Quantification of Polyfunctional Thiols in Wine by HS-SPME-GC-MS Following Extractive Alkylation
Abstract
:1. Introduction
2. Results and Discussion
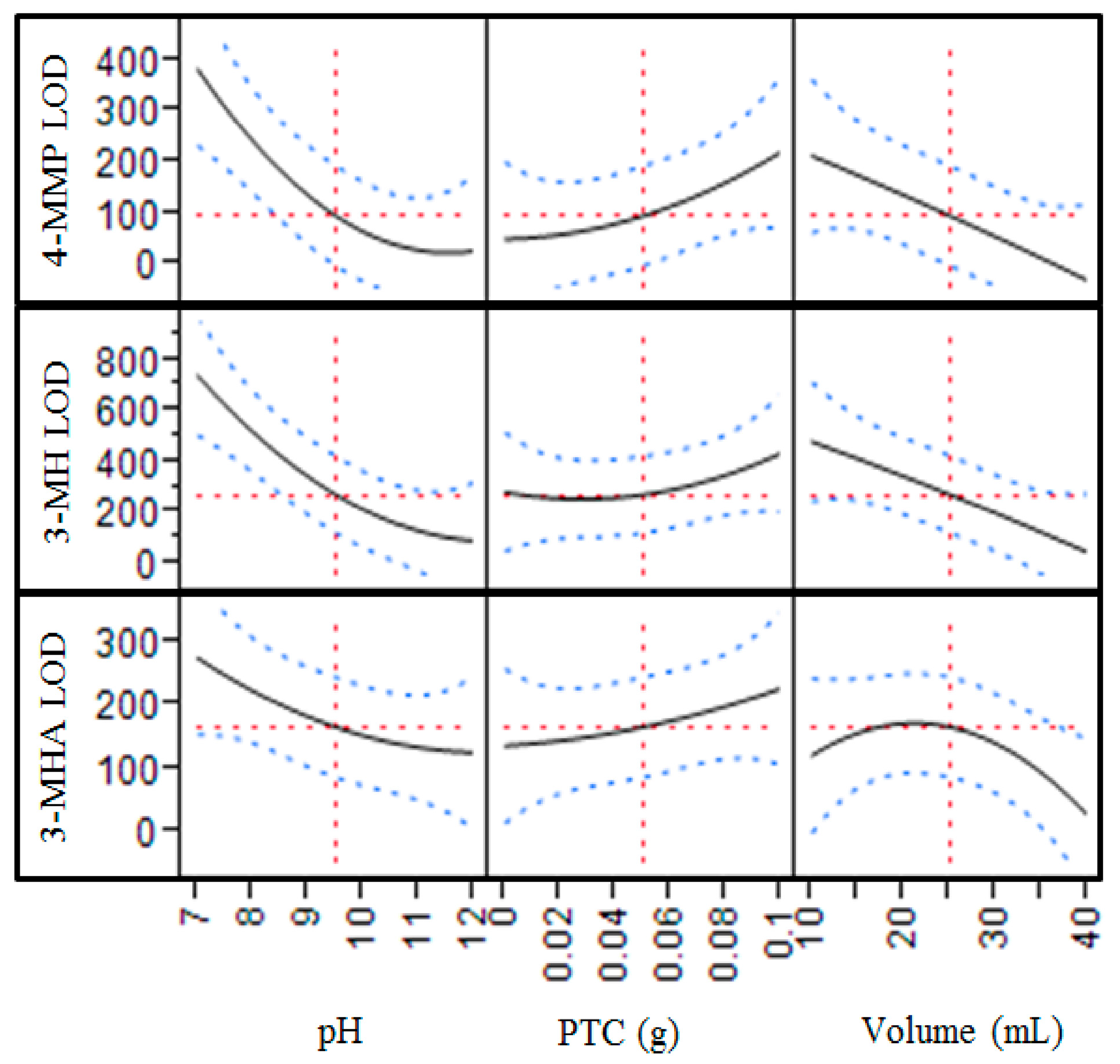
2.1. Optimization of Extractive Alkylation Conditions
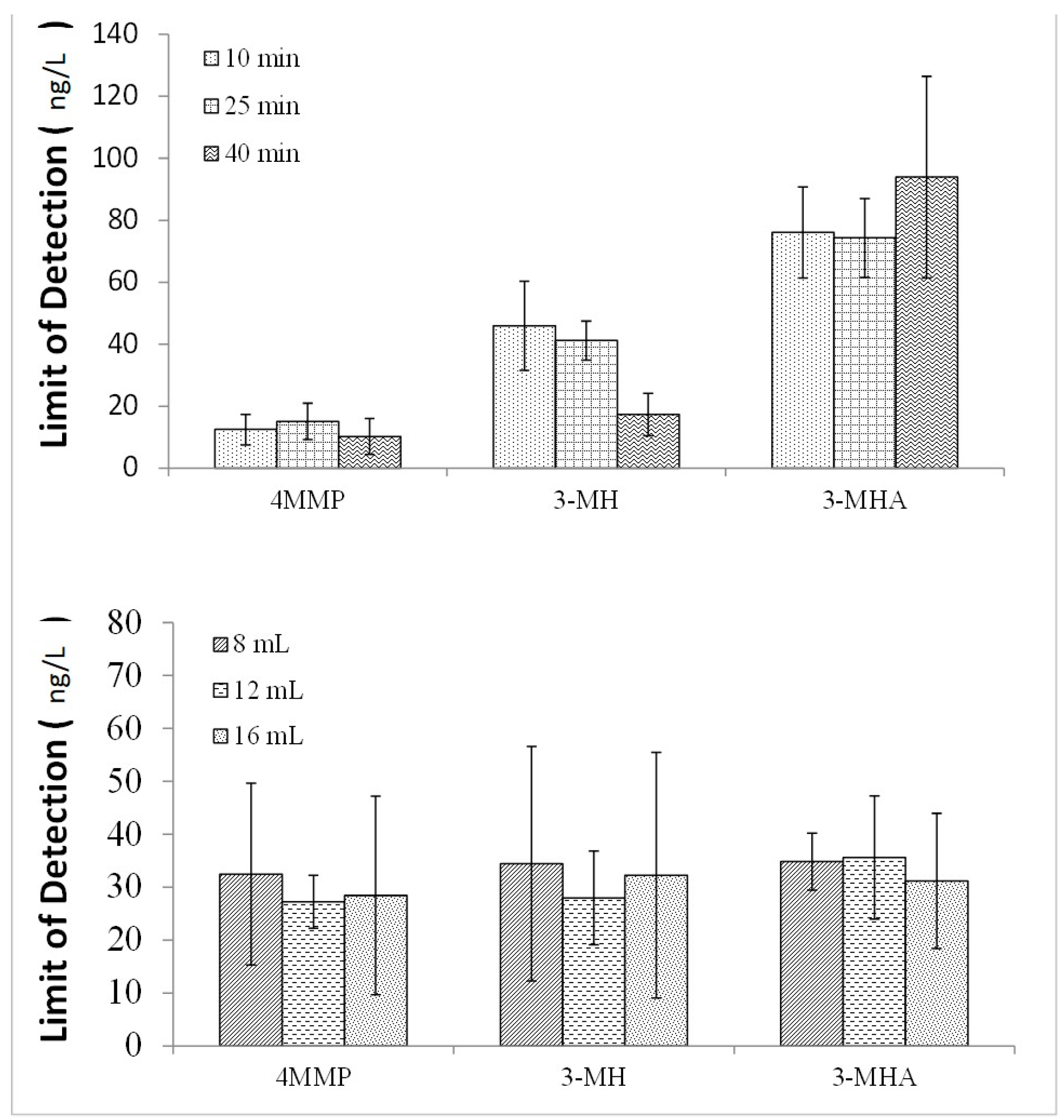
2.1.1. Reaction Time and Organic Solvent Volume
2.1.2. Sample pH, Sample Volume, PTC Concentration
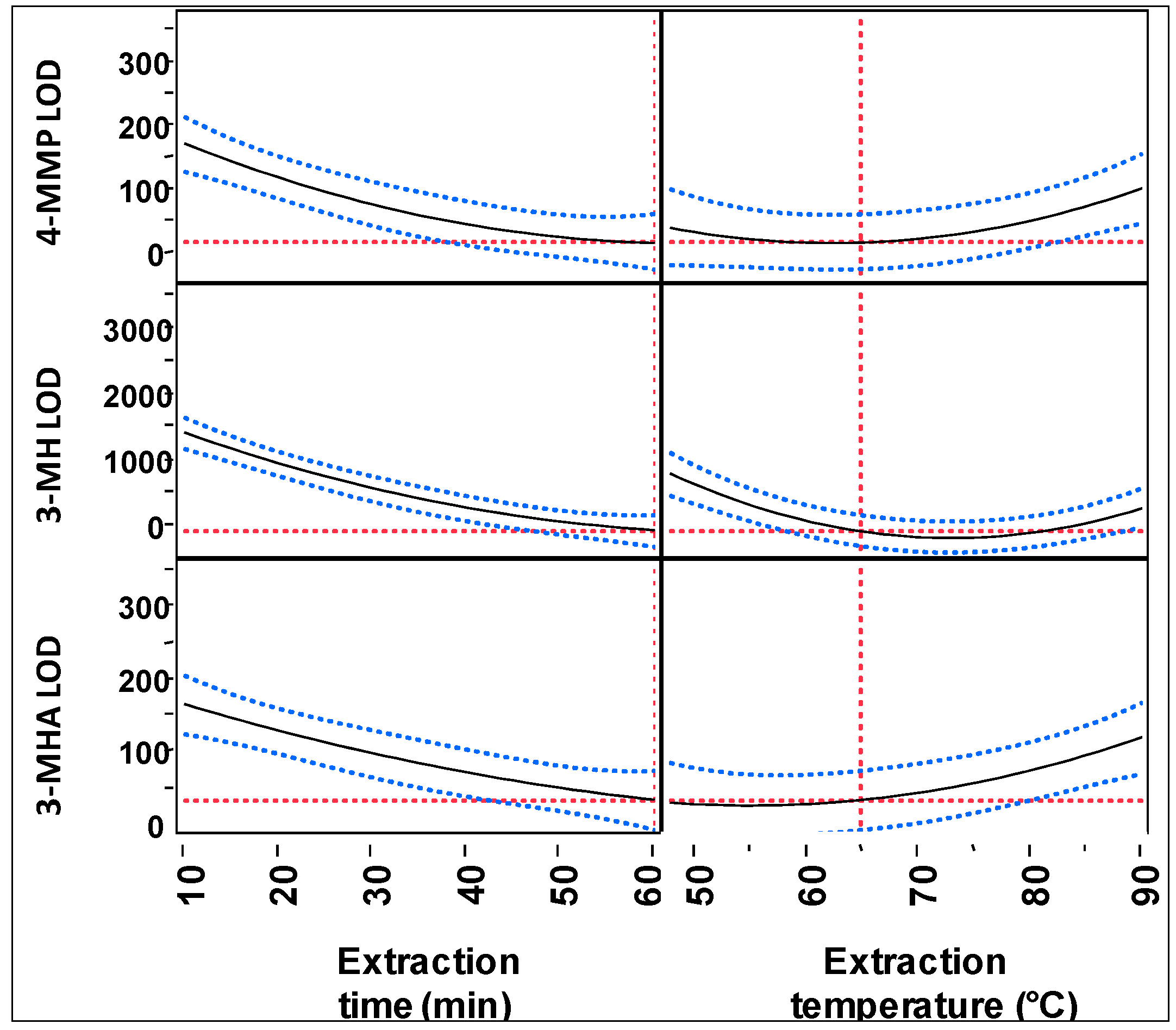
2.2. Optimization of HS-SPME Conditions
2.2.1. Reconstitution Volume

2.2.2. HS-SPME Extraction Time and Temperature
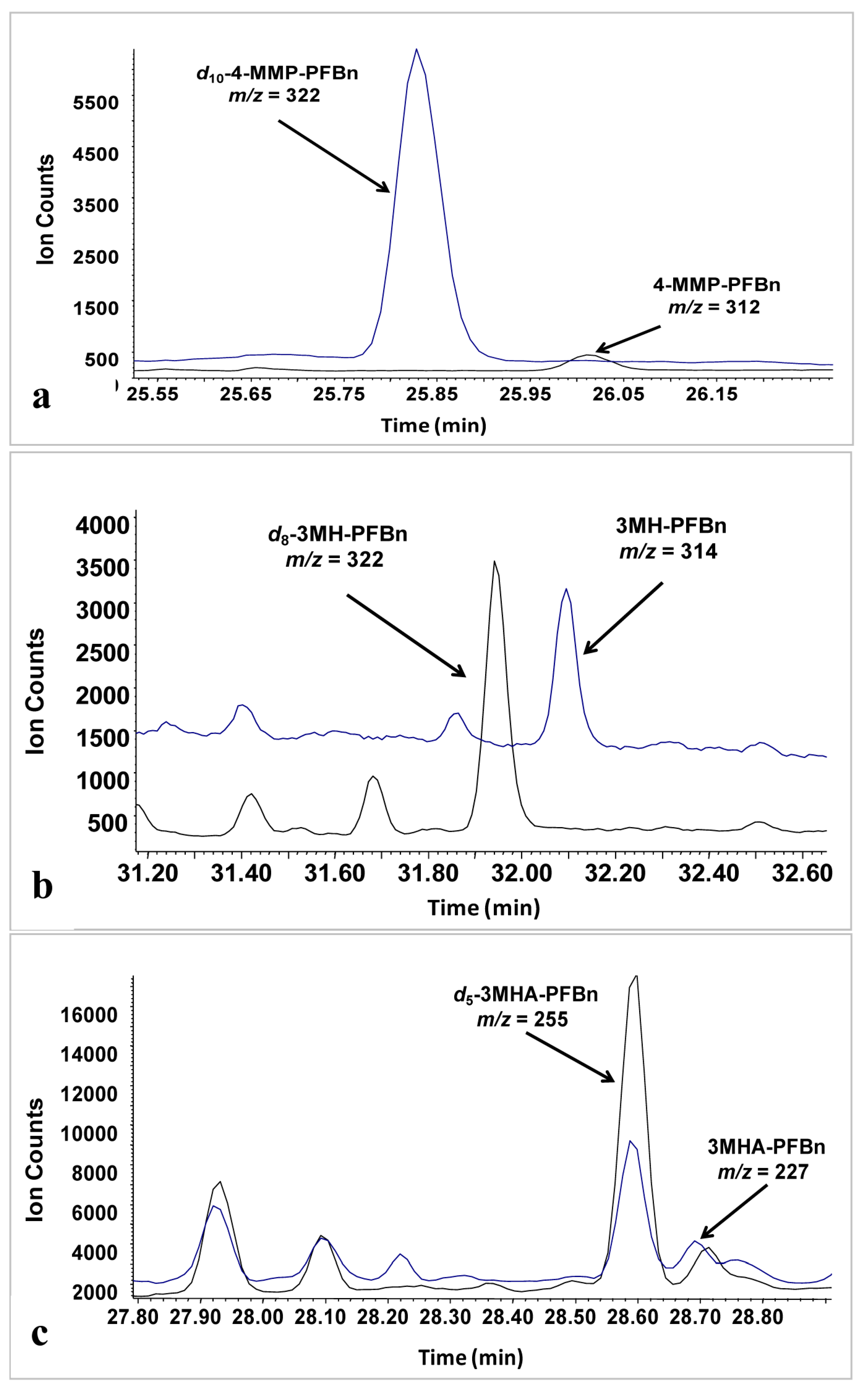
2.3. Figures of Merit
2.3.1. Linearity and Limits of Detection
| Spike Level (ng/L) | ng/L of 4-MMP | ng/L of 3-MH | ng/L of 3-MHA |
|---|---|---|---|
| 6 | 8.0 (±0.3) | 4.1 (±0.1) | n.d. |
| 20 | 19 (±0.7) | 19 (±2.1) | n.d. |
| 63 | 50 (±3.2) | 66 (±3.3) | 72 ( ± 4.9) |
| 200 | 179 (±12.2) | 202 (±11.3) | 178 (±10.0) |
| 632 | 525 (±50.1) | 722 (±5.7) | 589 (±26.7) |
| 2000 | 1792 (±133.2) | 2245 (±16.4) | 2080 (±42.5) |
| 6325 | 5848 (±219.4) | 6335 (±88.8) | 6301 (±218.5) |
| 20,000 | 20826 (±2599.2) | 19652 (±191.0) | n.d. |
| σi, ng/L | 0.31 | 0.33 | 5.8 |
| LOD, ng/L | 0.9 | 1.0 | 17.3 |
| r2 | 0.996 | 0.998 | 0.999 |
| Lack of Fit Test (p value) | 0.9946 | 0.0741 | 0.8969 |
2.3.2. Accuracy and Precision
| Analyte | Recovery (%) | RSD (%) | ||
|---|---|---|---|---|
| Low Level | High Level | Low Level | High Level | |
| 4-MMP | 104.9 | 108.7 | 9.8 | 6.6 |
| 3-MH | 102.6 | 90.5 | 6.9 | 5.4 |
| 3-MHA | 90.2 | 100.5 | 11.1 | 5.6 |
2.4. Comparison of Current Method to Existing GC-MS Methods for Wine Polyfunctional Thiol Analysis
2.5. Quantification of Polyfunctional Thiols in Commercial Wines
| Variety | Region | Concentration (ng/L) | ||
|---|---|---|---|---|
| 4-MMP | 3-MH | 3-MHA | ||
| Cayuga White | Finger Lakes, NY (n = 5) | <LOD | 195 ± 38 | <LOD |
| Niagara | Finger Lakes, NY (n = 5) | 18 ± 13 | 230 ± 159 | <LOD |
| Riesling | Finger Lakes, NY (n = 5) | 2.3 ± 2.5 | 569 ± 334 | <LOD |
| Gewürztraminer | Finger Lakes, NY (n = 5) | <LOD | 373 ± 134 | <LOD |
| Rosé | Finger Lakes, NY (n = 5) | <LOD | 296 ± 116 | a |
| Sauvignon blanc | Finger Lakes, NY (n = 5) | 27 ± 8 | 446 ± 154 | a |
| Napa Valley, CA (n = 4) | 44 ± 22 | 438 ± 87 | b | |
| Sonoma County, CA (n = 4) | 43 ± 18 | 712 ± 342 | b | |
| Central Coast, CA (n = 3) | 50 ± 25 | 835 ± 137 | b | |
| Cabernet Sauvignon | Napa Valley, CA (n = 8) | <LOD | 765 ± 396 | 57 ± 16 |
| Sonoma County, CA (n = 5) | <LOD. | 405 ± 106 | 60 ± 21 | |
| Central coast, CA (n = 6) | <LOD | 498 ± 113 | 67 ± 24 | |
| Lodi (n = 1) | <LOD | 396 | 46 | |
3. Experimental Section
3.1. Chemical Reagents and Standards
3.2. GC-MS Conditions
| Compound | Retention Time (min) | Quantifying Ion | Qualifying Ions |
|---|---|---|---|
| 4-MMP | 26.0 | m/z: 312 | m/z: 181 |
| d10-4-MMP | 25.8 | m/z: 322 | m/z: 181 |
| 3-MH | 31.7 | m/z: 314 | m/z: 181 |
| d8-3-MH | 31.5 | m/z: 322 | m/z: 181, 229 |
| 3-MHA | 28.7 | m/z: 227 | m/z: * |
| d5-3-MHA | 28.6 | m/z: 255 | m/z: 117 |
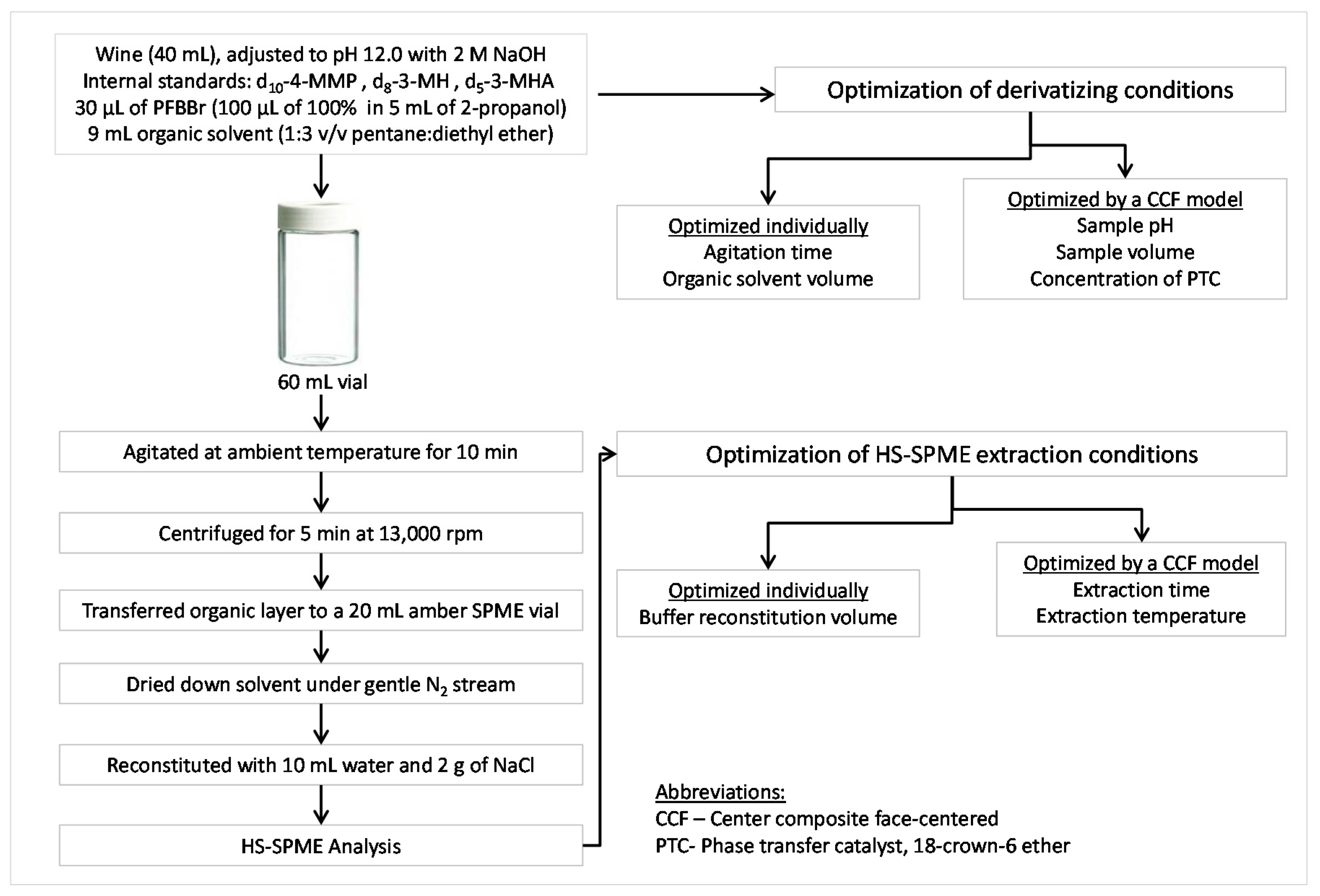
3.3. Optimization of Sample Preparation Conditions
3.3.1. Overview
| Parameters | Values | Description |
|---|---|---|
| Derivatization Parameters | ||
| Agitation time a | 10, 25, 40 min | Agitation time during extractive alkylation |
| Solvent Volume a | 8, 12, 16 mL | Volume of organic solvent used for the extractive alkylation |
| pH b | 6, 9.5, 12 | Sample pH following adjustment with 2 M NaOH |
| Catalyst b | 0, 0.05, 0.1 g | Amount of phase transfer catalyst (18-crown-6 ether) |
| Volume b | 10, 25, 40 mL | Volume of wine sample |
| HS-SPME Parameters | ||
| Reconstitution Volume a | 0, 5, 10 mL | Volume of 17% w/w NaCl solution added to the dried down SPME vial prior to HS-SPME analysis |
| Time c | 10, 30, 60 min | HS-SPME extraction time |
| Temperature c | 50, 70, 90 °C | HS-SPME incubation and extraction temperature |
3.3.2. Optimization of Sample Preparation Conditions
3.3.3. Optimization of Sample Extraction Method
3.3.4. Optimization of HS-SPME Analysis Conditions
3.4. Method Validation
3.4.1. Linearity and Limits of Detection
3.4.2. Accuracy
3.4.3. Precision
3.5. Quantification of Thiols in Commercial Wines
3.6. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Parameter(s) Optimized | Model Wine Volume | Thiol Concentration | No. of Replicates | Constant Parameters |
|---|---|---|---|---|
| Agitation time | 20 mL | 200 ng/L | 3 | 10 min extraction at 90 °C, pH 10, 0.1 g PTC, 4 mL solvent, 10 mL buffer |
| Solvent Volume | 40 mL | 3000 ng/L | 3 | 60 min extraction at 70 °C, pH 12, 0 g PTC, 10 mL buffer, 10 min agitation |
| Reconstitution Volume | 40 mL | 200 ng/L | 2 | 60 min extraction at 70 °C, pH 12, 0 g PTC, 9 mL solvent, 10 min agitation |
| pH, Volume, Catalyst | -- | 2000 ng/L | 2 | 10 min extraction at 90 °C, 10 min agitation, 9 mL solvent |
| SPME Time, Temperature | 40 mL | 3000 ng/L | 2 | pH 10, 0.1 g PTC, 10 min agitation, 9 mL solvent |
References
- Roland, A.; Schneider, R.; Razungles, A.; Cavelier, F. Varietal thiols in wine: Discovery, analysis and applications. Chem. Rev. 2011, 111, 7355–7376. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology. The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons: Chichester, UK, 2006; Volume 2. [Google Scholar]
- Tominaga, T.; Baltenweck-Guyot, R.; Des Gachons, C.P.; Dubourdieu, D. Contribution of volatile thiols to the aromas of white wines made from several vitis vinifera grape varieties. Am. J. Enol. Vitic. 2000, 51, 178–181. [Google Scholar]
- Tominaga, T.; Murat, M.L.; Dubourdieu, D. Development of a method for analyzing the volatile thiols involved in the characteristic aroma of wines made from vitis vinifera l. Cv. Sauvignon blanc. J. Agric. Food Chem. 1998, 46, 1044–1048. [Google Scholar] [CrossRef]
- Ferreira, V.; Cacho, J. Identification of Impact Odorants of Wines. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 393–415. [Google Scholar]
- Fedrizzi, B.; Versini, G.; Lavagnini, I.; Nicolini, G.; Magno, F. Gas chromatography-mass spectrometry determination of 3-mercaptohexan-1-ol and 3-mercaptohexyl acetate in wine a comparison of headspace solid phase microextraction and solid phase extraction methods. Anal. Chem. Acta 2007, 596, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Wesselink, W.; de Jong, C. Comparison of the sensitivity of different aroma extraction techniques in combination with gas chromatography-mass spectrometry to detect minor aroma compounds in wine. J. Chromatogr. A 2013, 1272, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Vivaracho, L.; Ferreira, V.; Cacho, J. Automated analysis of 2-methyl-2-furanthiol and 3-mercaptohexyl acetate at ng l-1 level by headspace solid-phase microextraction with on-fibre derivatisation and gas chromatography-negative chemical ionization mass spectrometric determination. J. Chromatogr. A 2006, 1121, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Vivaracho, L.; Cacho, J.; Ferreira, V. Quantitative determination of wine polyfunctional mercaptans at nanogram per liter level by gas chromatography-negative ion mass spectrometric analysis of their pentafluorobenzyl derivatives. J. Chromatogr. A 2007, 1146, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Vivaracho, L.; Cacho, J.; Ferreira, V. Improved solid-phase extraction procedure for the isolation and in-sorbent pentafluorobenzyl alkylation of polyfunctional mercaptans. Optimized procedure and analytical applications. J. Chromatogr. A 2008, 1185, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Vivaracho, L.; Zapata, J.; Cacho, J.; Ferreira, V. Analysis, occurrence, and potential sensory significance of five polyfunctional mercaptans in white wines. J. Agric. Food Chem. 2010, 58, 10184–10194. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bencomo, J.J.; Schneider, R.; Lepoutre, J.P.; Rigou, P. Improved method to quantitatively determine powerful odorant volatile thiols in wine by headspace solid-phase microextraction after derivatization. J. Chromatogr. A 2009, 1216, 5640–5646. [Google Scholar] [CrossRef] [PubMed]
- Capone, D.L.; Sefton, M.A.; Jeffery, D.W. Application of a modified method for 3-mercaptohexan-1-ol determination to investigate the relationship between free thiol and related conjugates in grape juice and wine. J. Agric. Food Chem. 2011, 59, 4649–4658. [Google Scholar] [CrossRef] [PubMed]
- Herbst-Johnstone, M.; Piano, F.; Duhamel, N.; Barker, D.; Fedrizzi, B. Ethyl propiolate derivatisation for the analysis of varietal thiols in wine. J. Chromatogr. A 2013, 1312, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Dagan, L.; Reillon, F.; Roland, A.; Schneider, R. Development of a routine analysis of 4-mercapto-4-methylpentan-2-one in wine by stable isotope dilution assay and mass tandem spectrometry. Anal. Chim. Acta 2014, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Capone, D.L.; Ristic, R.; Pardon, K.H.; Jeffery, D.W. Simple quantitative determination of potent thiols at ultratrace levels in wine by derivatization and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) analysis. Anal. Chem. 2015, 87, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Piano, F.; Fracassetti, D.; Buica, A.; Stander, M.; du Toit, W.J.; Borsa, D.; Tirelli, A. Development of a novel liquid/liquid extraction and ultra-performance liquid chromatography tandem mass spectrometry method for the assessment of thiols in south african sauvignon blanc wines. Aust. J. Grape Wine Res. 2015, 21, 40–48. [Google Scholar] [CrossRef]
- Danielson, N.D.; Gallagher, P.A.; Bao, J.J. Chemical reagents and derivatization procedures in drug analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rodríguez-Bencomo, J.J.; Muñoz-González, C.; Andújar-Ortiz, I.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Assessment of the effect of the non-volatile wine matrix on the volatility of typical wine aroma compounds by headspace solid phase microextraction/gas chromatography analysis. J. Sci. Food Agric. 2011, 91, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Fiamegos, Y.C.; Stalikas, C.D. Phase-transfer catalysis in analytical chemistry. Anal. Chim. Acta 2005, 550, 1–12. [Google Scholar] [CrossRef]
- Sala, C.; Mestres, M.; Marti, M.P.; Busto, O.; Guasch, J. Headspace solid-phase microextraction method for determining 3-alkyl-2-methoxypyrazines in musts by means of polydimethylsiloxane-divinylbenzene fibres. J. Chromatogr. A 2000, 880, 93–99. [Google Scholar] [CrossRef]
- Herbert, P.; Morais, S.; Paíga, P.; Alves, A.; Santos, L. Development and validation of a novel method for the analysis of chlorinated pesticides in soils using microwave-assisted extraction-headspace solid phase microextraction and gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2006, 384, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Mac Berthouex, P. Statistics for Environmental Engineers, 2 ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Benkwitz, F.; Tominaga, T.; Kilmartin, P.A.; Lund, C.; Wohlers, M.; Nicolau, L. Identifying the chemical composition related to the distinct aroma characteristics of new zealand sauvignon blanc wines. Am. J. Enol. Vitic. 2012, 63, 62–72. [Google Scholar] [CrossRef]
- Nakamura, S.; Hwee Sian, T.; Daishima, S. Determination of estrogens in river water by gas chromatography-negative-ion chemical-ionization mass spectrometry. J. Chromatogr. A 2001, 919, 275–282. [Google Scholar] [CrossRef]
- Capone, D.L.; Sefton, M.A.; Jeffery, D.W. Analytical investigations of wine odorant 3-mercaptohexan-1-ol and its precursors. In Flavor Chemistry of Wine and other Alcoholic Beverages; American Chemical Society: Washington, DC, USA, 2012; Volume 1104, pp. 15–35. [Google Scholar]
- Bouchilloux, P.; Darriet, P.; Henry, R.; Lavigne-Cruège, V.; Dubourdieu, D. Identification of volatile and powerful odorous thiols in bordeaux red wine varieties. J. Agric. Food Chem. 1998, 46, 3095–3099. [Google Scholar] [CrossRef]
- Capone, D.L.; Black, C.A.; Jeffery, D.W. Effects on 3-mercaptohexan-1-ol precursor concentrations from prolonged storage of sauvignon blanc grapes prior to crushing and pressing. J. Agric. Food Chem. 2012, 60, 3515–3523. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.; Edwards, P.B.; Jouanneau, S.; Kilmartin, P.; Gardner, R.; Villas-Boas, S. Sauvignon blanc metabolomics: Grape juice metabolites affecting the development of varietal thiols and other aroma compounds in wines. Metabolomics 2014, 10, 556–573. [Google Scholar] [CrossRef]
- Baumes, R. Wine aroma precursors. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 251–274. [Google Scholar]
- Makhotkina, O.; Kilmartin, P.A. Hydrolysis and formation of volatile esters in new zealand sauvignon blanc wine. Food Chem. 2012, 135, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, M.A.; Martinson, T.E.; Mansfield, A.K. Accumulation and prediction of yeast assimilable nitrogen in new york winegrape cultivars. Am. J. Enol. Vitic. 2014, 65, 325–332. [Google Scholar] [CrossRef]
- Acree, T.E.; Lavin, E.H.; Nishida, R.; Watanabe, S. The serendipitous discovery of o-amino acetophenone as the foxy smelling component of labruscana grapes. Abstr. Pap. Am. Chem. Soc. 1990, 200, 7. [Google Scholar]
- Nelson, R.R.; Acree, T.E.; Lee, C.Y.; Butts, R.M. Methyl anthranilate as an aroma constituent of american wine. J. Food Sci. 1977, 42, 57–59. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Ray, J.L.; Augier, C.; Baumes, R. Quantitative determination of sulfur containing wine odorants at sub-ppb levels. 1. Synthesis of deuterated analogs. J. Agric. Food Chem. 2000, 48, 5819–5823. [Google Scholar] [CrossRef] [PubMed]
- Pardon, K.H.; Graney, S.D.; Capone, D.L.; Swiegers, J.H.; Sefton, M.J.; Elsey, G.M. Synthesis of the individual diastereomers of the cysteine conjugate of 3-mercaptohexanol (3-mh). J. Agric. Food Chem. 2008, 56, 3758–3763. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Some of the frozen wine samples are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musumeci, L.E.; Ryona, I.; Pan, B.S.; Loscos, N.; Feng, H.; Cleary, M.T.; Sacks, G.L. Quantification of Polyfunctional Thiols in Wine by HS-SPME-GC-MS Following Extractive Alkylation. Molecules 2015, 20, 12280-12299. https://doi.org/10.3390/molecules200712280
Musumeci LE, Ryona I, Pan BS, Loscos N, Feng H, Cleary MT, Sacks GL. Quantification of Polyfunctional Thiols in Wine by HS-SPME-GC-MS Following Extractive Alkylation. Molecules. 2015; 20(7):12280-12299. https://doi.org/10.3390/molecules200712280
Chicago/Turabian StyleMusumeci, Lauren E., Imelda Ryona, Bruce S. Pan, Natalia Loscos, Hui Feng, Michael T. Cleary, and Gavin L. Sacks. 2015. "Quantification of Polyfunctional Thiols in Wine by HS-SPME-GC-MS Following Extractive Alkylation" Molecules 20, no. 7: 12280-12299. https://doi.org/10.3390/molecules200712280
APA StyleMusumeci, L. E., Ryona, I., Pan, B. S., Loscos, N., Feng, H., Cleary, M. T., & Sacks, G. L. (2015). Quantification of Polyfunctional Thiols in Wine by HS-SPME-GC-MS Following Extractive Alkylation. Molecules, 20(7), 12280-12299. https://doi.org/10.3390/molecules200712280





