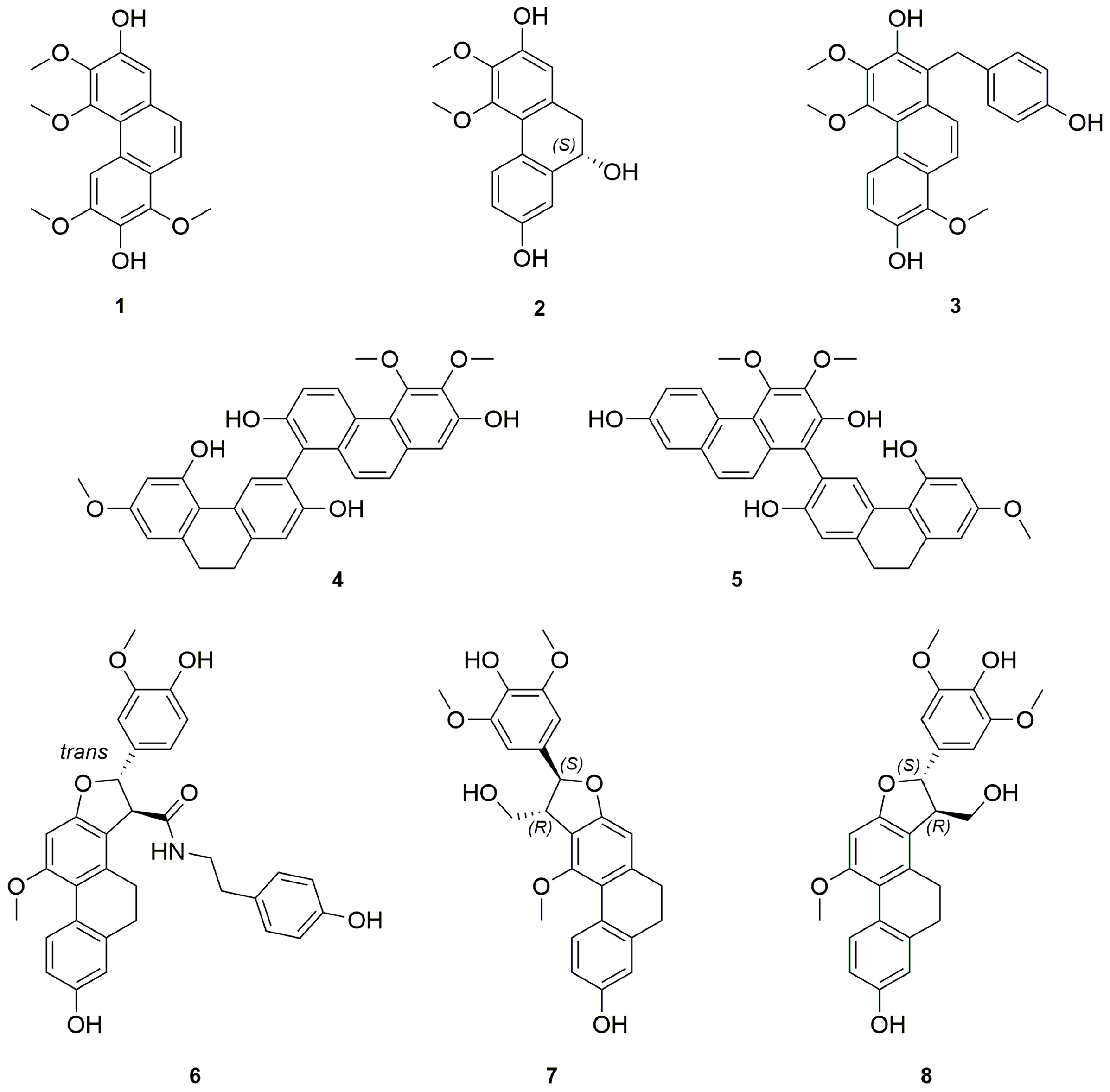
Abstract
We report the first phytochemical study of the neotropical orchid Cyrtopodium paniculatum. Eight new compounds, including one phenanthrene 1, one 9,10-dihydro-phenanthrene 2, one hydroxybenzylphenanthrene 3, two biphenanthrenes 4–5, and three 9,10 dihydrophenanthrofurans 6–8, together with 28 known phenolic compounds, mostly stilbenoids, were isolated from the CH2Cl2 extract of its leaves and pseudobulbs. The structures of the new compounds were established on the basis of extensive spectroscopic methods.
1. Introduction
The family Orchidaceae comprises 820 genera with almost 35,000 described species and can be regarded as the largest family of flowering plants. The phytochemical and biological investigations of this family has been mainly conducted in relation with their traditional uses and focused on a large number of Asian orchids from the genus Arundina, Bletilla, Dendrobium, Gastrodia and Pleione at the expense of the New World orchids In fact, the first report on a phytochemical study of South American orchids was in the late 1990s and was mainly focused on species in the genus Scaphyglottis [1,2,3], Maxillaria [4,5,6,7] and Cyrtopodium [8,9,10].
This genus Cyrtopodium includes 47 endemic species, distributed from Southern Florida to Central America, and they are terrestrial or epiphytic orchids, well recognized by their ovoid to fusiform pseudobulbs as well as their showy flowers [11,12,13,14]. To the best of our knowledge, only three Cyrtopodium species have been explored: C. cardiochilum Lindl. and C. andersonnii R.Br. for their polysaccharidic content, which displays anti-inflammatory and gastroprotective activities [8,10], and C. macrobulbon (La Llave & Lex.) G.A. Romero & Carnevali, a species traditionally used for treating urinary infections and whose stilbenoids are considered as its bioactive constituents [9]. C. paniculatum (Ruiz & Pav.) Garay has not yet received any attention since no report on its medicinal uses or chemical composition has been found in the literature. This provides a substantial basis for a detailed phytochemical investigation of this unexplored species. Therefore, in our continuing search for bioactive secondary metabolites from orchids [15,16,17], we carried out an investigation on the CH2Cl2 extracts from the leaves and pseudobulbs of C. paniculatum. This investigation led to the isolation of eight new compounds 1–8, together with 28 known compounds. In this paper, we describe the structure elucidation of these new compounds, which was performed by means of NMR, HRESIMS data and CD spectrum determination.
2. Results and Discussion
Structure Elucidation
The pseudobulbs and the leaves from C. paniculatum were studied separately. The freshly cut pseudobulbs (7.5 kg) were ground and macerated in water to get rid of their sugar content, and then in ethanol to afford a crude extract (60 g). The alcoholic crude extract was suspended in water and sequentially extracted with cyclohexane, CH2Cl2 and n-BuOH. The CH2Cl2 extract was concentrated to dryness and subjected to silica gel column chromatography, Sephadex LH-20 and preparative thin layer chromatography (PTLC) to afford eight new compounds 1–8 (Figure 1) together with 28 previously described compounds 9–35 which were identified as para-ethoxybenzyl alcohol (9) [18,19], 3,4,6-trimethoxyl-9,10-dihydrophenanthrene-2,7-diol (10) [20], confusarin (11) [21], erianthridin (12) [22], para-hydroxybenzaldehyde (13) [18], nudol (14) [23], cephathrene-B (15) [24], gigantol (16) [25], batatasin III (17) [26], ephemeranthoquinone (18) [27], coelonin (19) [28], 2,4-dimethoxy-phenanthrene-3,7-diol (20) [29], lusianthridin (21) [30], densiflorol B (22) [31], denthyrsinin (23) [32], bleformins A (24) and B (25) [33], chrysoeriol (26) [34,35], vanillic alcool (27) [36], gastrodigenin (28) [37,38], 1-(4-hydroxybenzyl)-4-methoxyl-9,10-dihydrophenanthrene-2,7-diol (29) [39], shancidin (30) [40], blestriarenes A (32) and B (31) [41], velutin (33) [42], (+)-syringaresinol (34) [43] and (+)-balanophonin (35) [44], respectively, by comparison of their UV, MS, and NMR data with literature data.
Figure 1.
Chemical structures of compounds 1–8 from C. paniculatum pseudobulbs.
In parallel, the leaves (70 g) were ground, lyophilized and successively extracted by maceration with cyclohexane, CH2Cl2 and n-BuOH. The CH2Cl2 extract (1.23 g) was fractionated using vacuum liquid chromatography (VLC) and semi-preparative RP-HPLC to afford gastrodigenin (28), coelonin (19), ephemeranthoquinone (18), lusianthridin (21), 1-(4-hydroxybenzyl)-4-methoxyl-9,10-dihydrophenanthrene-2,7-diol (29) and trans-feruloyltyramine (36) [45].
Compound 1 was obtained as a brown amorphous powder and showed a [M + H]+ peak at m/z 331.1188 (calcd. for C18H19O6 331.1176) in the HRESIMS, indicating a molecular formula of C18H18O6 and suggesting the existence of ten degrees of unsaturation. The UV maximal absorptions at 216, 261, 281, 312 and 344 nm indicated the presence of a phenanthrene derivative [46,47,48]. The IR spectrum exhibited a broad peak at 3370 cm−1, characteristic of hydroxyl groups, and 1616, 1576, 953 and 860 cm−1, characteristic of aromatic rings. Comparison of the HRMS and the 1H-NMR data of 1 to those of cephathrene-B (15) [16] indicated substantial similarities between the two compounds, thus suggesting that compound 1 is structurally related to cephathrene-B. Extensive analysis of the 1D NMR of 1 (Table 1) indicated the presence of two mutually ortho-coupled aromatic protons at δH 7.88 (1H, d, J = 8.9 Hz, H-10) and 7.50 (1H, d, J = 8.9 Hz, H-9), thus suggesting the presence of a tetra-substituted aromatic ring. Additional aromatic proton signals at δH 8.90 (1H, s, H-4) and 7.17 (1H, s, H-8) afforded two penta-substituted aromatic rings. Two hydroxyl signals at δH 7.91 (1H, s, 2-OH) and 8.39 (1H, s, 7-OH) were observed. Four methoxyl groups were presumed based on the 1H-NMR resonances at δH 3.99 (3H, s, 1-OCH3), 4.06 (3H, s, 3-OCH3), 4.03 (3H, s, 5-OCH3) and 4.02 (3H, s, 6-OCH3) and was supported by the corresponding 13C-NMR signals at δC 61.1, 56.3, 60.5 and 61.4, respectively.

Table 1.
1H (500 MHz) and 13C (125 MHz), HMBC and NOESY spectroscopic data of compounds 1–3 (δ in ppm, J in Hz) in acetone-d6.
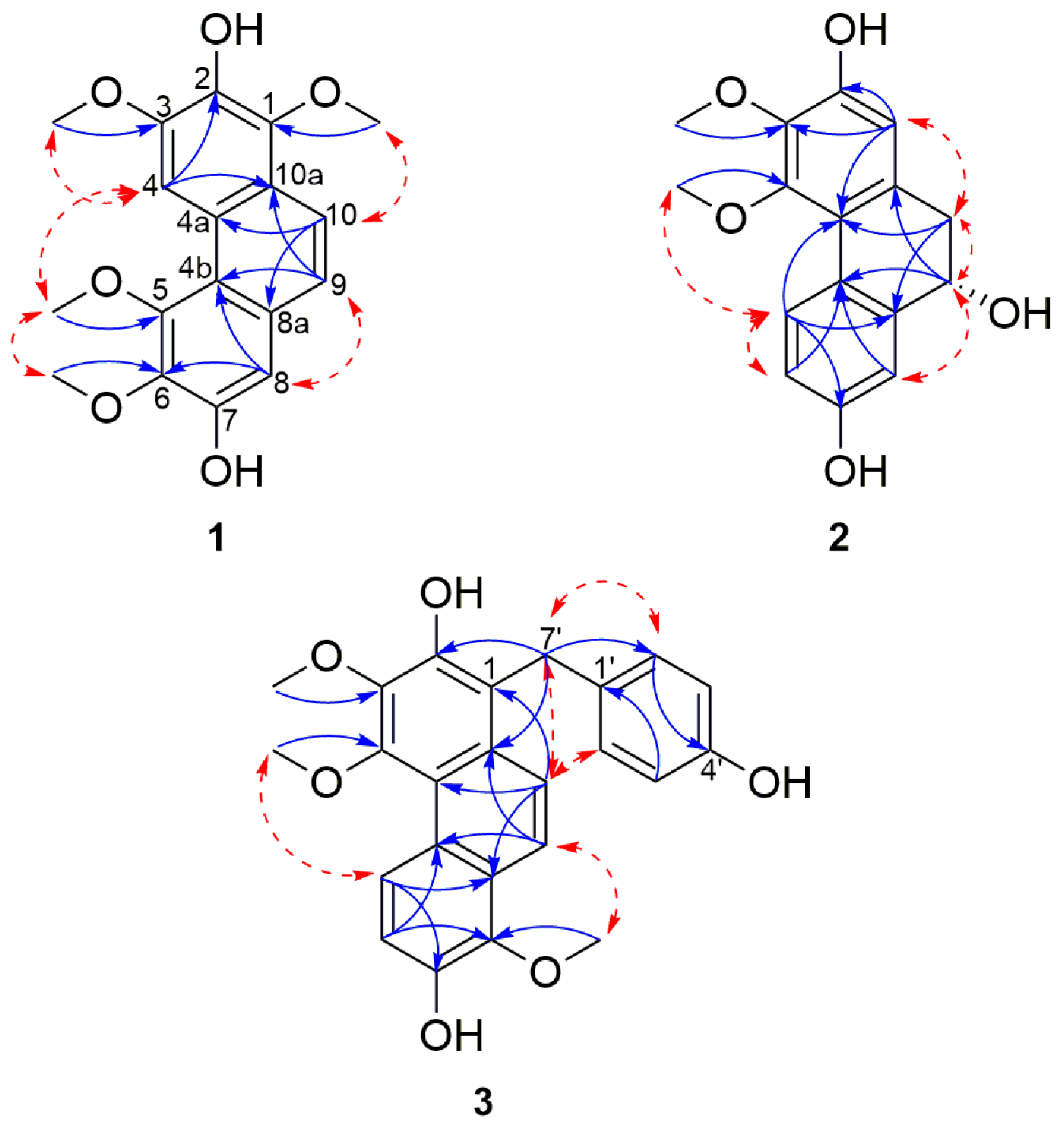
HMBC correlations from 1-OCH3 to C-1, 3-OCH3 to C-3, 5-OCH3 to C-5 and 6-OCH3 to C-6 confirmed the position of the methoxyls assigned to C-1, C-3, C-5 and C-6 respectively. In the same way, the locations of the hydroxyl signals at δH 7.91 (1H, s, 2-OH) and 8.39 (1H, s, 7-OH) were positioned on C-2 and C-7 respectively, due to HMBC correlations from 2-OH to C-1, C-2 and C-3 and 7-OH with C-6, C-7, and C-8. Furthermore, NOESY correlations between 2-OH and 1/3-OCH3; 7-OH with 6-OCH3 and H-8 confirmed the positions of the hydroxyl and methoxyl substituents. Additional HMBC correlations from H-4 to C-2, C-3, C-4b, and C-10a; H-8 with C-4b, C-5, C-6, C-7 and C-9; from H-9 to C-4b, C-8 and C-10a and H-10 to C-1, C-4a and C-8a established the link between the three aromatic moieties. Therefore, 1 was assigned as 1,3,5,6-tetramethoxylphenanthrene-2,7-diol and named cyrtopodin.
Compound 2 was obtained as a white amorphous powder. Its molecular formula was determined as C16H16O5 as deduced from HRESIMS at m/z 311.0884 [M + Na]+; (calcd. for C16H16NaO5 311.8900), suggesting the presence of nine degrees of unsaturation. The UV spectrum showed absorption maxima at 220, 262 and 282 nm suggesting a 9,10 dihydrophenanthrene moiety [49,50]. The IR spectrum showed a broad absorption at 3227 cm−1, indicating the presence of hydroxyl groups and at 1611, 1584, 999, 946, 870 and 828 cm−1, characteristic of aromatic rings. The 1H-NMR spectra (Table 1) showed a close resemblance to those of erianthridin (12) [22], except for the presence of an additional hydroxyl at position 9. This was supported by signals of the 1H and 13C resonance belonging to the methylene protons at δH 2.70 (1H, dd, J = 14.3, 10.6 Hz, H-10 ax) and 2.83 (1H, dd, J = 14.3, 4.6 Hz, H-10 eq), a deshielded methine resonance at δH 4.63 (1H, dd, J = 10.6, 4.6 Hz, H-9 ax), and deshielded carbon resonance at δC 68.9 (C-9). The absolute configuration of compound 2 was obtained with the help of circular dichroism (CD). The CD spectrum of 2 showed a negative Cotton effect at 236 nm and a positive Cotton effect at 281 nm, suggesting a 9S configuration for compound 2 which agrees with previous reports [51,52]. Therefore, 2 was identified as (+)-(S)-3,4-dimethoxyl-9,10-dihydrophenanthrene-2,7,9-triol, and named (S)-9-hydroxyerianthridin.
Compound 3 was obtained as a yellow amorphous powder. It showed a [M + H]+ signal at m/z 407.1508 (calcd. for C24H23O6 407.1890) in the HRESIMS, indicating a molecular formula of C24H22O6 and fourteen degrees of unsaturation. Its UV spectrum showed maximal absorptions at 212, 268, 289, 297, 301 and 314 nm suggesting a phenanthrene moiety. The 1D-NMR data indicated that the structure of 3 comprised a confusarin (11) [21] and gastrodigenin (28) [38] subunits. This hypothesis was supported by the 1D and 2D-NMR spectral data (Table 1). In the 1H-NMR spectrum, six aromatic protons signals at δH 9.21 (1H, d, J = 9.4 Hz, H-5), 7.24 (1H, d, J = 9.4 Hz, H-6), 7.87 (1H, d, J = 9.5 Hz, H-9), 7.90 (1H, d, J = 9.5 Hz, H-10) and three methoxyl proton signals at δH 4.06 (3H, s, 3-OCH3), 3.95 (3H, s, 4-OCH3) and 3.90 (3H, s, 8-OCH3) were observed, which corresponds to the resonances attributable to the confusarin moiety.
Additional aromatic signals of two equivalent set of mutually coupling protons at δH 7.08 (2H, d, J = 8.6 Hz, H-2′/6′) and 6.68 (2H, d, J = 8.6 Hz, H-3′/5′) indicated the presence of a di-substituted aromatic ring which was attributed to the resonance belonging to the gastrodigenin moiety. The linkage between the confusarin and gastrodigenin subunits took place via a methylene bridge at δH 4.41 (s, H-7′) and was confirmed by the HMBC correlations from H-7′ to C-2, C-10a, C-2′/6′ (Figure 2). Thus, compound 3 was identified as 1-(4′-hydroxybenzyl)-3,4,8-trimethoxylphenanthrene-2,7-diol and named gastrodiconfusarin.
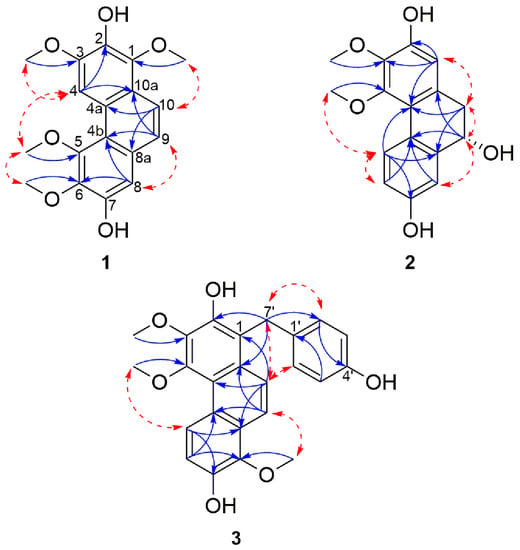
Figure 2.
Selected NOESY (red dashed arrows) and HMBC (blue arrows) correlations of compounds 1–3.
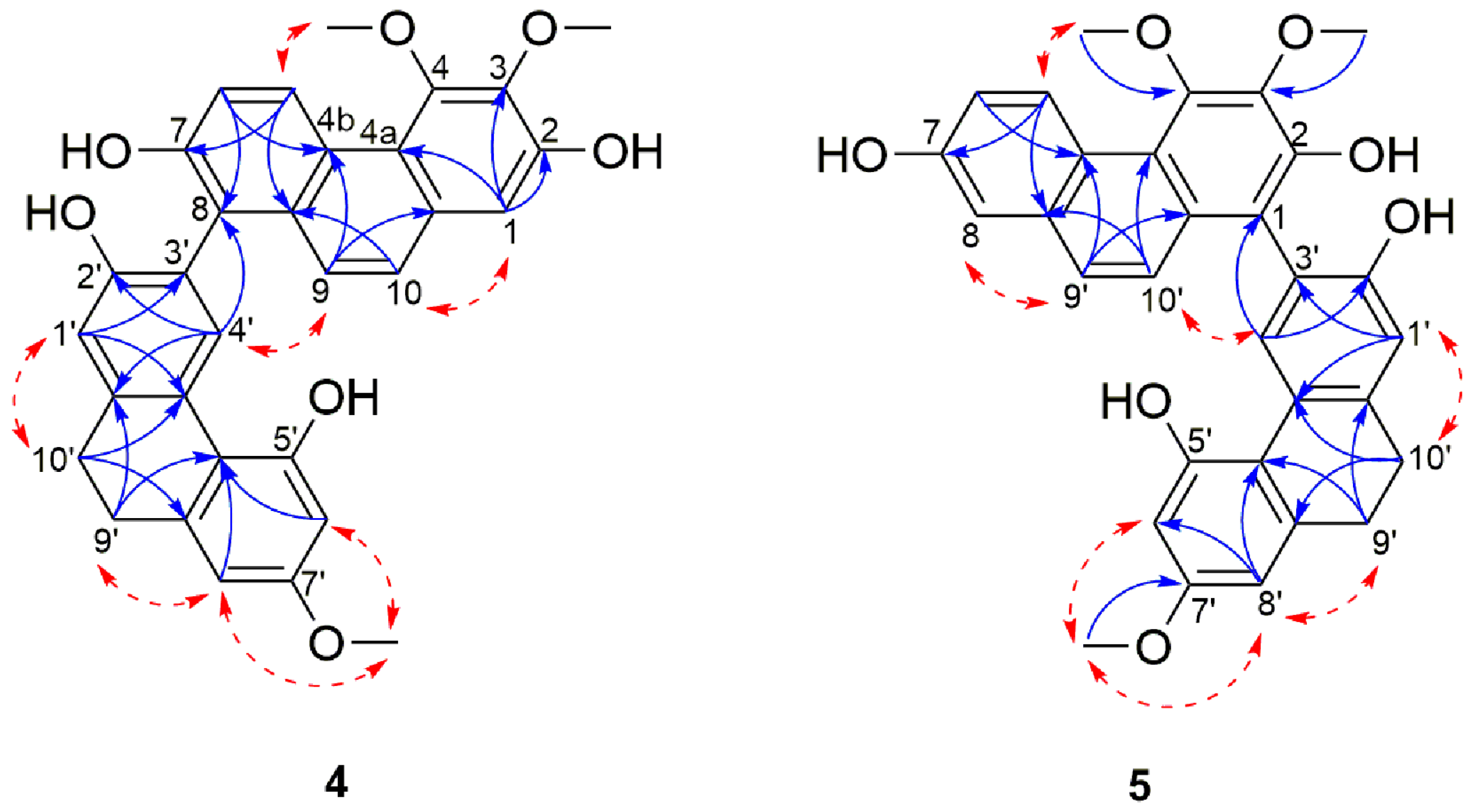
Compound 4 was obtained as a pale yellow amorphous powder. The HRESIMS showed a [M + H]+ molecular ion peak at m/z 511.1742 indicating a molecular formula of C31H26O7 (calcd. C31H27O7 for 511.1751) which is consistent with nineteen degrees of unsaturation. Its UV spectrum showed maximal absorptions at 213, 263, 296, 310, 349 and 367 nm. The IR spectrum showed typical absorption bands of a hydroxyl group at 3245 cm−1 and of aromatic rings at 1608, 1585, 948, 865 and 830 cm−1. In 1D-NMR spectrum (Table 2), the presence of two deshielded aromatic proton signals at δH 9.42 (1H, d, J = 9.2 Hz, H-5) and δH 8.28 (1H, s, H-4′) suggested that 4 was a dimeric molecule formed from the fusion of a phenanthrene unit [23,46,53] and a 9,10-dihydrophenanthrene subunit [28,54]. The 1H spectrum showed resonances for a pair of ortho-coupled aromatic protons at δH 9.42 (1H, d, J = 9.2 Hz, H-5) and 7.31 (1H, d, J = 9.2 Hz, H-6), a broad singlet integrated for two aromatic protons at δH 7.43 (2H, s, H-9, H-10), two meta-coupled aromatic protons at δH 6.39 (1H, d, J = 2.6 Hz, H-6′) and 6.42 (d, J = 2.6 Hz, H-8′) and three isolated aromatic protons at δH 7.11 (1H, s, H-1), 6.92 (1H, s, H-1′) and 8.28 (1H, s, H-4′). Additional signals belonging to three methoxyl groups at δH 4.01 (3H, s, 3-OCH3), 4.00 (3H, s, 4-OCH3), 3.73 (3H, s, 7′-OCH3) and two methylene groups emerging as a broad singlet at δH 2.80 (4H, s, H-9′ and H-10′), were also observed. The position of the hydroxyl and methoxyl functions and the linkage between the two subunits were ascertained based on the comprehensive analysis of 13C, HMBC and NOESY spectra (Figure 3). HMBC correlations from H-1 and 3-OCH3 to C-3, as well as HMBC cross peak from 4-OCH3 to C-4 and NOESY cross peak between 4-OCH3 and H-5 confirmed the location of the two methoxyl group on position C-3 and C-4 of the phenanthrene unit.

Table 2.
1H (500MHz) and 13C (125 MHz), HMBC and NOESY spectroscopic data of compounds 4–5 (δ in ppm, J in Hz) in acetone-d6.
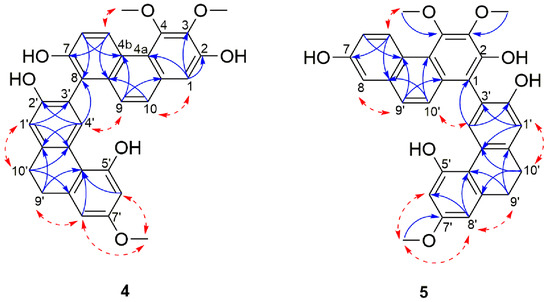
Figure 3.
Selected NOESY (red dashed arrows) and HMBC (blue arrows) correlations of compounds 4–5.
The positioning of the substituents on the 9,10-dihydrophenanthrene unit was deduced based on a HMBC cross peak from H-4′ to C-2′, as well as H-8′ and 7′-OCH3 to C-7′ that ascertained the position of the hydroxyl on C-2 and the methoxyl on C-7′. The remaining carbon (C-7), because of its proton-free environment, didn’t allow any HMBC correlations and was thus directly assigned based on the 13C chemical shift of C-7 (δC 153.5) which favors a hydroxyl substitution. The two monomers were connected through a C-8-C-3′ linkage as indicated by HMBC correlations from H-4′ to C-8 and nOe correlations between H-4′ and H-9 (Figure 3).
This was also supported by the downfield shift in the 13C-NMR signal of C-8 and C-3′ as compared to their respective monomeric units (113.0 to 121.0 ppm for nudol (14) and 113.3 to 120.6 ppm for lusianthridin (21)). The optical rotation of compound 4 was zero, and no Cotton effects was observed in the CD spectrum, inferring that 4 is a racemic mixture. On the basis of the above data, 4 was identified as 3,4,7′-trimethoxy-9′,10′-dihydro-[1,3′-biphenanthrene]-2,2,7,7′-tetraol and named lusidol A.
Compound 5 was obtained as a pale yellow amorphous powder. Its molecular formula was determined as C31H26O7 by the HRESIMS [M + H]+ peak at m/z 511.1740 (calcd. for C31H27O7 511.1751) which indicated that it has the same molecular formula as compound 4. The UV and IR spectra were identical to those of 4. Comparison of the NMR spectral data of these two compounds revealed their structural similarities, suggesting that the structure of 5 comprises the same phenanthrene (nudol) and 9,10-dihydrophenanthrene (lusianthridin) moieties (Table 3). The only difference was observed in the linkage that connects the two units: in 6 the connection was via a C-1-C-3′ linkage as opposed to the C-8-C-3′ linkage in 4 (Figure 3). This was confirmed by the presence of 1H signal observed at δH 7.21 (d, J = 2.7 Hz, H-8) in 5. The NOESY correlations from H-8 to H-9, H-10 to H-4′, as well as subsequent HMBC correlations from H-10 and H-4′ to C-1; H-8 to C-4b, C-6 and C-9 confirmed the position of this linkage. The optical rotation experiment result was zero, thus suggesting that 5 is a racemic compound. Therefore, compound 5 was identified as a 3,4,7′-trimethoxy-9′,10′-dihydro-[1,3′-biphenanthrene]-2,2′,5′,7-tetraol and named lusidol B.

Table 3.
1H (500 MHz), 13C (125 MHz), HMBC and NOESY spectroscopic data of compound 6 (δ in ppm, J in Hz) in acetone-d6.
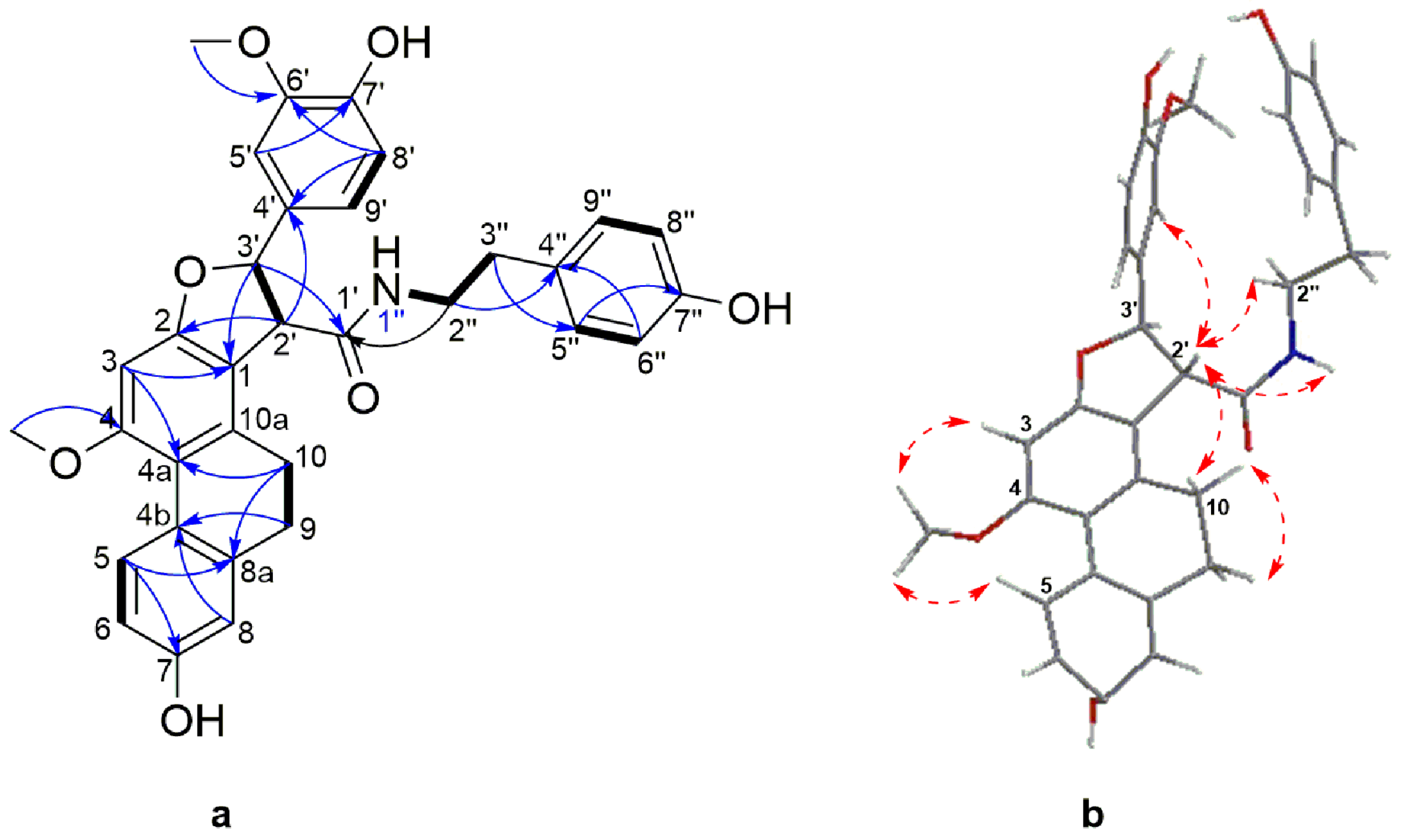
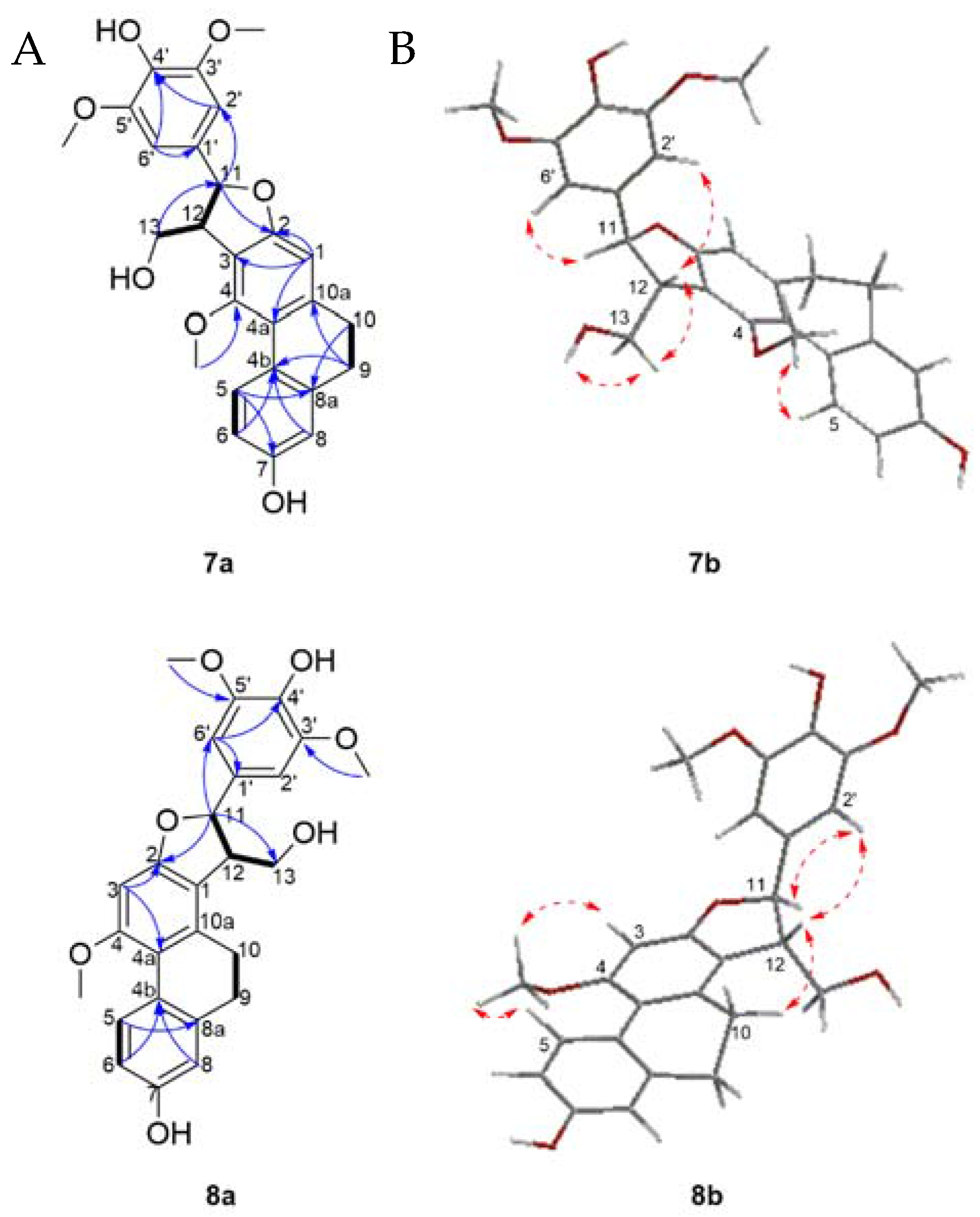
Compound 6 was obtained as a white amorphous powder. Its molecular formula was determined to be C33H32NO7 based on its HRESIMS [M + H]+ molecular ion peak at m/z 554.2169 (calcd for C33H32NO7 554.2173). Its UV spectrum showed maximal absorption bands at 208, 281, 305 and 318 nm, indicative of a dihydrophenanthrene derivative. Its IR spectrum exhibited absorption bands at 3241 cm−1 (hydroxyl), 1704 and 1519 cm−1 (amide), 1199, 1118, 1015, 950, 814 and 773 cm−1 (aromatic rings). Analysis of the 13C-NMR and DEPT-135 spectra of 6 disclosed the presence of signals belonging to 24 aromatic carbons, comprising of eleven methine, seven quaternary, and six oxygenated tertiary carbons. The 1H-NMR spectrum (Table 3) showed resonances for three aromatic protons as an ABX system owing to a 9,10-dihydrophenanthrene moiety at δH 8.04 (1H, d, J = 9.4 Hz, H-5), 6.68 (1H, dd, J = 9.4, 2.5 Hz, H-6), 6.68 (1H, d, J = 2.5 Hz, H-8) and one aromatic proton singlet at δH 6.54 (1H, s, H-3). Two methylene proton signals at δH 2.45–2.55 (2H, m, H-9) and 2.55–2.60 (2H, m, H-10) and the corresponding carbon signals at δC 30.7 and 27.5, respectively, were characteristic of the methylene groups of a 9,10-dihydrophenanthrene skeleton. A 4′-hydroxy-3′-methoxyphenyl moiety comprising of three aromatic protons at δH 6.82 (1H, dd, J = 8.3, 1.5 Hz, H-5′), 6.82 (1H, d, J = 8.3 Hz, H-6′) and 6.99 (1H, d, J =1.5 Hz, H-9′) that formed the second ABX system. The presence of an A2B2 system characteristic of a symmetrical hydroxybenzyl ring was inferred by the resonance at δH 7.01 (2H, d, J = 8.4 Hz, H-5′′/9′′), 6.73 (2H, d, J = 8.4 Hz, H-6′′/8′′), as well as signals attributed to an ethylamide moiety at δH 3.46 (1H, td, J = 7.1, 5.5 Hz, H-2′′), 2.72 (1H, brt, J = 7.1 Hz, H-3′′), 7.28 (1H, t, J = 7.28, 1′-NH) and a amide carbonyl at δC 172.3, thus achieving a hydroxybenzylethylamide moiety. The connection between the three partial structures of 9,10-dihydrophenanthrene, 4′-hydroxy-3′-methoxyphenyl and hydroxybenzylethylamide moiety was achieved through a central furan ring as observed by the presence of two coupled methines at δH 4.11 (1H, d, J = 6.6 Hz, H-2′) and the second being oxygenated at δH 5.67 (1H, d, J = 6.6 Hz, H-3′). This was confirmed by extensive analysis of HMBC and NOESY spectra (Figure 4). The oxymethine proton at H-3′ (δH 5.67) showed HMBC correlations to C-2, C-4′, C-5′, C-9′ and the amide carbonyl at C-1′; H-2′ to C-1, C-2, C-1′, C-3′, and C-4′. Additional HMBC correlations were observed from the two aromatic protons at H-5′ to C-3′ and H-9′ to C-3′.
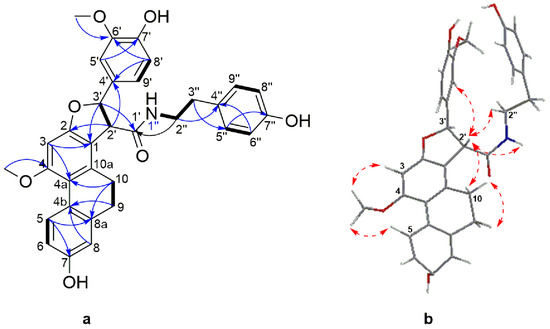
Figure 4.
(a) Selected COSY (plain bonds) and HMBC (blue arrows) correlations of 6 (b) Energy minimized 3D structure and selected NOESY correlations (red dashed arrows) of 6.
This was further supported by NOESY correlations between H-2′ to H-10, H-5′, H-9′ and 1′′-NH; H-3′ to H-5′, H-9′, and 1′′-NH. The assignments and locations of the two methoxyl groups at δH 3.88 (3H, s, 4-OCH3) and 3.82 (3H, s, 8′-OCH3) as well as the three hydroxyl groups on position C-7, C7′ and C-7′′ were confirmed by HMBC and NOESY analysis and supported by the characteristic chemical shift of the carbon bearing the groups. The hydroxyl bearing carbon position was settled in C-7 based on the HMBC correlation between H-5 and C-7 and by the characteristic chemical shift at δC 156.2. The positioning of the methoxyl on C-4 was affirmed from the NOESY cross peaks between H-3, H-5 and 4-OCH3.
The relative configuration of the methine protons H-2′ and H-3′ on the furan ring was defined as trans- based on NOESY correlations between H-2′ and H-5′′/9′′, suggesting these protons are on the same side of the furan ring. This was also supported by the coupling constant of 6.6 Hz, typical of trans-dihydrophenanthrenofuran derivatives [55,56], which allows the possibility of two enantiomeric stereoconfigurations, either (2′R, 3′R) or (2′S, 3′S). The absolute configurations of compound 6 could not be ascertained as its optical rotation was zero, suggesting a racemization of the trans form. The 3D structure of 6 (Figure 4) obtained from a minimized energy MM2 algorithm suggested that a S,S-trans configuration was more appropriate for this compound. Thus, the structure of 6 should be 7-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-N-(4-hydroxyphenethyl)-10-methoxy-2,3,4,5-tetrahydrophenanthro[2,1-b]furan-3-carboxamide, and it was named moupilonin.
Compound 7 was obtained as a white amorphous powder. Its molecular formula was determined as C26H26O7 as deduced from its HRESIMS [M + H]+ ion peak at m/z 451.1753 (calcd. for C26H27O7 451.1751), suggesting the presence of fourteen degrees of unsaturation. The UV spectrum showed absorption maxima at 210, 274 and 284 nm, suggesting that compound 7 has a dihydrophenanthrene skeleton. The IR absorption bands at νmax at 3303, 1601, 1452 and 773 cm−1 were characteristic to a hydroxyl and aromatic moieties.
The 1H-NMR spectrum (Table 4) showed resonances for four aromatic protons at δH 8.03 (1H, d, J = 9.2 Hz, H-5), 6.73 (1H, dd, J = 9.2, 2.8 Hz, H-6), 6.73 (1H, d, J = 2.8 Hz, H-8), and 6.56 (1H, s, H-1), as well as two methylenes at δH 2.69 (2H, m, H-9) and 2.67 (2H, m, H-10), which corresponds to resonances attributed to a 9,10-dihydrophenanthrene ring. A singlet aromatic proton integrating for two protons at δH 6.75 (2H, s, H-2′/H-6′) suggested the presence of a symmetrical 1′,3′,4′,5-tetrasubstituted aromatic ring. Three methoxy groups at δH 3.80 (6H, s, 3′/5′-OCH3), 3.60 (3H, s, 4-OCH3) and two hydroxyl groups at δH 8.29 (1H, s, 7-OH) and 7.24 (1H, s, 4′-OH), were also noticed. The linkage between the 9,10-dihydrophenanthrene unit and 1′,3′,4′,5-tetrasubstituted aromatic moiety of compound 7 was achieved through a furan ring as supported by signals assigned to an oxymethine proton at δH 5.66 (1H, d, J = 5.4 Hz, H-11), a methine at δH 3.73 (1H, m, H-12) and an oxygenated methylene at δH 3.83 (1H, m, H-13) and δH 4.08 (1H, ddd, J = 10.4, 4.7, 4.2 Hz, H-13) which coupled to an hydroxyl group at δH 4.17 (1H, dd, J = 6.1, 4.7 Hz, 13-OH). Analysis of the COSY spectrum (Figure 5) validated the OH-CH2(13)-CH(12)-CH(11) proton spin systems. Furthermore, HMBC correlations from H-11 to C-2, C-2′/6′; H-2′/6′ to C-11 as well as NOESY cross peaks between H-11 and H-2′/6′; H-2′/6′ and H-12 supported the structure as shown, connecting the phenyl to the furan ring at C-11.

Table 4.
1H (500MHz), 13C (125 MHz), HMBC and NOESY spectroscopic data of compounds 7–8 (δ in ppm, J in Hz) in acetone-d6.
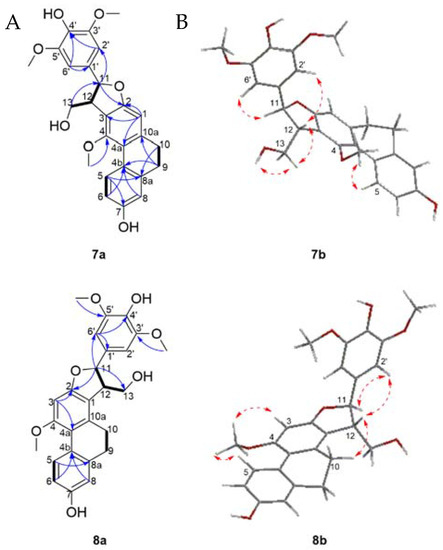
Figure 5.
(A) Selected COSY (plain bonds) and HMBC (blue arrows) correlations of compounds 7–8; (B) Energy minimized 3D structures and NOESY correlations (red dashed arrows) of compounds 7–8.
The assignment of the substitution pattern was achieved based on HMBC correlations from 4-OCH3 to C4 and 3′/5′-OCH3 to C-3′/5′ suggested that the methoxy groups were attached to C-4 and C-3′/5′, respectively. NOESY correlations between 4-OCH3 and H-5, and between H-2′/H-6′ to 3′/5′-OCH3 also validated this assignment. The hydroxyl groups were assigned to positions C-7 and C-4′ based on HMBC long range correlations between 7-OH to C-7 (and additional correlation to C6 and C8), and 4′-OH to C-4′ (and additional correlations to C3′/5′). The relative stereoconfiguration of 7 was established on the basis of NOESY experiments, which displayed cross peaks between H-12 on the furan ring and H-2′/6′ indicated that both protons reside on the same side. In addition, the coupling constant of 5.4 Hz between H-11 and H-12 was in agreement with the reported literature values for the relative trans-configuration [57]. The absolute configurations of C-11 and C-12 were confirmed by CD determination: a negative Cotton effect at 284 nm suggested a 11S,12R form, which is consistent with previous reports [58,59]. The 3D structure was generated with an optimized energy minimization procedure using MM2, and suggested also a trans form as a 11S,12R. Therefore compound 7 was identified as (+)-(9S,10R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-10-(hydroxymethyl)-11-methoxy-5,6,9,10-tetrahydrophenanthro[2,3-b]furan-3-ol and named cyrtonesin A .
Compound 8 was obtained as a white amorphous powder and its molecular formula was established as C26H26O7 by the HREIMS peak at m/z 451.1748 [M + H]+ (calcd. for C26H27O7 451.1751). The mass, UV, IR and NMR spectra (Table 4) indicated the striking similarity between compounds 7 and 8. The difference between the two compounds is the positioning of the furan ring on the dihydrophenanthrene chore. In compound 7, the furan ring was positioned as -C2-C3-C12-C-11-O-, while in compound 8 it is positioned as a -C-1-C2-O-C-11-C-12-. This was corroborated by the presence of a singlet proton resonance at δH 6.56 (1H, s, H-3) as well as HMBC correlations (Figure 5a) from H-3 to C-2 and C-4a as well as NOESY correlations (Figure 5b) between 4-OCH3, H-3 and H-5 also validated the proton in position C-3.
The methylene at position C-13 also had NOESY correlation with the proton H-10 on the dihydrophenanthrene subunit. The relative configuration between H-11 and H-12 was assumed to be trans because of the NOESY correlation between H-12 to H-2′/H-6′ as well as the coupling constant of 3.3 Hz supporting a trans-configuration as previously reported in dihydrophenanthrenofuran derivatives [54]. The CD spectrum of 8 showed a negative Cotton effect at 273 nm, allowing the assignment of a 11S, 12R. Based on the above evidence, the structure of compound 8 was determined to be (+)-(2S,3R)-2-(4-hydroxy-3,5-dimethoxyphenyl)-3-(hydroxymethyl)-10-methoxy-2,3,4,5-tetra-hydrophenanthro[2,1-b]furan-7-ol. Compound 8 was named cyrtonesin B.
3. Experimental Section
3.1. General Procedures
Optical rotations were measured with a P-2000 polarimeter (Jasco, Lisses, France) and circular dichroïsm spectra were recorded on a Jasco J-510 spectropolarimeter apparatus (Jasco). UV spectra were recorded on a UV-2401 PC spectrometer (Shimadzu, Kyoto, Japan). IR spectra were obtained on a 380 FT-IR spectrophotometer (Thermo Electron Corporation, Saint-Herblain, France).The 1D and 2D NMR spectra were recorded on a Bruker 500 MHz Avance III spectrometer (Bruker BioSpin, Rheinstetten, Germany) equipped with a DCH 13C/1H Cryoprobe (Bruker Biospin, Fallanden, Switzerland). Acetone-d6 and methanol-d4 (Euriso-Top, Saint-Aubin, France) were used as deuterated solvents and their protonated residual signals were used as internal standard at 2.05 ppm and 3.31 ppm respectively, relative to TMS. The HRESIMS analysis were performed on a HPLC-DAD/UV-MS Agilent 1200 series coupled to a 6520 Q-ToF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The acquisition of mass spectra was conducted in ESI positive ion mode. Column chromatography and vacuum liquid chromatography were carried out using 40–60 µm silica gel (Sigma Aldrich, St-Louis, MO, USA). The obtained fractions were monitored by TLC and the spots were visualized under UV light (254 nm) and by 2% sulfuric vanillin reagent. Sephadex LH-20 (Sigma Aldrich, St-Louis, MO, USA), was used for gel chromatography. RP-HPLC experiments were conducted on a Gilson LC system (Gilson Inc., Limburg an der Lahn, Germany) equipped with a semi preparative Kinetex Axia C-18 column (100 mm × 21.2 mm i.d, 5 µm; Phenomenex, Torrance, CA, USA). Experiments were conducted at a wavelength of 280 nm. Preparative TLC was performed on a glass supported silica gel 60F254 (0.25 mm thickness; Merck, Darmstadt, Germany). Analytical grade solvents of HPLC quality were purchased from Sigma Aldrich.
3.2. Plant Material
Fifteen fresh specimens of C. paniculatum (Ruiz & Pav.) Garay were purchased from the orchid farm Orquidea del Valle, Ginebra, Colombia in October 2013 and imported to France, according to the Convention of Natural Trades in Endangered Species (CITES). The voucher specimens (No 58054 and 58056) were deposited at the Herbarium of CUVC, Universidad del Valle, Cali, Colombia.
3.3. Extraction and Isolation
The pseudobulbs (7.5 kg) were cut into small pieces, ground and soaked in demineralized water for 30 min to get rid of the mucilage content. The remaining part was then macerated with ethanol and the filtrates were evaporated under reduced pressure to obtain a crude ethanolic extract (60 g). The ethanolic extract was suspended in water and successively partitioned with cyclohexane, CH2Cl2 and n-BuOH to yield after solvent removal a cyclohexane extract (0.60 g), a CH2Cl2 extract (1.67 g) and an n-BuOH extract (7.88 g). The CH2Cl2 extract was subjected to silica column chromatography (cyclohexane/EtOAc; 100:0 to 0:100, EtOAc/CH3OH 100:0 to 0:100) to afford 24 fractions (A–X). Fraction D (24.4 mg) was subjected to preparative thin layer chromatography (PTLC; CHCl3/CH3OH 94:6) to afford compound 9 (6.3 mg). Fraction F (51.6 mg) was fractionated on Sephadex LH-20 (CH3OH) to obtain sub-fractions F1-F8. Sub-fractions F4 (7.4 mg) and F5 (25.7 mg) were further purified using PTLC (CHCl3/CH3OH 94:6) to afford 10 (4 mg), 11 (5 mg), 12 (5 mg) and 13 (0.9 mg). Fraction G (165.4 mg) was purified using Sephadex LH-20 (CH3OH) to obtain eight sub-fractions (G1–G8). Sub-fraction G8 was obtained as a pure compound 14 (2.8 mg). Fraction H (71.6 mg) was also subjected to Sephadex LH-20 (CH3OH) to obtain four sub-fractions (H1–H4). H1 (12.2 mg) led to the isolation of 15 (1.4 mg) and 16 (1.3 mg) on PTLC eluting with CHCl3/CH3OH (94:6). Sub-fraction H2 (4.7 mg) led to the isolation of 17 (1 mg) and 18 (0.9 mg) using PTLC (CHCl3/CH3OH). Fraction I (87 mg) was purified on Sephadex LH-20 (CH3OH) to obtain seven sub-fractions (I1-I7). Sub-fraction I1 (25.6 mg) led to the isolation of 19 (13.1 mg), while sub-fraction I2 (13.3 mg) afforded compounds 20 (5.3 mg) and 21 (4.1 mg) using PTLC eluting with CHCl3/CH3OH (94:6). Sub-fraction I7 (4.2 mg) was purified on a PTLC (CHCl3/CH3OH; 94:6) to obtain compound 1 (1.3 mg). Fraction J (32.2 mg) was fractioned on Sephadex LH-20 (CH3OH) and PTLC (CHCl3/CH3OH; 94:6) to obtain 22 (0.9 mg) and 23 (1 mg). Fraction K (29.5 mg) was purified on Sephadex LH-20 (CH3OH) affording 7 sub-fractions (K1–K7). Sub-fraction K6 (4.7 mg) was subjected to a PTLC eluting with CHCl3/CH3OH (94:6) affording compounds 3 (0.6 mg), 24 (2 mg) and 25 (0.6 mg). Fraction L (26.6 mg) was also subjected to Sephadex LH-20 (CH3OH) to yield 8 sub-fractions (L1–L8). Sub-fraction L5 (2.2 mg) was purified using PTLC with CHCl3/CH3OH (94:6) as the eluting solvent to yield 26 (0.6 mg). Fraction M (90.0 mg) was subjected to Sephadex LH-20 and eluted with CH3OH to afford 10 sub-fractions (M1–M10). Compounds 27 (2.4 mg) and 28 (6.7 mg) were obtained from sub-fraction M2 (19.9 mg) by PTLC using CHCl3/CH3OH as the mobile phase. Sub- fraction M7 (13.2) was further purified by PTLC using CHCl3/CH3OH (94:6) to obtain 29 (2.1 g) and 30 (2.6 mg). Sub-fraction M8 (2.9 mg) was passage over PTLC that was eluted with CHCl3/CH3OH (94:6) to yield compound 31 (1.1 mg). Sub-fraction M9 (11.6 mg) was purified by PTLC with CHCl3/CH3OH (94:6) to yield compounds 4 (0.7 mg), 5 (0.6 mg) and 32 (0.9 mg). Fraction O (71.7 mg) was subjected to Sephadex LH-20 eluting with CH3OH, to obtain 5 sub-fractions (O1–O5). Sub-fraction O3 (9.8 mg) was purified by PTLC with CHCl3/CH3OH (94:6) to obtain compound 2 (2.8 mg). Sub-fraction O5 (2.4 mg) was purified by PTLC with CHCl3/CH3OH (94:6) to give compound 33 (0.7 mg). Fraction P (63 mg) was fractionated on Sephadex LH-20 (CH3OH) to obtain six sub-fractions (P1–P6). Sub-fraction P4 (3.7 mg) was further subjected to PTLC eluting with CHCl3/CH3OH (94:6) to obtain compounds 6 (0.6 mg), 7 (0.9 mg) and 8 (0.6 mg). Fraction Q (19.5 mg) was purified on Sephadex LH-20 (CH3OH) affording six sub-fractions. Compounds 34 (3 mg) and 35 (0.8 mg) were obtained from sub-fraction Q4 (7 mg) through a PTLC with CHCl3/CH3OH (94:6) as the eluting solvent.
Fresh leaves were lyophilized and grinded to afford 70 g of dried powder and were successively extracted with cyclohexane, CH2Cl2 and CH3OH (1 g/15 mL × 3) to afford a cyclohexane extract (6.99 g), a CH2Cl2 extract (1.23 g) and a CH3OH extract (4.98 g). The CH2Cl2 extract was subjected to vacuum liquid chromatography (VLC) using cyclohexane, EtOAc and CH3OH to afford six main fractions (A–F). Fraction E (98 mg) was subjected to semi-preparative HPLC (40%–45% B in 5 min, 45% isocratic mode for 30 min, B = CH3OH/0.05% formic acid, A = H2O/0.05% formic acid) to afford compounds 13 (3.5 mg), 18 (0.7 mg), 19 (1 mg), and 21 (1.7 mg). Fractions F (158.4 mg) was also subjected to semi- preparative RP-HPLC (35% B for 35 min) to afford compound 36 (9.6 mg).
3.4. Compound Characterization
Cyrtopodin (1). Brown amorphous powder (1.3 mg); UV (CH3OH) λmax (log ε): 216 (3.96), 261 (4.38), 281 (4.02), 312 (3.48), 344 (2.52); IR (FT-IR) νmax: 3370, 2935, 2831, 1616, 1573, 1465, 1268, 1118, 1061, 1021, 953, 860, 770 cm−1; 1H-NMR and 13C-NMR see Table 1; HRESIMS: m/z 331.1188 [M + H]+ (Calcd. for C18H19O6, 331.1176).
(+)-9S-Hydroxyerianthridin (2). White amorphous powder (2.8 mg); + 6.4 (c 0.09, CH3OH); CD (CH3OH): nm (Δε): 236.2 (−20.69), 280.9 (+5.39); (UV (CH3OH) λmax (log ε): 220 (4.01), 262 (3.91), 282 (4.00), 348 (2.28), 364 (2.26); IR (FT-IR) νmax: 3227, 2936, 2834, 1611, 1584, 1446, 1412, 1348, 1213, 1112, 1066, 999, 946, 870, 828 cm−1; 1H-NMR and 13C-NMR see Table 1; HRESIMS: m/z 311.0884 [M + Na]+; (Calcd. for C16H16O5Na, 311.8900).
Gastrodiconfusarin (3). Yellow amorphous powder (0.6 mg); UV (CH3OH) λmax (log ε): 212 (4.11), 268 (4.09), 289 (3.63), 297 (4.00), 301 (3.59), 314 (3.45); IR (FT-IR) νmax: 3336, 2931, 2936, 1606, 1512, 1454, 1352, 1221, 1106, 1017, 822, 773 cm−1; 1H-NMR and 13C-NMR see Table 1; HRESIMS m/z 407.1508 [M + H]+ (Calcd. for C24H23O6, 407.189).
Lusidol A (4). Pale yellow amorphous powder (0.7 mg); 0 (c 0.04, CH3OH); UV (CH3OH) λmax (log ε): 213 (4.80), 263 (4.82), 296 (4.47), 310 (4.40), 349 (3.58), 367 (3.55); IR (FT-IR) νmax: 3245, 2931, 2835, 1608, 1585, 1451, 1393, 1351, 1210, 1162, 1118, 1076, 999, 948, 865, 830 cm−1; 1H-NMR and 13C-NMR see Table 2; HRESIMS: m/z 511.1742 [M + H]+ (Calcd. for C31H27O7, 511.1751).
Lusidol B (5). Pale yellow amorphous powder (0.6 mg); 0 (c 0.04, CH3OH); UV (CH3OH) λmax (log ε): 212 (4.72), 264 (4.66), 297 (4.37), 309 (4.29), 350 (3.28), 367 (3.27). IR (FT-IR) νmax: 3271, 2931, 2835, 1608, 1585, 1451, 1393, 1351, 1210, 1162, 1118, 1076, 999, 948, 865, 830; 527 cm−1; 1H-NMR and 13C-NMR see Table 2; HRESIMS: m/z 511.1740 [M + H]+ (Calcd. for C31H27O7, 511.1751).
(±)-Moupilonin (6). White amorphous powder (0.9 mg); 0 (c 0.07, CH3OH); UV (CH3OH) λmax (log ε): 208 (4.37), 281 (3.97), 305 (3.73), 318 (3.51); IR (FT-IR) νmax : 3241, 2935, 2829, 1704, 1519, 1447, 1349, 1237, 1199, 1118, 1015, 950, 814, 773 cm−1; 1H-NMR and 13C-NMR see Table 3; HRESIMS: m/z 554.2169 [M + H]+ (Calcd. for C33H32NO7, 554.2173).
(+)-Cyrtonesin A (7). White amorphous powder (0.6 mg); 27 (c 0.04, CH3OH); CD (CH3OH): nm (Δε): 233.1 (+0.84), 284.2 (−0.95); UV (CH3OH) λmax (log ε): 210 (4.30), 274 (3.73), 284 (3.76); IR (FT-IR) νmax: 3303, 2935, 2831, 1601, 1452, 1359, 1239, 1217, 1114, 1068, 1033, 1008, 977, 951, 824, 773 cm−1; 1H-NMR and 13C-NMR see Table 4; HRESIMS: m/z 451.1753 [M + H]+ (Calcd. for C26H27O7 451.1751).
(+)-Cyrtonesin B (8). White amorphous powder (0.6 mg); 26 (c 0.02, CH3OH); CD (CH3OH): nm (Δε): 235.0 (+1.11), 272.8 (−7.26); UV (CH3OH) λmax (log ε): 207 (4.29), 292 (3.73), 301 (3.77); IR (FT-IR) νmax: 3320, 2936, 2825, 1599, 1460, 1354, 1239, 1221, 1060, 1035, 1008, 977, 955, 825, 773 cm−1; 1H-NMR and 13C-NMR see Table 4; HRESIMS: m/z 451.1748 [M + H]+ (Calcd. for C26H27O7 451.1751).
4. Conclusions
Due to extreme environmental and territorial conditions as well as predation, plant species have evolved to produce complex secondary metabolites as a means of survival. In orchids, stilbenoids and phenanthrene derivatives are produced as major phytoalexins produced in response to various biotic and abiotic stressors, mostly fungal, bacterial and worm attacks [60,61,62,63,64,65,66]. The phytochemical study of CH2Cl2 extract from the aerial parts of C. paniculatum led us to the isolation of an long list of polyphenolic derivatives, mainly stilbenoids. Monomeric stilbenoid derivatives, comprising bibenzyls, 9,10-dihydrophenanthrenes, phenanthrenes and phenanthrenequinones, are well represented in this orchid. The second set of isolated compounds consisted of phenolic adducts (4-hydroxybenzyl) coupled to a 9,10-dihydrophenanthrene or phenanthrene derivative. This type of 4-hydroxybenzyl adduct (gastrodigenin) is very common in orchids and mostly found in the genus Arundina [67,68], Bletilla [69,70,71,72] and Pleione [40,73,74]. The third set of isolated compounds was a combination of a 9,10-dihydrophenanthrene and a phenylpropane derivative (trans-feruloyltyramine for 6, synapyl alcohol for 7 and 8). The connection between the two units allows a new cyclization leading to a furan ring. It is noteworthy that this kind of dihydrophenanthrene derivative is considered as a chemotaxonomic marker for the species in the genus Pleione [59,73,75,76,77], but has been also found in the genus Bulbophyllum [55] and Cremastra [54]. The last set of compounds were the dimers, occurring as either homo- (two units of 9,10-dihydrophenanthrene or phenanthrene) or as heterodimers (combinations of a 9,10-dihydrophenanthrene and a phenanthrene unit) having a C-C bond linkage. These biphenanthrene derivatives occur mostly in species of the genus Bletilla [33,41,71,78,79,80], Bulbophyllum [55,81,82,83] and Cremastra [54,84,85,86,87,88]. The existence of dimers together their respective monomers in the same species provides support for a proposed biogenetic pathway for biaryl-derived compounds occurring from their corresponding monomers as a result of free radical [56] or an enzymatic oxidative phenolic coupling reaction [82,89,90,91]. This study reveals for the first time the chemical diversity of phenolic constituents produced by an endemic South-American orchid, Cyrtopodium paniculatum.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at www.mdpi.com/1420-3049/21/10/1418/s1.
Acknowledgments
This study was financially supported by the French Ministry of Education and Research, LVMH Recherche and Guerlain. The authors wish to thank Olivier de la Roque for its implication for the raw material importing process to France, and Maurice Coppe, University of Strasbourg, for the NMR experiment acquisitions.
Author Contributions
F.A. conceived and designed the whole experiments (chromatographic isolation and spectroscopic identification of all compounds) with the help of D.R., F.A. and O.J.O. wrote the manuscript. G.H., D.R. and C.A. provided significant help during the identification process. B.S. and F.B. contributed to the selection and the raw material collection and its international import. A.L. supervised the research work. All authors have read and revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Estrada, S.; Rojas, A.; Mathison, Y.; Israel, A.; Mata, R. Nitric oxide/cGMP mediates the spasmolytic action of 3,4′-dihydroxy-5,5′-dimethoxybibenzyl from Scaphyglottis livida. Planta Med. 1999, 65, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Rivero-Cruz, J.F. Bioactive secondary metabolites from selected mexican medicinal plants: Recent progress. In Bioactive Constituents from Natural Sources: Isolation, Characterization and Biological properties; Tringali, C., Ed.; Taylor & Francis Publishing: London, UK, 2001; pp. 129–158. [Google Scholar]
- Estrada, S.; Acevedo, L.; Rodriguez, M.; Toscano, R.A.; Mata, R. New triterpenoids from the orchids Scaphyglottis livida and Nidema boothii. Nat. Prod. Lett. 2002, 16, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Estrada, S.; Toscano, R.A.; Mata, R. New phenanthrene derivatives from Maxillaria densa. J. Nat. Prod. 1999, 62, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Islas, N.A.; Paul, R.N.; Shier, W.T.; Mata, R.; Abbas, H.K. Phytotoxicity and ultrastructural effects of gymnopusin from the orchid Maxillaria densa on duckweed (Lemna pausicostata) frond and root tissues. Phytochemistry 2002, 61, 141–148. [Google Scholar] [CrossRef]
- Deciga-Campos, M.; Palacios-Espinosa, J.F.; Reyes-Ramirez, A.; Mata, R. Antinociceptive and anti-inflammatory effects of compounds isolated from Scaphyglottis livida and Maxillaria densa. J. Ethnopharmacol. 2007, 114, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rendon-Vallejo, P.; Hernandez-Abreu, O.; Vergara-Galicia, J.; Millan-Pacheco, C.; Mejia, A.; Ibarra-Barajas, M.; Estrada-Soto, S. Ex vivo study of the vasorelaxant activity induced by phenanthrene derivatives isolated from Maxillaria densa. J. Nat. Prod. 2012, 75, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Barreto, D.W.; Parente, J.P. Chemical properties and biological activity of a polysaccharide from Cyrtopodium cardiochilum. Carbohyd. Polym. 2006, 64, 287–291. [Google Scholar] [CrossRef]
- Morales-Sanchez, V.; Rivero-Cruz, I.; Laguna-Hernandez, G.; Salazar-Chavez, G.; Mata, R. Chemical composition, potential toxicity, and quality control. Procedures of the crude drug of Cyrtopodium macrobulbon. J. Ethnopharmacol. 2014, 154, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Parente, J.P.; Adao, C.R.; da Silva, B.P.; Tinoco, L.W. Structural characterization of an acetylated glucomannan with antiinflammatory activity and gastroprotective property from Cyrtopodium andersonii. Carbohyd. Res. 2014, 391, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Cogniaux, A. Orchidaceae. In Cyrtopodium in Flora Brasiliensis; Martius, C.F.P., Eichler, A.G.I., Eds.; R. Oldenbourg Verlag GmbH: Munich, Germany, 1898–1902; Volume 3, pp. 356–375. [Google Scholar]
- Romero-González, G.A.; Fernández-Concha, G.C. Notes on the species on Cyrtopodium (Cyrtopodinae, Orchidaceae) from Florida, the Greater Antilles, Mexico, Central and Northern America. Harv. Pap. Bot. 1999, 4, 327–341. [Google Scholar]
- Romero-González, G.A. Cyrtopodium. In Flora of North-America; Oxford Univ. Press New York: New York, NY, USA, 2002; Volume 26, pp. 642–643. [Google Scholar]
- Romero-González, G.A.; Batista, J.A.; Bianchetti, L.D.B. A synopsis of the genus Cyrtopodium (Catasetinae: Orchidaceae). Harv. Pap. Botany 2008, 13, 189–206. [Google Scholar] [CrossRef]
- Simmler, C.; Antheaume, C.; Lobstein, A. Antioxidant biomarkers from Vanda coerulea stems reduce irradiated HaCaT PGE-2 production as a result of COX-2 inhibition. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Antheaume, C.; Andre, P.; Bonte, F.; Lobstein, A. Glucosyloxybenzyl eucomate derivatives from Vanda teres stimulate HaCaT cytochrome c oxidase. J. Nat. Prod. 2011, 74, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Cakova, V.; Urbain, A.; Antheaume, C.; Rimlinger, N.; Wehrung, P.; Bonte, F.; Lobstein, A. Identification of phenanthrene derivatives in Aerides rosea (Orchidaceae) using the combined systems HPLC-ESI-HRMS/MS and HPLC-DAD-MS-SPE-UV-NMR. Phytochem. Anal. 2015, 26, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Barl, M.D.C.D.; Siegel, H.D.C.D.; Degner, D.D.C.D.; Mercker, H.J.D.C. Benzyl alcohols. DE3107147A1, 9 September 1982. [Google Scholar]
- Duan, J.; Li, W.; Hu, X.; Koike, K.; Fu, H. Chemical constituents of Silene rubicunda Franch. Zhong Cao Yao 2009, 40, 528–530. [Google Scholar]
- Letcher, R.M.; Nhamo, L.R.M. Chemical constituents of the Combretaceae. Iii. Substituted phenanthrenes, 9,10-dihydrophenanthrenes, and bibenzyls from the heartwood of Combretum psidioides. J. Chem. Soc. 1972, 2941–2946. [Google Scholar] [CrossRef]
- Majumder, P.L.; Kar, A. Confusarin and confusaridin, two phenanthrene derivatives of the orchid Eria confusa. Phytochemistry 1987, 26, 1127–1129. [Google Scholar] [CrossRef]
- Majumder, P.L.; Joardar, M. Erianthridin, a new 9,10-dihydrophenanthrene derivative from the orchids Eria carinata and Eria stricta. Ind. J. Chem. Sect. B 1985, 24B, 1192–1194. [Google Scholar]
- Bhandari, S.R.; Kapadi, A.H.; Mujumder, P.L.; Joardar, M.; Shoolery, J.N. Nudol, a phenanthrene of the orchids Eulophia nuda, Eria carinata and Eria stricta. Phytochemistry 1985, 24, 801–804. [Google Scholar] [CrossRef]
- Chang, C.-F.; Hsu, Y.-L.; Lee, C.-Y.; Wu, C.-H.; Wu, Y.-C.; Chuang, T.-H. Isolation and cytotoxicity evaluation of the chemical constituents from Cephalantheropsis gracilis. Int. J. Mol. Sci. 2015, 16, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Juneja, R.K.; Sharma, S.C.; Tandon, J.S. A substituted 1,2-diarylethane from Cymbidium giganteum. Phytochemistry 1985, 24, 321–324. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hasegawa, K.; Kawarada, A. Batatasins: New dormancy-inducing substances of yam bulbils. Planta 1972, 108, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, Y.; Hirano, H.; Kikuchi, T.; Xu, G. Constituents of orchidaceous plants. X. Constituents of Ephemerantha lonchophylla; isolation and structure elucidation of new phenolic compounds, ephemeranthol-a, ephemeranthol-b, and ephemeranthoquinone, and of a new diterpene glucoside, ephemeranthoside. Chem. Pharm. Bull. 1991, 39, 593–598. [Google Scholar] [CrossRef]
- Majumder, P.; Laha, S.; Datta, N. Coelonin, a 9,10-dihydrophenanthrene from the orchids Coelogyne ochracea and C. elata. Phytochemistry 1982, 21, 478–480. [Google Scholar] [CrossRef]
- Yamaki, M.; Honda, C. The stilbenoids from Dendrobium plicatile. Phytochemistry 1996, 43, 207–208. [Google Scholar] [CrossRef]
- Majumder, P.L.; Lahiri, S. Lusianthrin and lusianthridin, two stilbenoids from the orchid lusia indivisa. Phytochemistry 1990, 29, 621–624. [Google Scholar] [CrossRef]
- Fan, C.Q.; Zhao, W.M.; Qin, G.W. New bibenzyl and phenanthrenedione from Dendrobium densiflorum. Chin. Chem. Lett. 2000, 11, 705–706. [Google Scholar]
- Zhang, G.-N.; Zhong, L.-Y.; Annie Bligh, S.W.; Guo, Y.-L.; Zhang, C.-F.; Zhang, M.; Wang, Z.-T.; Xu, L.-S. Bi-bicyclic and bi-tricyclic compounds from Dendrobium thyrsiflorum. Phytochemistry 2005, 66, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Hwang, T.-L.; Chen, F.-A.; Huang, C.-H.; Hung, H.-Y.; Wu, T.-S. Chemical constituents of the rhizomes of Bletilla formosana and their potential anti-inflammatory activity. J. Nat. Prod. 2016, 79, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.O.; Varshney, I.P.; Rahman, W.; Gangwar, P.C. The synthesis of chrysoeriol (3′-methyl ether of luteolin) and homoeriodictyol (5,7,4′-trihydroxy-3′-methoxyflavanone). Naturwissenschaften 1959, 46, 76. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, Y.H.; Park, S.M.; Lee, K.E.; Lee, J.J.; Lee, B.C.; Pyo, H.B.; Song, K.S.; Park, H.D.; Yun, Y.P. Antioxidants and inhibitor of matrix metalloproteinase-1 expression from leaves of Zostera marina L. Arch. Pharm. Res. 2004, 27, 177–183. [Google Scholar] [CrossRef]
- Menke, A.E.; Bentley, W.B. Preliminary note on some new derivatives of vanillin. J. Am. Chem. Soc. 1898, 20, 316–317. [Google Scholar] [CrossRef]
- Li, S.; Lundquist, K. A new method for the analysis of phenolic groups in lignins by proton NMR spectrometry. Nord. Pulp Pap. Res. J. 1994, 9, 191–195. [Google Scholar] [CrossRef]
- Cai, J.-Y.; Zhao, L.; Zhang, D.-Z. Chemical constituents from Bletilla ochracea Schltr. Chem. Res. Chin. Univ. 2007, 23, 705–707. [Google Scholar] [CrossRef]
- Takagi, S.; Yamaki, M.; Inoue, K. Antimicrobial agents from Bletilla striata. Phytochemistry 1983, 22, 1011–1015. [Google Scholar] [CrossRef]
- Bai, L.; Yamaki, M.; Takagi, S. Stilbenoids from Pleione bulbocodioides. Phytochemistry 1996, 42, 853–856. [Google Scholar] [CrossRef]
- Yamaki, M.; Bai, L.; Inoue, K.; Takagi, S. Biphenanthrenes from Bletilla striata. Phytochemistry 1989, 28, 3503–3505. [Google Scholar] [CrossRef]
- Das, K.C.; Farmer, W.J.; Weinstein, B. Phytochemical studies. IX. A new flavone, velutin. J. Org. Chem. 1970, 35, 3989–3990. [Google Scholar] [CrossRef]
- Lodhi, M.A.; Choudhary, M.I.; Malik, A.; Ahmad, S. Α-chymotrypsin inhibition studies on the lignans from Vitex negundo Linn. J. Enzyme Inhib. Med. Chem. 2008, 23, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Haruna, M.; Koube, T.; Ito, K.; Murata, H. Balanophonin, a new neo-lignan from Balanophora japonica Makino. Chem. Pharm. Bull. 1982, 30, 1525–1527. [Google Scholar] [CrossRef]
- Hussain, S.F.; Goezler, B.; Shamma, M.; Goezler, T. Feruloyltyramine from Hypecoum. Phytochemistry 1982, 21, 2979–2980. [Google Scholar] [CrossRef]
- Majumder, P.L.; Basak, M. Cirrhopetalin, a phenanthrene derivative from Cirrhopetalum andersonii. Phytochemistry 1990, 29, 1002–1004. [Google Scholar] [CrossRef]
- Majumder, P.L.; Sen, R.C. Pendulin, a polyoxygenated phenanthrene derivative from the orchid Cymbidium pendulum. Phytochemistry 1991, 30, 2432–2434. [Google Scholar] [CrossRef]
- Honda, C.; Yamaki, M. Phenanthrenes from Dendrobium plicatile. Phytochemistry 2000, 53, 987–990. [Google Scholar] [CrossRef]
- Majumder, P.L.; Pal, S. Rotundatin, a new 9,10-dihydrophenanthrene derivative from Dendrobium rotundatum. Phytochemistry 1992, 31, 3225–3228. [Google Scholar] [CrossRef]
- Majumder, P.L.; Sen, S.; Majumder, S. Phenanthrene derivatives from the orchid Coelogyne cristata. Phytochemistry 2001, 58, 581–586. [Google Scholar] [CrossRef]
- Resnick, S.M.; Gibson, D.T. Regio- and stereospecific oxidation of 9,10-dihydroanthracene and 9,10-dihydrophenanthrene by naphthalene dioxygenase: Structure and absolute stereochemistry of metabolites. Appl. Environ. Microbiol. 1996, 62, 3355–3359. [Google Scholar] [PubMed]
- Lin, Y.; Wang, F.; Yang, L.-J.; Chun, Z.; Bao, J.-K.; Zhang, G.-L. Anti-inflammatory phenanthrene derivatives from stems of Dendrobium denneanum. Phytochemistry 2013, 95, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.L.; Pal, A.; Joardar, M. Cirrhopetalanthrin, a dimeric phenanthrene derivative from the orchid Cirrhopetalum maculosum. Phytochemistry 1990, 29, 271–274. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, S.-H.; Meng, Y.-H.; Zhang, Y.-B.; Cheng, C.-R.; Shi, Y.-Y.; Feng, R.-H.; Zeng, F.; Wu, Z.-Y.; Zhang, J.-X.; et al. Phenanthrenes, 9,10-dihydrophenanthrenes, bibenzyls with their derivatives, and malate or tartrate benzyl ester glucosides from tubers of Cremastra appendiculata. Phytochemistry 2013, 94, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.-W.; Harrison, L.J. A biphenanthrene and a phenanthro[4,3-b]furan from the orchid Bulbophyllum vaginatum. J. Nat. Prod. 2004, 67, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Tang, C.-P.; Li, X.-Q.; Ye, Y. Phochinenins a–f, dimeric 9,10-dihydrophenanthrene derivatives, from Pholidota chinensis. Helv. Chim. Acta 2008, 91, 2122–2129. [Google Scholar] [CrossRef]
- Ali, Z.; Tanaka, T.; Iliya, I.; Iinuma, M.; Furusawa, M.; Ito, T.; Nakaya, K.; Murata, J.; Darnaedi, D. Phenolic constituents of Gnetum klossii. J. Nat. Prod. 2003, 66, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Antus, S.; Kurtan, T.; Juhasz, L.; Kiss, L.; Hollosi, M.; Majer, Z. Chiroptical properties of 2,3-dihydrobenzo[b]furan and chromane chromophores in naturally occurring O-heterocycles. Chirality 2001, 13, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, C.; Li, Y.; Guo, S.; Yang, J. Complete assignments of 1H and 13C-NMR data of three new dihydrophenanthrofurans from Pleione yunnanensis. Magn. Reson. Chem. 2010, 48, 256–260. [Google Scholar] [PubMed]
- Fisch, M.H.; Flick, B.H.; Arditti, J. Orchid phytoalexins. I. Structure and antifungal activity of hircinol, loroglossol, and orchinol. Phytochemistry 1973, 12, 437–441. [Google Scholar] [CrossRef]
- Ward, E.W.B.; Unwin, C.H.; Stoessl, A. Loroglossol. Orchid phytoalexin. Phytopathology 1975, 65, 632–633. [Google Scholar] [CrossRef]
- Fritzemeier, K.H.; Kindl, H. 9,10-dihydrophenanthrenes as phytoalexins of Orchidaceae. Biosynthetic studies in vitro and in vivo proving the route from l-phenylalanine to dihydro-m-coumaric acid, dihydrostilbene and dihydrophenanthrenes. Eur. J. Biochem. 1983, 133, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Hills, K.A.; Stoessl, A.; Oliva, A.P.; Arditti, J. Effects of orchinol, loroglossol, dehydroorchinol, batatasin III, and 3,4′-dihydroxy-5-methoxydihydrostilbene on orchid seedlings. Bot. Gaz. 1984, 145, 298–301. [Google Scholar] [CrossRef]
- Reinecke, T.; Kindl, H. Characterization of bibenzyl synthase catalyzing the biosynthesis of phytoalexins of orchids. Phytochemistry 1994, 35, 63–66. [Google Scholar] [CrossRef]
- Coxon, D.T.; Ogundana, S.K.; Dennis, C. Antifungal phenanthrenes in yam tubers. Phytochemistry 1982, 21, 1389–1392. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant. Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Majumder, P.L.; Ghosal, S. Two stilbenoids from the orchid Arundina bambusifolia. Phytochemistry 1993, 32, 439–444. [Google Scholar] [CrossRef]
- Liu, M.-F.; Han, Y.; Xing, D.-M.; Shi, Y.; Xu, L.-Z.; Du, L.-J.; Ding, Y. A new stilbenoid from Arundina graminifolia. J. Asian Nat. Prod. Res. 2004, 6, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Chen, W.-P.; Macabalang, A.D. Dihydrophenanthrenes from Bletilla formosana. Chem. Pharm. Bull. 2005, 53, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, C.; Zhao, P.; Shu, G.; Mei, Z. Antimicrobial constituents from the tubers of Bletilla ochracea. Planta Med. 2012, 78, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Kato, T.; Inoue, K.; Yamakai, M.; Takagi, S. Nonpolar constituents from Bletilla striata. Part 6. Blestrianol A, B and C, biphenanthrenes from Bletilla striata. Phytochemistry 1991, 30, 2733–2735. [Google Scholar] [CrossRef]
- Feng, J.-Q.; Zhang, R.-J.; Zhao, W.-M. Novel bibenzyl derivatives from the tubers of Bletilla striata. Helv. Chim. Acta 2008, 91, 520–525. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Yuan, Q.-Y.; Guo, Y.-Q. Two new stilbenoids from Pleione bulbocodioides. J. Asian Nat. Prod. Res. 2009, 11, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, S.-W.; Cui, B.-S.; Wang, X.-J.; Li, S. Chemical constituents from Pleione bulbocodioides. Zhongguo Zhongyao Zazhi 2014, 39, 442–447. [Google Scholar] [PubMed]
- Bai, L.; Yamaki, M.; Takagi, S. Flavan-3-ols and dihydrophenanthropyrans from Pleione bulbocodioides. Phytochemistry 1998, 47, 1125–1129. [Google Scholar] [CrossRef]
- Liu, X.Q.; Gao, W.Y.; Guo, Y.Q.; Zhang, T.J.; Yan, L.L. A new phenanthro[2,3-b]furan from Pleione bulbocodioides. Chin. Chem. Lett. 2007, 18, 1089–1091. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Guo, Y.-Q.; Gao, W.-Y.; Zhang, T.-J.; Yan, L.-L. Two new phenanthrofurans from Pleione bulbocodioides. J. Asian Nat. Prod. Res. 2008, 10, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.-D.; Jiang, F.-S.; Yu, H.-S.; Shen, Y.; Fu, Y.-H.; Cheng, D.-Q.; Gan, L.-S.; Ding, Z.-S. Antibacterial biphenanthrenes from the fibrous roots of Bletilla striata. J. Nat. Prod. 2015, 78, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Liu, J.; Pang, S.; Lin, J.; Xu, R. Two novel phenanthraquinones with anti-cancer activity isolated from Bletilla striata. Bioorg. Med. Chem. Lett. 2016, 26, 2375–2379. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Xu, C.; Lv, J.; Wang, C.; Song, P. Antimicrobial stilbenoids from Bletilla yunnanensis. Chem. Nat. Compd. 2016, 52, 19–22. [Google Scholar] [CrossRef]
- Majumder, P.L.; Pal, S.; Majumder, S. Dimeric phenanthrenes from the orchid Bulbophyllum reptans. Phytochemistry 1999, 50, 891–897. [Google Scholar] [CrossRef]
- Majumder, P.L.; Bandyopadhyay, S.; Pal, S. Rigidanthrin, a new dimeric phenanthrene derivative of the orchid Bulbophyllum rigidum. J. Indian Chem. Soc. 2008, 85, 1116–1123. [Google Scholar]
- Xu, J.; Yu, H.; Qing, C.; Zhang, Y.; Liu, Y.; Chen, Y. Two new biphenanthrenes with cytotoxic activity from Bulbophyllum odoratissimum. Fitoterapia 2009, 80, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Li, S.; Wang, S.; Wang, Y.; Yang, Y.; Shi, J.; He, L. Mono-, bi-, and triphenanthrenes from the tubers of Cremastra appendiculata. J. Nat. Prod. 2006, 69, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Q.; Li, X.-P.; Yuan, Q.-Y. A new biphenanthrene glucoside from Cremastra appendiculata. Chem. Nat. Compd. 2015, 51, 1035–1037. [Google Scholar] [CrossRef]
- Yuan, Q.-Y.; Liu, X.-Q. Chemical constituents from Cremastra appendiculata. Zhongyaocai 2015, 38, 298–301. [Google Scholar] [PubMed]
- Liu, X.-Q.; Li, X.-P.; Yuan, W.-K.; Yuan, Q.-Y.; Qin, B.-H. Two new phenanthrene glucosides from Cremastra appendiculata and their cytotoxic activities. Nat. Prod. Commun. 2016, 11, 477–479. [Google Scholar] [PubMed]
- Liu, L.; Zeng, K.-W.; Jiang, Y.; Tu, P.-F.; Liu, L.; Liu, L.; Li, J. Five new biphenanthrenes from Cremastra appendiculata. Molecules 2016, 21, 1089. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.; Cohen, T. Some biogenetic aspects of phenol oxidation. Festschr. Arthur. Stoll. 1957, 117–143. [Google Scholar]
- Stadler, R.; Zenk, M.H. The purification and characterization of a unique cytochrome P-450 enzyme from Berberis stolonifera plant cell cultures. J. Biol. Chem. 1993, 268, 823–831. [Google Scholar] [PubMed]
- Cottyn, B.; Kollmann, A.; Waffo-Teguo, P.; Ducrot, P.H. Rationalization and in vitro modeling of the chemical mechanisms of the enzymatic oxidation of phenolic compounds in planta: From flavonols and stilbenoids to lignins. Chem. Eur. J. 2011, 17, 7282–7287. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).