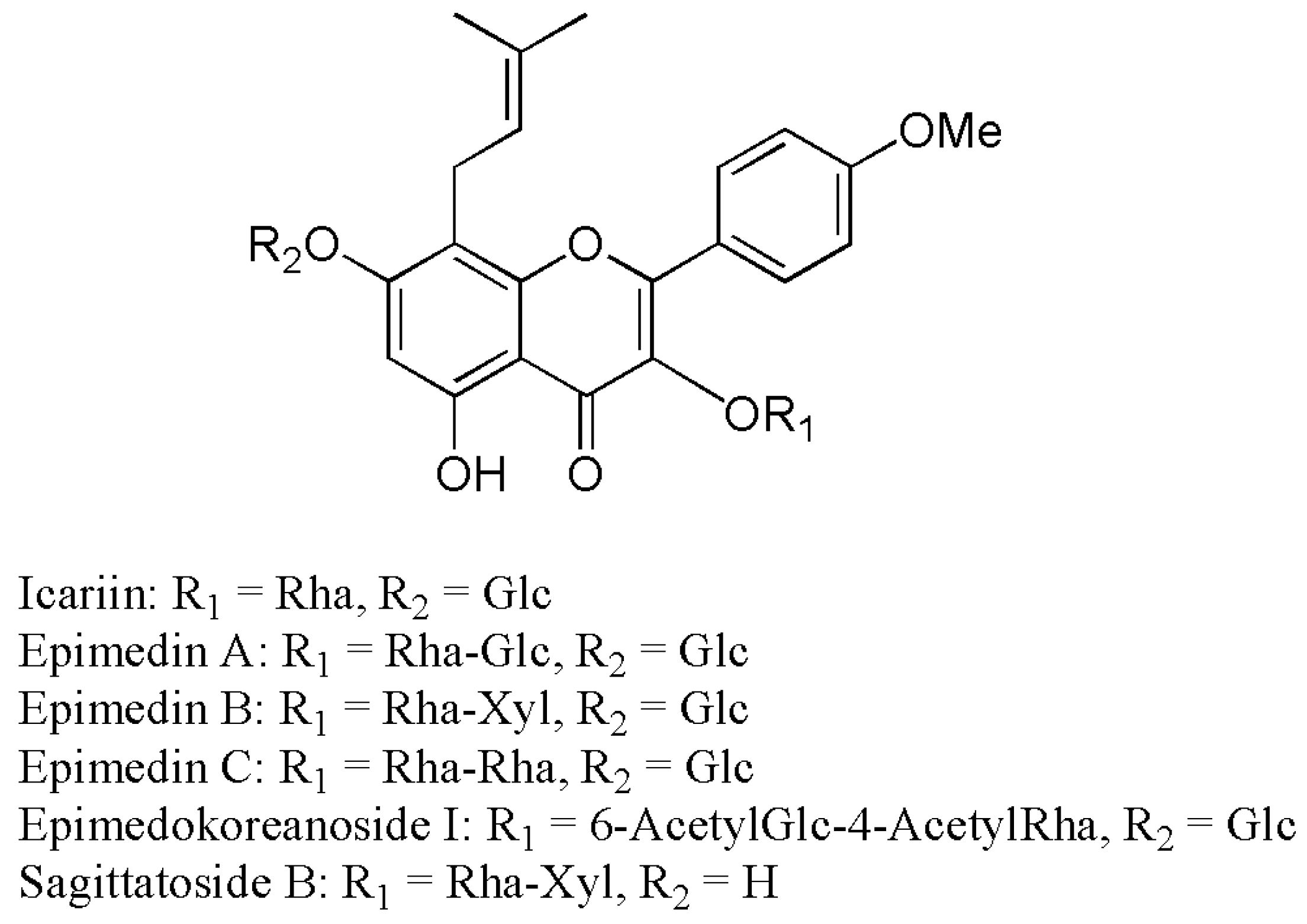
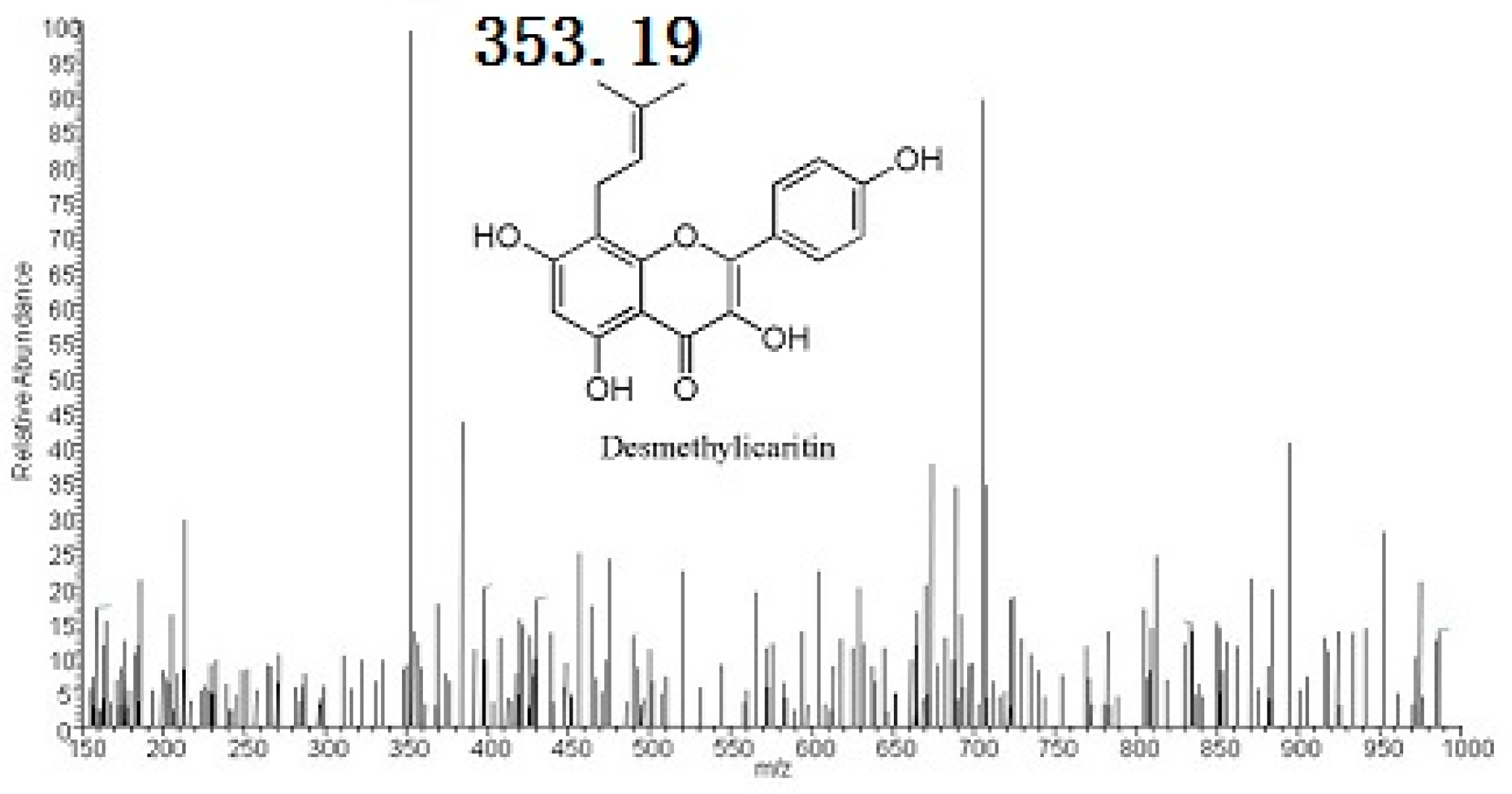
2.2. Metabolites Resulting from Icariin Metabolism by Intestinal Bacteria
2.3. Biotransformation Rate of Icariin by Intestinal Bacteria
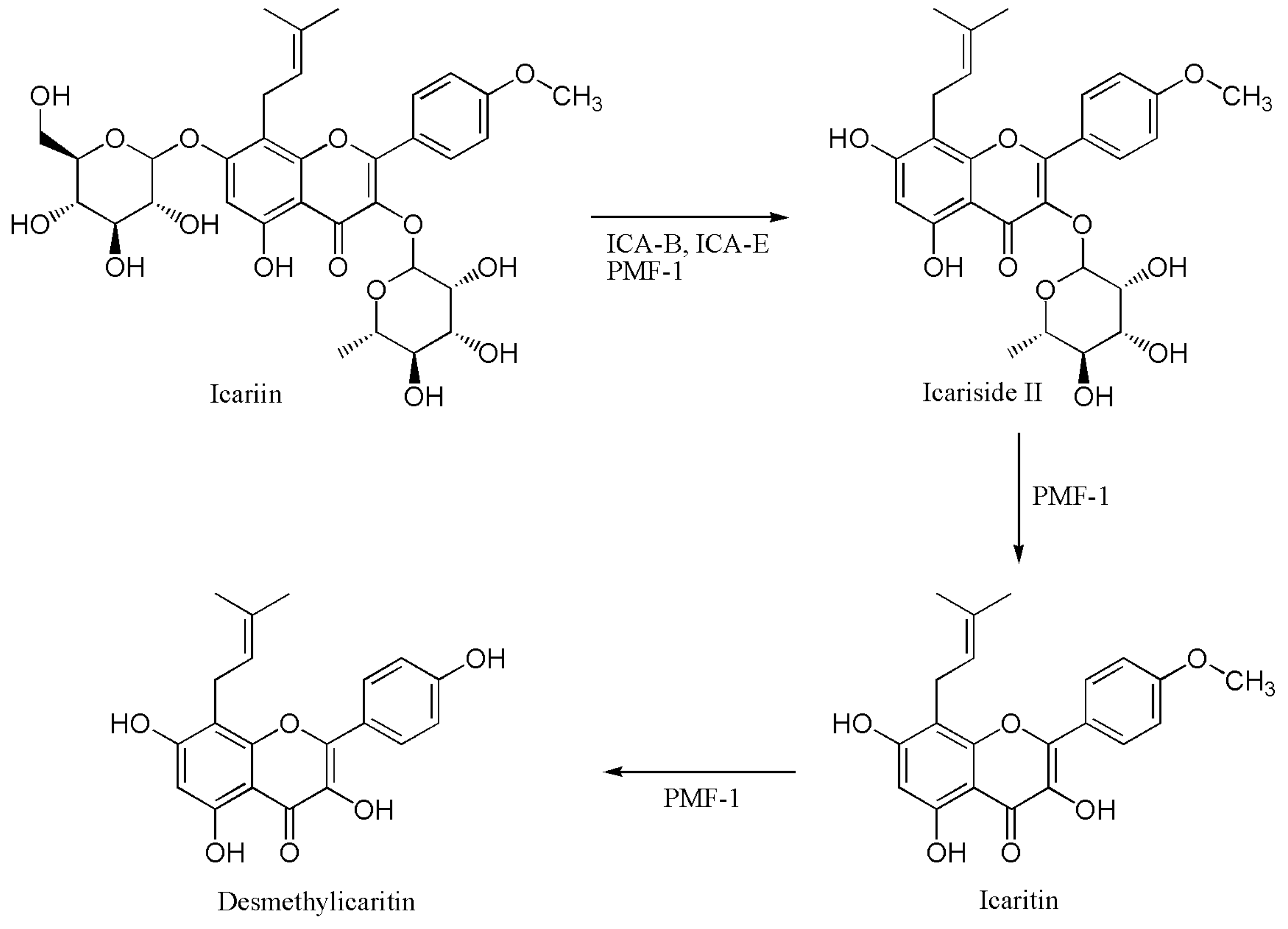
2.4. Proposed Metabolic Pathway of Icariin in Human Intestine
3. Discussion
4. Materials and Methods
4.1. Chemicals and Bacteria
4.2. General HPLC Methods
4.3. Icariin Metabolism by Human Intestinal Bacteria
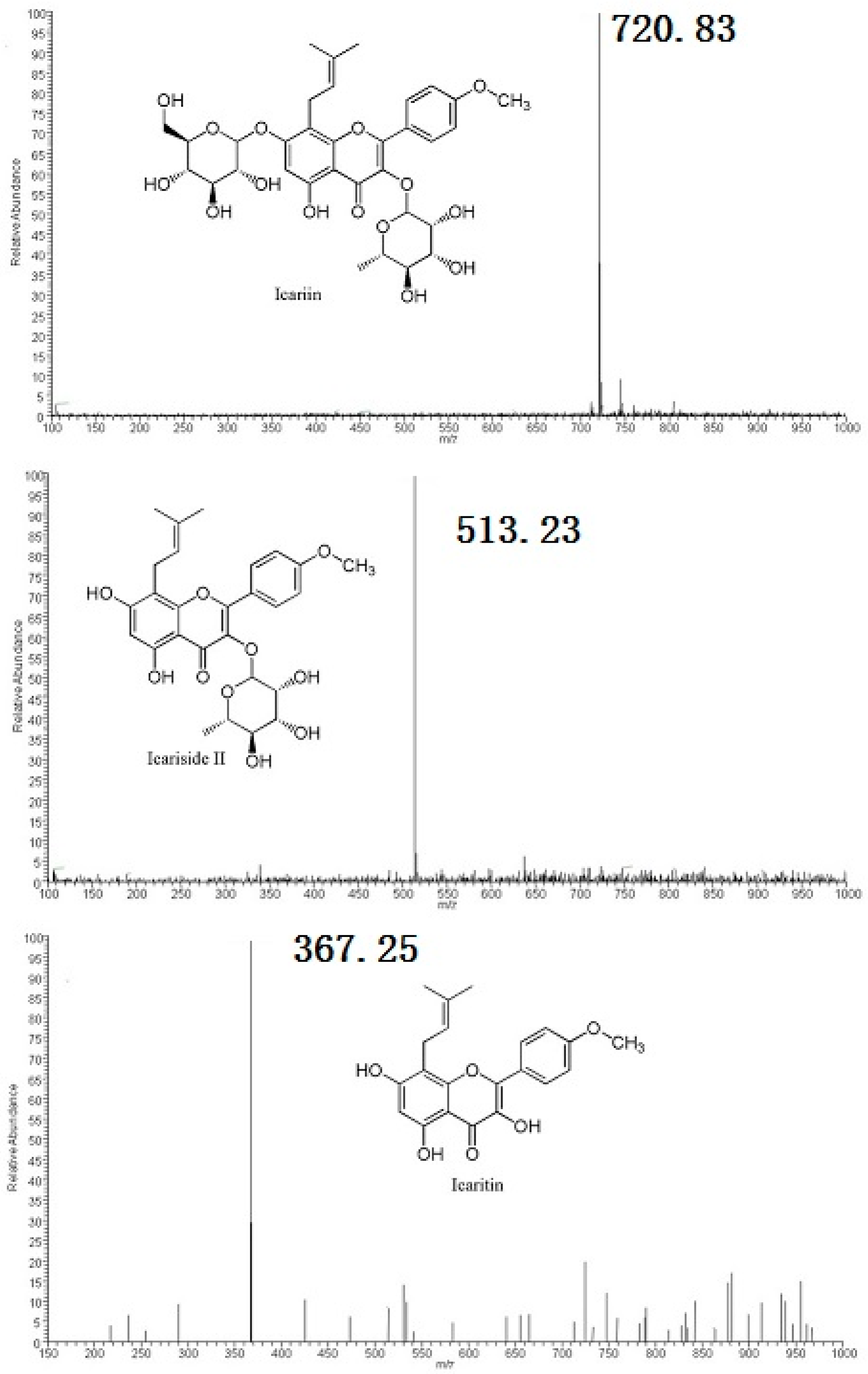
4.4. Structural Analyses of Icariin Metabolites by HPLC-MS
4.5. Biotransformation Kinetics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, W.; Eisenbrand, G. Handbook of Chinese Medicinal Plants; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Wang, L.; Wang, X.; Kong, L. Automatic authentication and distinction of Epimedium koreanum and Epimedium wushanense with HPLC fingerprint analysis assisted by pattern recognition techniques. Biochem. Syst. Ecol. 2012, 40, 138–145. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Mei, Q.; Lu, T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015, 126, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, M.S.; Ding, T.; Wang, J.; Oh, D.H. Biological activities of isolated icariin from Epimedium koreanum Nakai. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1397–1403. [Google Scholar] [CrossRef]
- Ma, H.R.; Wang, J.; Chen, Y.F.; Chen, H.; Wang, W.S.; Aisa, H.A. Icariin and icaritin stimulate the proliferation of SKBr3 cells through the GPER1-mediated modulation of the EGFR-MAPK signaling pathway. Int. J. Mol. Med. 2014, 33, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H.; Liu, M.H. Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine 2010, 17, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.K.; Guo, X.Y.; Ge, B.F.; Zhen, P.; Ma, X.N.; Zhou, J.; Ma, H.P.; Xian, C.J.; Chen, K.M. Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone 2014, 66, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.Y.; Lou, Y.J. Estrogenic effects of two derivatives of icariin on human breast cancer MCF-7 cells. Phytomedicine 2005, 12, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.T.T.; Choi, R.C.Y.; Chu, G.K.Y.; Cheung, A.W.H.; Gao, Q.T.; Li, J.; Jiang, Z.Y.; Dong, T.T.X.; Tsim, K.W.K. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: A comparison of different flavonoids in activating estrogenic effect and in preventing β-amyloid-induced cell death. J. Agric. Food Chem. 2007, 55, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Choi, Y.H.; Kwon, H.; Lee, S.B.; Kim, D.H.; Sung, C.K.; Park, Y.I.; Dong, M.S. Estrogenic/antiestrogenic activities of a Epimedium koreanum extract and its major components: In vitro and in vivo studies. Food Chem. Toxicol. 2012, 50, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Li, S.L.; Sun, E.; Zhang, K.R.; Tan, X.B.; Wei, Y.J.; Fan, H.W.; Cui, L.; Jia, X.B. Metabolite profiles of icariin in rat plasma by ultra-fast liquid chromatography coupled to triple-quadrupole/time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2012, 66, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Lin, C.W.; Lin, L.C.; Chiu, A.W.; Chen, K.K.; Tsai, T.H. Analysis of biliary excretion of icariin in rats. J. Agric. Food Chem. 2010, 58, 9905–9911. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, Y.; Wang, Y.; Gao, X.; Qu, D.; Liu, C. A comparative study on the metabolism of Epimedium koreanum Nakai-Prenyllated flavonoids in rats by an intestinal enzyme (lactase phlorizin hydrolase) and intestinal flora. Molecules 2014, 19, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.; Han, J. Deglycosylation of isoflavone C-glycosides by newly isolated human intestinal bacteria. J. Sci. Food Agric. 2015, 95, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, N.; Han, J. Deglycosylation of flavonoid O-glucosides by human intestinal bacteria Enterococcus sp. MRG-2 and Lactococcus sp. MRG-IF-4. Appl. Biol. Chem. 2016, 59, 443–449. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.I.; Han, J.; Wang, X.L.; Song, D.G.; Kim, S.U. Stereospecific biotransformation of dihydrodaidzein into (3S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl. Environ. Microbiol. 2009, 75, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Marsh, E.N.G.; Kim, S.U.; Han, J. Conversion of (3S,4R)-tetrahydrodaidzein to (3S)-equol by THD reductase: Proposed mechanism involving a radical intermediate. Biochemistry 2010, 49, 5582–5587. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Won, D.; Han, J. Absolute configuration determination of isoflavan-4-ol stereoisomers. Bioorg. Med. Chem. Lett. 2010, 20, 4337–4341. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, M.; Han, J. Stereospecific microbial production of isoflavanones from isoflavones and isoflavone glucosides. Appl. Microbiol. Biotechnol. 2011, 91, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Han, J. Absolute configuration of (−)-2-(4-hydroxyphenyl)propionic acid: Stereochemistry of soy isoflavone metabolism. Bull. Korean Chem. Soc. 2014, 35, 1883–1886. [Google Scholar] [CrossRef]
- Kim, M.; Han, J. Chiroptical study and absolute configuration of (−)-O-DMA produced from daidzein metabolism. Chirality 2014, 26, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.H.; Liu, M.Y.; Dai, Y.; Zhang, Y.; Qin, Z.F.; Tu, F.J.; Yao, X.S. Metabolism of Epimedium-derived flavonoid glycosides in intestinal flora of rabbits and its inhibition by gluconolactone. Chin. J. Natl. Med. 2011, 9, 461–465. [Google Scholar]
- Cho, N.J.; Sung, S.H.; Lee, H.S.; Jeon, M.H.; Kim, Y.C. Anti-hepatotoxic activity of icariside II, a constituent of Epimedium koreanum. Arch. Pharm. Res. 1995, 18, 289–292. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Lou, Y.J. Proliferation-stimulating effects of icaritin and desmethylicaritin in MCF-7 cells. Eur. J. Pharmacol. 2004, 504, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, W.; Qu, L.; Wu, J.; Si, J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and P-glycoprotein expression. Mol. Med. Rep. 2013, 8, 1883–1887. [Google Scholar] [PubMed]
- Wu, T.; Wang, S.; Wu, J.; Lin, Z.; Sui, Z.; Xu, X.; Shimizu, N.; Chen, B.; Wang, X. Icaritin induces lytic cytotoxicity in extranodal NK/T-cell lymphoma. J. Exp. Clin. Cancer Res. 2015, 34, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wang, N.; Zheng, L.Z.; Xie, X.H.; Yao, D.; Liu, M.Y.; Yao, Z.H.; Dai, Y.; Zhang, G.; Yao, X.S.; et al. Phytoestrogenic molecule desmethylicaritin suppressed adipogenesis via Wnt/β-catenin signaling pathway. Eur. J. Pharmacol. 2013, 714, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, N.; Han, J. Metabolism of Kaempferia parviflora polymethoxyflavones by human intestinal bacterium Bautia sp. MRG-PMF1. J. Agric. Food Chem. 2014, 62, 12377–12383. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of icariin, icariside II, and icaritin are available from the authors and commercial sources.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Kim, M.; Han, J. Icariin Metabolism by Human Intestinal Microflora. Molecules 2016, 21, 1158. https://doi.org/10.3390/molecules21091158
Wu H, Kim M, Han J. Icariin Metabolism by Human Intestinal Microflora. Molecules. 2016; 21(9):1158. https://doi.org/10.3390/molecules21091158
Chicago/Turabian StyleWu, Hailong, Mihyang Kim, and Jaehong Han. 2016. "Icariin Metabolism by Human Intestinal Microflora" Molecules 21, no. 9: 1158. https://doi.org/10.3390/molecules21091158
APA StyleWu, H., Kim, M., & Han, J. (2016). Icariin Metabolism by Human Intestinal Microflora. Molecules, 21(9), 1158. https://doi.org/10.3390/molecules21091158