Abstract
Catharanthus roseus is a medicinal plant belonging to the family Apocynaceae which produces terpenoid indole alkaloids (TIAs) of high medicinal importance. Indeed, a number of activities like antidiabetic, bactericide and antihypertensive are linked to C. roseus. Nevertheless, the high added value of this plant is based on its enormous pharmaceutical interest, producing more than 130 TIAs, some of which exhibit strong pharmacological activities. The most striking biological activity investigated has been the antitumour effect of dimeric alkaloids such as anhydrovinblastine, vinblastine and vincristine which are already in pre-, clinical or in use. The great pharmacological importance of these indole alkaloids, contrasts with the small amounts of them found in this plant, making their extraction a very expensive process. To overcome this problem, researches have looked for alternative sources and strategies to produce them in higher amounts. In this sense, intensive research on the biosynthesis of TIAs and the regulation of their pathways has been developed with the aim to increase by biotechnological approaches, the production of these high added value compounds. This review is focused on the different strategies which improve TIA production, and in the analysis of the beneficial effects that these compounds exert on human health.
1. Introduction
Catharanthus is a perennial tropical medicinal plant belonging to the Family Apocynaceae which comprises eight species, seven endemic to Madagascar (C. coriaceus, C. lanceus, C. longifolius, C. ovalis, C. roseus, C. scitulus, C. trichophyllus), and one, C. pusillus, from India. Specifically, C. roseus is a decorative and curing plant of enormous pharmaceutical interest because it is nothing less than a chemical factory, producing more than 130 different terpenoid indole alkaloids (TIAs), some of which exhibit strong and important pharmacological activities [1]. In fact, vinblastine and vincristine are commercial TIAs used in anticancer chemotherapy, and together with a number of related semi-synthetic compounds, are collectively named the Vinca alkaloids. Currently, vinblastine, vincristine, vinorelbine and vindesine have been used in clinical trials, although only vinblastine, vincristine and vinorelbine have been approved for medical treatment in the United States [2]. Vinflunine, a fluorinated analogue of vinorelbine, has been approved in Europe [3,4]. Vincristine and vinblastine also show a strong antimicrobial activity [5]. In addition, C. roseus also produces ajmalicine and serpentine, which are monoterpenic indole alkaloids used as anti-hypertensive and anti-neuro-inflammatory agents, yohimbine which is mainly used in treatments for erectile dysfunction, and vindolicine used for the development of antidiabetic therapeutics.
The large interest in the anticancer compounds vinblastine and vincristine, which derive from the coupling of catharanthine and vindoline, contrasts with the low amounts of these compounds found in the plants, making their extraction a very expensive process. These low levels are mainly associated to the spatial separation of biosynthetic sites where these compounds are produced in the plant and to the high degree of specialization of some leaf cells where the assembly of specific steps of the TIA biosynthetic pathway occurs [6]. In fact, catharanthine is accumulated almost exclusively in the wax exudates on the leaf surface, whereas vindoline is produced in specialized internal leaf cells, suggesting that an involvement of transport processes are needed for their coupling to take place [7]. Recently, an ABC transporter, CrTPT2, whose primary function consists in enhancing the transport and hence, the accumulation of catharanthine in the leaf epidermal surface, has been identified [6]. However, the physical separation of catharanthine and vindoline observed by Yu and DeLuca [6] is probably a limiting factor in very young leaves, where AVLB was actually shown to be absent [8], but definitely not in developed leaves, where the dimer AVLB was repeatedly reported to be abundant [8,9,10,11,12]. In fact, Carqueijeiro et al. [12] demonstrated that catharanthine, vindoline and AVLB were accumulated in the vacuoles of mesophyll cells by a specific proton antiport system, dependent on the transtonoplast pH gradient generated by V-H+-ATPase and V-H+-PPase using vacuoles isolated from leaves of adult plants.
In addition, researchers have looked for alternative sources and strategies to produce TIAs in high amounts. In fact, the low levels of the TIAs with anticancer activity found in plants have stimulated an intense research effort aiming to obtain in vitro C. roseus cultures with a higher production of these TIAs. Technologically, Zhao and Verpoorte showed that although C. roseus cells can be cultivated in bioreactors, the TIA biosynthesis is extremely low, which prevents their industrial production. To increase this production, several approaches were tried [13] using C. roseus cell cultures, being genetic modification or metabolic engineering the most promising biotechnological alternatives for producing these compounds [1].
After providing an overview of pharmacological activities of some TIAs and semi-synthetic Vinca alkaloids, this review aims to summarize and highlight the key issues of TIA-related research in the 21st century, with particular emphasis on the empirical strategies developed for improving TIA production using plants and in vitro cultures of shoots, hairy roots and cells. Special attention is also focused on rational approaches, which are the most promising strategies to improve TIA production in the future.
2. Pharmacological Activities
2.2. Antidiabetic and Antioxidant Properties
Diabetes mellitus is considered one of the most important causes of mortality and to date, there is not a completely effective treatment for its healing [20,21,22]. Due to the side effects of insulin and oral hypoglycemic agents, currently bioactive plant natural metabolites which have antidiabetic activity are a promising alternative. In this way, Tiong et al. [23] examined the hypoglycemic and antioxidant activity of vindoline, vindolidine, vindolicine and vindolinine obtained from C. roseus (L.) G. Don leaves. These authors indicated that these compounds provoked a rise of glucose absorption in pancreatic or myoblast cells, being vindolicine the compound that showed the highest activity. In addition, vindolidine, vindolicine and vindolinine had a high inhibitory activity against protein tyrosine phosphatase-1B inhibitory activity, indicating that these compounds could be used for diabetes. Vindolicine also exhibited the highest antioxidant effects in both the oxygen radical absorbance capacity and 1,1-diphenyl-2-picrylhydrazyl tests, and this compound also decreased H2O2-induced oxidative damage to pancreatic cells. Therefore, in the future, vindolicine could be used as antidiabetic agent.
2.3. Potential Effects of Yohimbine on Erectile Dysfunction
Up until the late 1990s, yohimbine, one α-2 adrenergic antagonist, was one of the few oral pharmacological agents prescribed for the treatment of erectile dysfunction. However, relatively few well-designed studies on the therapeutic effect of yohimbine have been completed [24]. In general, the treatment with yohimbine alone presents low efficacy in patients with erectile dysfunction, while the combined therapy of yohimbine and L-arginine (that enhances the nitric oxide pathway) is more effective to improve the erectile function [25].
2.4. Potential Effects of Ajmalicine and Serpentine as Anti-Neuro-Inflammatory Agents
Inflammation of the central nervous system or neuro-inflammation is the most important feature of neurodegenerative diseases such as Alzheimer’s [26,27]. In this sense, irregular action of the central nervous system triggers an increase in cyclooxygenase-II levels, which provokes neuroinflammation [28]. Cyclooxygenases catalyse an early step in the biosynthesis of prostanoids, which potentiate the inflammatory cascade causing neuronal damage. Manigandan et al. [29] evaluated the inhibitory effect of ajmalicine, vindoline, catharanthine, serpentine and tabersonine on cyclooxygenase-II, observing that particularly serpentine acted as a potent anti-neuroinflammatory agent since it inhibited the receptor of cyclooxygenase-II.
2.5. Potential Effects of Ajmalicine on Vascular Disorders
Adrenergic receptor antagonists have been widely used for the treatment of hypertension. In this sense, ajmalicine has been used as a hypotensive agent since it acted as an α-adrenergic receptor antagonist [30]. Similarly, serpentine is also considered a hypotensive agent [31].
2.6. Antitumour Properties
The Vinca alkaloids have generally been used in the treatment of cancer [2,32]. These compounds repress cell growth because they alter the microtubular dynamics, and ultimately this provokes apoptosis. Semi-synthetic compounds similar to vinblastine and vincristine have been developed to increase their therapeutic action [33].
Vinblastine is used in particular for the treatment of Hodgkin’s disease, besides lymphosarcoma, choriocarcinoma, neuroblastoma, carcinoma of breast and lung, and lymphocytic leukemia [34,35]. Anhydrovinblastine, the direct precursor of vinblastine, also showed significant in vitro cytotoxic effect against human non-small cell lung cancer C4 and human cervical carcinoma, human leukemic cells, and A431 human carcinoma cells [36].
Vincristine is an oxidised form of vinblastine that arrests mitosis in metaphase and is very effective for treating acute lymphoblastic leukaemia in both children and adults. It is also used against Hodgkin’s disease, Wilkins’s tumour, neuroblastoma, and reticulum cell sarcoma [2,32]. In addition, vincristine has also been used in the treatment of multiples non-malignant hematologic disorders like autoimmune and thrombotic thrombocytopenia, and hemolytic uremic syndrome [2,32].
On the other hand, the cytotoxic effect of catharoseumine, which is a monoterpenic indole alkaloid isolated from the whole plant of C. roseus, was tested in different human tumour cell lines showing only a moderate cytotoxic effect against HL-60 cell line [19].
2.6.1. Semi-Synthetic Derivatives of Dimeric Alkaloids
Vinblastine and vincristine are present at low concentration levels in C. roseus plants, however their production is more than enough to generate derivative compounds with improved pharmacological properties. Vindesine is a semi-synthetic derivative from vinblastine, which provokes the arrest of cells in metaphase since vindesine inhibits the function of tubulin. This drug is used to treat diseases such as acute leukaemia, malignant lymphoma, Hodgkin's disease, acute erythraemia and acute panmyelosis. Nowadays, this compound is being examined for its potential to synergize with interferon, and for its value as therapeutic in preventing metastasis [37]. Vindesine was also shown to be effective in combined therapies of soft tissue sarcomas. In fact, Rhomberg et al. [38] analyzed the combination of razoxane, vindesine and radiotherapy on the dynamics of metastasis in advanced soft tissue sarcomas. The combination inhibited the development of metastasis in a majority of the patients and prolonged their survival.
Vinorelbine is other semi-synthetic derivative obtained from vinblastine, although it is less neurotoxic than its precursor. This compound showed a high antitumour effect on patients with breast or prostate cancer because vinorelbine inhibits mitosis through its interaction with tubulin. In addition, vinorelbine has been approved by Food and Drug Administration to treat patients with advanced lung cancer in the United States [39]. Because of this efficacy and its good safety, vinorelbine has been administered in combination with other agents (capecitabine, epirubicin, docetaxel etc...) for the treatment of different types of cancers [40].
Vinflunine is the first fluorinated microtubule inhibitor synthesised from vinorelbine. Vinflunine is different from others Vinca alkaloids because this compound binds weakly to tubuline, showing an improved tolerance profile as a result of its less neurotoxicity. Three possible effects of vinflunine have been described: its action against tubulin and microtubules, its capacity to disrupt newly formed blood vessels, and its ability to reduce the metastatic process. These findings support the hypothesis that vinflunine might have not only a tumour-cytostatic effect but also a specific antiangiogenic effect. Clinically, vinflunine is being seen for its potential role for treating of mammary carcinoma and non-small cell lung cancer, amongst others [4,41].
2.6.2. Mechanism of Action of Dimeric Alkaloids
Microtubules (MTs) are components of the cytoskeleton and play very important role in a great numbers of cellular processes. They are involved in chromosome separation during mitosis and meiosis, and are the major constituents of mitotic spindles, besides they are involved in maintaining cell structure, transport and many others cell functions. MTs are synthesized from α,β-tubulin heterodimers which polymerize end-to-end into linear protofilaments. These structures are, in a dynamic polymerization and depolymerization, at their ends. The assembly and disassembly of the MT polymers are regulated by the binding of tubulin and guanosine 5-triphosphate. Any interference with this MT dynamics can provoke a cell cycle arrest and lead to programmed cell death or apoptosis [42].
MT dynamics, and therefore cell division, can also be perturbed by small molecules, which are usually divided into two groups: (i) MT-stabilizing agents that prevent then depolymerization; and (ii) MT-depolymerizing agents that inhibit their formation. Vinca alkaloids fall inside the second group, as they arrest tumour cells during mitosis by binding at the surface between two tubulin heterodimers next to the exchangeable guanosine 5-triphosphate-binding site [43] and depolymerizing the MTs. This leads to cell cycle arrest in mitosis [44,45]. At high concentration Vinca alkaloids lead to the formation of large tubulin polymers called “Paracristal” and as a result, the tumoral cells are stopped in mitosis and immediately they die [46]. However, when the levels of Vinca alkaloids are low, the cells are arrested in mitosis and the cells die after a long time of incubation.
Since the binding of natural Vinca alkaloids, vinblastine and vincristine, as well as their semi-synthetic analogues, vinorelbine and vinflunine, is linked to tubulin self-association, their affinities can be determined and their binding are entropically driven so that the overall affinities decrease in the following order: vincristine > vinblastine > vinorelbine > vinflunine (Figure 1).
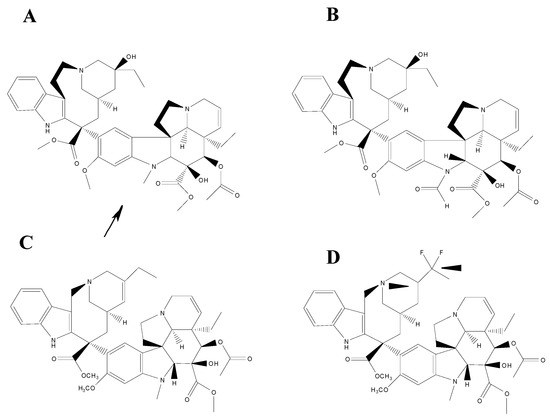
Figure 1.
Chemical structures of the four Vinca alkaloids vincristine (A), vinblastine (B), vinorelbine (C) and vinflunine (D). The arrow indicates the principal difference between vincristine (A) and vinblastine (B). The arrow heads indicate the principal difference between vinorelbine (C) and vinflunine (D).
The affinity of the four Vinca alkaloids for tubulin heterodimers appears to be almost identical however [47], the major differences amongst them are due to the distinct affinities of the resulting ligand heterodimers for polymerized spirals. All of Vinca alkaloids, in their respective complexes with α,β-tubulin, exhibit overall similar van der Waals forces and electrostatic interaction energy terms thereby defining a common binding site. However, the vindoline domain is used to bind tubulin heterodimers, while the catharanthine domain provides a cytotoxic effect [48]. Their binding site to MTs is lined by the side chains of a number of mostly non-polar amino acids from both tubulin monomers. The formyl group on the indole ring of vincristine that replaces the methyl in vinblastine is relevant for different spectra of pharmacological activity and dose-related toxicities, as well as differential cellular uptake and retention characteristics. On the other hand, the saturated double bond and the two fluorine atoms that differentiate vinflunine from vinorelbine provide only a marginally improved electrostatic interaction [49]. Even though their interaction with tubulin has been characterized, additional studies have revealed new mechanisms of action, such as their interplay with MT associated protein, inhibition of amino acid metabolism and interaction with calmodulin. This interaction with calmodulin could explain the differences in efficacy of vinflunine that, in spite of its binds to tubulin is lower than vinblastine or vincristine, displays a better activity than vinblastine against murine tumours and human tumour xenografts. Vinflunine turned out to be a better inhibitor binding to calmodulin, therefore presents higher efficiency and lower toxicity in vivo [50]. A recent study based on vinorelbine supports the hypothesis about the importance of impact of “secondary” targets in overall action of Vinca alkaloids [51]. This could explain why, despite of very similar binding parameters on tubulin, vinorelbine and vinblastine had such a different use in clinics [52].
3. Keynotes Based on the Use of C. roseus Plants as Biofactories for Enhancing the Production of Terpenoid Indole Alkaloids
As stated above, the production of TIAs by C. roseus in vitro cultures is a feasible technology pursued by industrial and academic interests. It is crucial to take into account, prior to the use of high producer in vitro cultures of TIAs, the right choice of the C. roseus cultivar which provides the best TIA levels. Although different studies based on HPLC metabolic profiling showed nearly equal amounts of TIAs in different cultivars of C. roseus, some C. roseus cell lines produced high levels of TIAs. In fact, the selection and development of a cell line which synthesizes and accumulates TIAs depends on critical steps like the selection of appropriated explants (type, size, age, amongst others.) which are used for initiating the cell culture as well as culture conditions (nutrients, growth regulators, pH, temperature, oxygen and light, amongst others), which in turn, are critical factors on which the success of in vitro cultures depends. In addition, screening of cell lines along successive subcultures [53,54,55] has also enabled the selection of high-yielding cell lines although they are usually unstable and tend to decrease their production after periodical subcultures. Mannonen et al. [56] used different cryopreservation methods, several cryoprotectants, and cooling and thawing processes. The use of mineral oil has been a useful strategy for preservating cells which restarted their growth in liquid fresh culture media [57].
On the other hand, several studies carried out using whole plants, shoot, callus and cell cultures of C. roseus demonstrated that TIA production is dependent on both cell differentiation and organogenesis [58]. Endo et al. [59] observed that the pattern of TIA production in root and shoot cultures of C. roseus was similar to that found in roots and leaves of whole plants. However, many TIAs cannot be synthesized by C. roseus cell cultures since the activation of biosynthetic pathways is tissue-specific and developmentally regulated. Shukla et al. [60] observed that levels of vindoline and catharanthine enhanced in C. roseus in vitro cultures as the level of cell differentiation increased. However, these compounds were only detected in elicited conditions, the highest amounts of catharanthine and vindoline occurring in shoots (0.0039% and 0.0013% on a dry weight (DW) basis, respectively) compared with the levels found in callus (0.00019% and 0.00015% on a DW basis, respectively). The culture of hairy roots is another strategy for TIA production, since roots grow rapidly in hormone-free liquid medium, and they are composed of differentiated cells genetically stable, able to produce a broad spectrum of TIAs. In fact, the production of ajmalicine, serpentine, and catharanthine has been repeatedly reported by different authors, sometimes reaching levels superior to non-transformed root cultures (around 0.2%–0.4% DW) [61,62]. Production of vindoline has also been detected in hairy roots, at very low levels (0.04%–0.08% DW) [61,62,63].
4. Empirical Strategies for Improving TIA Production
4.1. Empirical Optimization of the Culture Medium and Culture Conditions
The composition of the culture medium as well as the physical environmental conditions, and the addition of specific compounds to the culture medium have been proved to increase TIA yields in C. roseus in vitro cultures, sometimes in a dramatic way. Thus, reducing or removing nitrate and/or phosphate ions from the medium leads to a significant decrease of cell growth but remarkably, an increase in TIA accumulation [64]. On the other hand, the addition of KCl (4 g/L) did not inhibit cell growth and increased threefold the production of ajmalicine and serpentine in comparison to the levels found in control compact callus clusters cultures of C. roseus [65] (Table 1). Similarly, the addition of NaCl (1.7 g/L) caused a rise in catharanthine levels (around 90%), while the same KCl concentration resulted in an increase of catharanthine around 300%. Moreover, the effect of a higher NaCl concentration (2.9 g/L) increased the content of vindoline, catharanthine, vinblastine and vincristine in C. roseus seedlings [66]. The highest levels of TIAs were obtained by adding 250 mM mannitol (four- to five-fold higher in comparison to the controls) to compact callus clusters cultures of C. roseus [65] (Table 1). The carbon source concentration is another limiting factor for the TIA biosynthesis. In fact, Schlatmann et al. [67] observed that the presence of a low glucose concentration correlated with a high ajmalicine production level. Zhao et al. [68] observed an increase of ajmalicine, serpentine and catharanthine (a total alkaloid yield of over 43 mg/L), when the concentration of sucrose increased in the culture medium of compact callus clusters of C. roseus (Table 1). Similarly, Jung et al. [69] observed an increase in the levels of catharanthine when the carbon source changed (fructose for sucrose) in C. roseus hairy root cultures.
Several authors have showed that the production of ajmalicine was significantly lower when a low C. roseus cell inoculum density was used although catharanthine levels were nearly the same at high and low cell densities [70,71].
The immobilization of plant cells provides resistance to the diffusion and it could increase the levels of TIAs, specially using a high cell density. In fact, several carriers have been used to immobilize cells of C. roseus [1]. In this sense, an increase of ajmalicine levels and its excretion to the medium when C. roseus cells were immobilized with calcium alginate was observed by Asada and Shuler [72]. Also, Lee and Shuler [71] observed that high inoculum density in C. roseus cultures in which cells are immobilized on alginate beads produced a high ajmalicine concentration (120 mg/L) [71] (Table 1).

Table 1.
Strategies to increase the production of TIAs in C. roseus.
| Material | Strategy | Total TIAs | Observations | Refs |
|---|---|---|---|---|
| Compact callus cluster | Medium supplemented with mannitol (250 mM) | 4.13-fold increase (75 mg/L) | 5.05-fold increase in catharanthine (19.7 mg/L) | [65] |
| 2.77-fold increase in serpentine (13.6 mg/L) | ||||
| 4.54-fold increase in ajmalicine (42.3 mg/L) | ||||
| Medium supplemented with KCl (4g/L) | 3.15-fold increase (57.8 mg/L) | 3.15-fold increase in catharanthine (12.3 mg/L) | ||
| 2.42-fold increase in serpentine (11.9 mg/L) | ||||
| 3.62-fold increase in ajmalicine (33.5 mg/L) | ||||
| Compact callus cluster | Medium supplemented with sucrose (50 g/L) | 1.16-fold increase (46.7 mg/L) | 1.25-fold increase in serpentine (10 mg/L) | [68] |
| 1.20-fold increase in ajmalicine (14.5 mg/L) | ||||
| Suspension cultured cells | High cell density (200 g FW/L) | ND | 120-fold increase in ajmalicine (60 mg/L) | [73] |
| Immobilized cells | High cell density (100 g FW/L) | ND | 2-fold increase in ajmalicine (120 mg/L) | [71] |
| Immobilized cells | Elicitation with Phytophthora cactorum | ND | 45-fold increase in ajmalicine (90 mg/L) | [72] |
| Shoot culture | MS medium supplemented with plant growth regulators | ND | The concentrations of 8.90 µM BA and 2.85 µM IAA increased the production of ajmalicine (0.85 g/L) | [74] |
| Callus | MS medium supplemented with plant growth regulators | ND | The concentrations of 2.21 µM BA and 5.7 µM IAA increased the production of catharanthine (0.12 mg/g DW) | [68] |
| ajmalicine (0.35 mg/g DW) | ||||
| vindoline (0.19 mg/g DW) | ||||
| serpentine (0.53 mg/g DW) | ||||
| Immobilized cells | Variation of O2 and CO2 concentration | ND | 1.1-fold increase in ajmalicine (275 g/L ) | [75] |
| Suspension cultured cells | Feeding with loganin and triptamine | ND | 17.73-fold increase in strictosidine (53.19 µmo/g DW) | [76] |
| 6.4-fold increase in ajmalicine (3.2 µmol/g DW) | ||||
| Compact callus cluster | Feeding with succinic acid (10 mM) | 4.86-fold increase (73 mg/L) | 3.5-fold increase in catharanthine (7 mg/L) | [65] |
| 7.5-fold increase in serpentine (15 mg/L) | ||||
| 16-fold increase in ajmalicine (32 mg/L) | ||||
| Feeding with tryptamine (3.12 mM) | 3.86-fold increase (58 mg/L) | 2.5-fold increase in catharanthine (5 mg/L) | ||
| 7-fold increase in serpentine (14 mg/L) | ||||
| 15.5-fold increase in ajmalicine (31 mg/L) | ||||
| Feeding with tryptophan (2.44 mM) | 5.53-fold increase (68 mg/L) | 2.5-fold increase in catharanthine (5 mg/L) | ||
| 6-fold increase in serpentine (12 mg/L) | ||||
| 14-fold increase in ajmalicine (28 mg/L) | ||||
| Hairy root culture | Feeding with geraniol (0.5 mM) | ND | 1.5-fold increase in tabersonine (1.4 mg/g DW) | [77] |
| Hairy root culture | Elicitation with sodium nitroprusside (0.1 mM) | 1.42-fold increase (3.7 mg/g DW) | 2-fold increase in lochnericine (1 mg/g DW) | [78] |
| 2.3-fold increase in tabersonine (0.7 mg/g DW) | ||||
| 2-fold increase in ajmalicine (0.7 mg/g DW) | ||||
| Suspension cultured cells | Elicitation with MeJA (101.9 µM) | 1.33-fold increase (2.2 mg/g DW) | 2-fold increase in tabersonine (3.8 mg/g DW) | [79] |
| Suspension cultured cells | Elicitation with MeJA (100 µM) | ND | 27.44-fold increase in ajmalicine (137.2 mg/L) | [80] |
| 11.12-fold increase in catharanthine (55.6 mg/L) | ||||
| Hairy root culture | Elicitation with MeJA (250 µM) | 1.32-fold increase (49 mg/L) | 7-fold increase in ajmalicine (6.34 mg/g DW) | [81] |
| 2.9-fold increase in serpentine (1.71 mg/g DW) | ||||
| 3-fold increase in ajmaline (12 mg/g DW) | ||||
| 3-fold increase in catharanthine (4.34 mg/g DW) | ||||
| Suspension cultured cells | Elicitation with Trichoderma viride | ND | 7.9-fold increase in ajmalicine (0.166 mg/g DW) | [82] |
| Suspension cultured cells | Elicitation with the protein of Phytophthora boehmeriae (BP90) | ND | 4-fold increase in catharanthine (20 mg/L) | [83] |
| Suspension cultured cells | Elicitation with CDs | ND | 40-fold increase in ajmalicine (200 mg/L) | [80] |
| 17-fold increase in catharanthine (85 mg/L) | ||||
| Suspension cultured cells | Elicitation with UV-B light | ND | 3-fold increase in catharanthine (0.12 mg/g DW) | [84] |
| 117.6-fold increase in vindoline (0.06 mg/g DW) | ||||
| Suspension cultured cells | Elicitation with UV-C light | ND | 18-fold increase in ajmalicine (90 mg/L) | [73] |
| 10-fold increase in catharanthine (50 mg/L) | ||||
| Plant | Elicitation with chromium (50 µM) | ND | 1.5-fold increase in vincristine (2 µg/g DW) | [35] |
| 2.16-fold increase in vinblastine (2.25 µg/g DW) | ||||
| Suspension cultured cells | Elicitation with Aspergillum niger mycelium and tetramethyl ammonium bromide | 3.84-fold increase (96 mg/L) | 21-fold increase in ajmalicine (63 mg/L) | [85] |
| 17-fold increase in catharanthine (17 mg/L) | ||||
| Elicitation with malate and sodium alginate | 3.28-fold increase (82 mg/L) | 13.6-fold increase in ajmalicine (41 mg/L) | ||
| 26-fold increase in catharanthine (26 mg/L) | ||||
| Suspension cultured cells | Elicitation with MeJA and CDs | ND | 90-fold increase in ajmalicine (450 mg/L) | [73] |
| 31-fold increase in catharanthine (155 mg/L) | ||||
| Elicitation with MeJA, CDs and UV-C light | ND | 2.3-fold increase in ajmalicine (1040 mg/L) (85 mg/g DW) | ||
| 1.26-fold increase in catharanthine (196 mg/L) (10 mg/g DW) | ||||
| Suspension cultured cells | Overexpression of STR | 24.6 fold in increase (123 mg/L) | _ | [86] |
| Suspension cultured cells | Overexpression of TDC and feeding with loganin and secologanin | 125 fold in increase (625 mg/L) | _ | [87] |
| Hairy root culture | Overexpression of DAT | ND | 4-fold increase in hörhammericine (0.16 mg/g DW) | [88] |
| Hairy root culture | Overexpression of CrPrx | 1.5-fold increase (85 mg/g DW) | 5-fold increase in serpentine (3.7 mg/g DW) | [89] |
| 3-fold increase in ajmalicine (0.35 mg/g DW) | ||||
| Hairy root culture | Overexpression of DXS | NV | 1.66-fold increase in ajmalicine (1.5 mg/g DW) | [90] |
| 1.66-fold increase in lochnericine (1 mg/g DW) | ||||
| Overexpression of ASα | NV | 1.25-fold increase in lochnericine (2.5 mg/g DW) | ||
| Overexpression of G10H/DXS | 0.0072 mg/g DW | 1.35-fold increase in tabersonine (0.9 mg/g DW) | ||
| 1.15-fold increase in lochnericine (1.4 mg/g DW) | ||||
| Overexpression of ASα/DXS | 0.015 mg/g DW | 1.16-fold increase in tabersonine (1.7 mg/g DW) | ||
| 1.18-fold increase in lochnericine (2 mg/g DW) | ||||
| Leaves | Transient overexpression of GPPS | ND | 1.6-fold increase in vindoline (2.5 mg/g DW) | [91] |
| Tobacco cell cultures | Overexpression of TDC/STR | ND | Enhancement in strictosidine (5.3 mg/L) | [92] |
| Morinda citrifolia cell cultures | Overexpression of TDC/STR | ND | Enhancement in strictosidine (21.2 mg/L) | |
| Cinchona officinalis hairy root culture | Overexpression of TDC/STR | ND | Enhancement in strictosidine (1.95 mg/g FW) | [93] |
| Saccharomyces cerevisiae | Overexpression of STR/SGD | ND | Enhancement in strictosidine (2000 mg/L) | [94] |
| Hairy root culture | Overexpression of transcription factor CrWRKY1 | ND | 3-fold increase in serpentine (0.291 mg/g DW) | [95] |
| 10-fold increase in ajmalicine (0.015 mg/g DW) | ||||
| Leaves | Transient overexpression of transcription factor CrMPK3 | ND | 3.52-fold increase in serpentine (0.061 mg/g DW) | [96] |
| 2.66-fold increase in vindoline (4.1 mg/g DW) | ||||
| 1.44-fold increase in catharanthine (1.3 mg/g DW) | ||||
| 2-fold increase in vincristine (1.75 mg/g DW) | ||||
| Hairy root culture | Overexpression of transcription factor ORCA3 | ND | 2.5-fold increase in catharanthine (5.6 mg/g DW) | [97] |
| Hairy root culture | Overexpression of transcription factor ORCA2 | ND | 2-fold increase in catharanthine (4.8 mg/g DW) | [98] |
| Transgenic plant | Overexpression of ORCA3 and G10H | ND | 3.03-fold increase in vindoline (2.1 mg/g DW) | [99] |
| 2.29-fold increase in catharanthine (4.6 mg/g DW) | ||||
| 6.30-fold increase in ajmalicine (0.315 mg/g DW) | ||||
| 1.08-fold increase in anhydrovinblastine (10.2 mg/g DW) | ||||
| 10.2-fold increase in vinblastine (0.27 mg/g DW) |
Abbreviations: DW, dry weight; FW, fresh weight; ND, not determined; NV, no variation; TIAs, Terpenoid indole alkaloids; STR, strictosidine synthase; TDC, tryptophan decarboxylase; DAT, deacetylvindoline 4-O-acetyltransferase; CrPrx, C. roseus peroxidase; DXS, 1-deoxy-D-xylulose-synthase; G10H, geraniol-10-hydroxylase; ASα, anthranilate synthase α subunit; GPPS, Geranyl diphosphate synthase; CrMPK3, C. roseus mitogen activated protein kinase 3, MeJA, methyl jasmonate; CDs, cyclodextrins; BA, benzyl adenine; IAA, indole-3-acetic acid.
As regards the effect of plant growth regulators, 2,4-dichlorophenoxyacetic acid inhibited TIA production [100,101], even when the precursors, loganin and tryptamine, are added to C. roseus cell cultures [76]. Similarly, the addition of ethylene inhibited the production of ajmalicine [102]. However, the combination of benzyladenine or kinetin with indole-3-acetic acid led to an enhancement of TIA production (total alkaloid yield of over 45 mg/L). Moreover, Satdive et al. [74] found high levels of ajmalicine (over 0.85 g/L) released in the spent media of C. roseus shoot grown in the presence of a high level of benzyladenine (8.90 mM) and a low level of indole-3-acetic acid (2.85 mM) (Table 1), whereas the addition of a high concentration of indole-3-acetic acid (11.42 mM) and a low concentration of benzyladenine (2.22 mM) resulted in high levels of ajmalicine (over 0.40 g/L) being accumulated in the shoots. In addition, TIA biosynthesis improved when C. roseus callus were treated with benzyladenine and indole-3-acetic acid and exposed to the light, especially vindoline (0.19 mg/g DW) and serpentine (0.53 mg/g DW) compared with callus that was not exposed to light [101].
On the other hand, Lee-Parsons and Shuler [75] showed that different O2 concentration decreased both cell growth and ajmalicine biosynthesis in C. roseus immobilized cells. However, the ajmalicine biosynthesis was unaffected when a high concentration of O2 and CO2 was used.
4.2. Feeding with Precursors and Elicitation as Empirical Strategies for Increasing the Production of TIAs
Sometimes, feeding with specific precursors has proved to be a successful strategy to increase the levels of TIAs using in vitro cultures. Specifically, tryptophane, tryptamine, geraniol, 10-hydroxygeraniol, loganin and secologanin have been the precursors most extensively added [65,76,77,100,103,104,105]. In fact, the combined use of tryptamine and loganin improved the accumulation of ajmalicine [100] and provoked a high accumulation of strictosidine [76] in C. roseus cell cultures. Also, the addition of succinic acid, tryptamine and tryptophan to the culture medium significantly increased ajmalicine and catharanthine levels [65] (Table 1). Similarly, the levels of ajmalicine and strictosidine were enhanced when C. roseus cell cultures were fed with loganic acid, loganin and secologanin [105]. More recently, feeding experiments with geraniol (0.5 mM), hydroxygeraniol or loganin provoked a marked increase in the accumulation of tabersonine (around 1.4 mg/g DW) in C. roseus hairy root cultures [77] (Table 1), but the most spectacular results were found in loganin feeding experiments where TIA accumulation improved significantly compared to non-transformed cells [104].
On the other hand, elicitation is one of the most effective established strategies to improve secondary metabolite production in plant platforms [106]. Both abiotic and biotic elicitors have been extensively used for improving TIA production in C. roseus in vitro cultures since the beginning of the 1990s [105,107,108,109]. In fact, abiotic elicitors like metal ions and UV light, as well as biotic elicitors such as fungal preparations and yeast extracts, hydrolytic enzymes, chemicals (cyclodextrins (CDs) and signalling molecules like nitric oxide, jasmonic acid and derived compounds such as methyl jasmonate (MeJA) and acetylsalicylic acid were commonly used [73,84,85,110,111,112]. In this way, the treatment of C. roseus hairy root cultures with 0.1 mM sodium nitroprusside (NO donor) caused a significant increase of serpentine, catharanthine, ajmalicine, hörhammericine, lochnericine and tabersonine [78] (Table 1). Also working with hairy roots of C. roseus treated with 101.9 µM of jasmonic acid, Goldhaber-Pasillas et al. [79] showed an increase of 190% in tabersonine (Table 1). In the same way, Lee-Parsons et al. [110] showed that the addition of 100 µM MeJA to C. roseus cell cultures increased the ajmalicine extracellular production (10.2 mg/L) in a dose and time-dependent manner. MeJA also triggered the production of ajmalicine (137.2 mg/L) and catharanthine (55.6 mg/L) in C. roseus cell cultures [73]. This elicitor was also effective in accumulating ajmalicine (6.34 mg/g DW) serpentine (1.71 mg/g DW), ajmaline (12 mg/g DW) and catharanthine (4.34 mg/g DW) in C. roseus hairy roots elicited with 250 μM MeJA [81] (Table 1). Likewise, the addition of 1 mM of acetylsalicylic acid provoked an increase of 505% total alkaloids in C. roseus cells transformed with Agrobacterium tumefaciens [113].
Several studies carried out with fungal preparations have proved to be a successful approach to increase TIA production in C. roseus in vitro cultures. Indeed, a maximum yield of ajmalicine (166 µg/g DW) was obtained from 20-d old cell cultures treated with Trichoderma viride [82]. In addition, the elicitation with a fungal preparation derived from Penicilium citrinum or PB90 (a protein elicitor from Phytophthora boehmeriae) also increased the production of catharanthine reaching levels over 20 mg/L in both cases in C. roseus cell cultures [83,111] (Table 1).
Another class of compounds that are gaining attention as potential elicitors of secondary metabolism in plants are cyclodextrins (CDs) which are modified cyclic oligosaccharides derived from starch. CDs have been able to induce defence responses in C. roseus cell cultures enhancing ajmalicine (over 200 mg/L) and catharanthine (85 mg/L) levels [73,80] (Table 1).
Abiotic elicitors including UV-light or heavy metals also induce TIA production in C. roseus. Thus, hairy roots exposed to UV-B increased significantly the levels of lochnericine (over 2.7 mg/g DW), serpentine (over 0.62 mg/g DW), and ajmalicine (over 0.31 mg/g DW) [114]. Ramani and Jayabaskaran [84] also observed an enhancement of catharanthine (0.12 mg/g DW) and vindoline (0.06 mg/g DW) when C. roseus cell cultures were exposed to UV-B light. Moreover, a short UV-C irradiation promoted the production of 90 mg/L ajmalicine and 50 mg/L catharanthine in C. roseus cell cultures [73] (Table 1).
In relation to heavy metals, the treatment of 50 µM chromium raised vincristine and vinblastine content in C. roseus plants, particularly in shoots reaching levels of around 2 and 2.25 µg/g DW, respectively [35] (Table 1).
Biotic and abiotic elicitors are associated to different mechanisms of elicitation and, when used in combination, could enhance metabolite accumulation in plant cell cultures. Indeed, the joint action of Aspergillus niger mycelium and tetramethylammonium bromide induced a synergism on TIA accumulation in C. roseus cell cultures, since they increased the levels of ajmalicine (63 mg/L) and catharanthine (17 mg/L). Also, the combination of malate and sodium alginate resulted in a high ajmalicine (41 mg/L) and catharanthine (26 mg/L) accumulation [65,68] (Table 1).
A new strategy for increasing TIA production in C. roseus cell cultures is the combined use of CDs and MeJA. In fact, when C. roseus cell cultures were elicited with both elicitors a high accumulation of ajmalicine and catharanthine was detected (450 and 155 mg/L, respectively). This effect was even greater when these elicitors were combined with short UV light exposure reaching a production of ajmalicine and catharanthine of 1040 mg/L (85 mg/g DW) and 196 mg/L (10 mg/g DW), respectively [73] (Table 1).
5. Rational Approaches to the Biotechnological Production of TIAs
If progress in the biotechnological production of TIAs is to continue, a rational approach to the molecular bioprocesses that take place in the producer cells is essential. In other words, it is necessary to know how the different empirical factors that increase yields of TIAs affect gene expression and metabolic profiles in C. roseus in vitro cultures. Such an approach can provide insight into biosynthetic pathways and their regulation. In this sense, the biosynthesis of TIAs involves a large number of enzymes which in turn, are highly regulated, and are located in different subcellular compartments acting in an organ and tissue-specific manner [4]. Precisely this compartmentalization and localization makes the TIA biosynthesis is considered a process tightly regulated, in which the different metabolic intermediates needs to be transported from one point to another for their transformation. TDC is found in the cytosol and G10H has membrane localization in endoplasmic reticulum as well as SLS which is anchored to the cytosolic face of the endoplasmic reticulum (Figure 2). As STR has been localized in the vacuole, secologanin and tryptamine can be translocated to this compartment to form strictosidine. This latter compound is transported out of the vacuole to form the strictosidine aglycone by SGD [115]. The next steps of the TIA pathway for the biosynthesis of tabersonine and catharanthine are quite poorly characterized, and nothing is known about their subcellular localization. As occur with G10H and SLS, the cytochrome P450, T16H are located in membranes, specifically in endoplasmic reticulum and the reactions catalyzed by the enzymes OMT, D4H and DAT to form vindoline are carried out in the cytoplasm, while the transformation to deacetoxyvindoline by NMT occur in the thylakoids (Figure 2) [116]. Ultimately, vindoline and catharanthine are transported to the vacuole, where a peroxidase (Prx) catalyzes their coupling to produce anhydrovinblastine, the precursor of vinblastine [117].
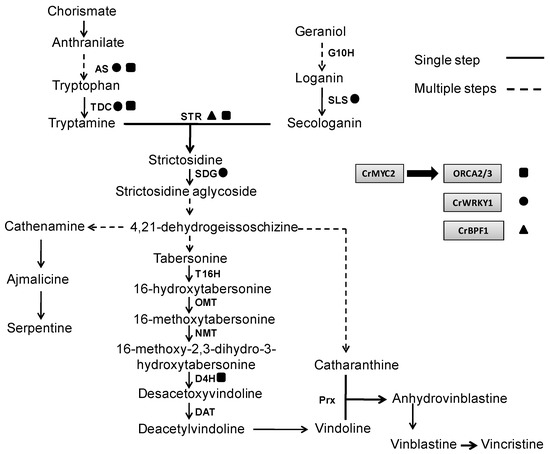
Figure 2.
Gene regulation scheme of transcription factors involved in the TIA biosynthethic pathways. AS, anthranilate synthase; TDC, tryptophan decarboxylase; G10H, geraniol 10-hydroxilase; SLS, secologanin synthase; STR, strictosidine synthase; SGD, strictosidine β-D-glucosidase, T16H, tabersonine 16-hydroxylase, OMT, 16-hydoxytabersonine 16-O-methyltransferase; NMT, N-methyltransferase; D4H, desacetoxyvindoline 4-hydroxylase; DAT, deacetylvindoline 4-O-acetyltransferase; Prx, peroxidase.
5.1. Elicitor Effects on the Expression of Genes Involved in TIA Metabolism
As mentioned above, empirical studies on C. roseus in vitro cultures have shown that TIA biosynthesis can be markedly induced by elicitors, however how the elicitors affect these specific metabolic pathways is not truly known [90]. In this way, different authors have studied the relationship between TIA accumulation and gene expression of G10H, SLS, TDC and STR under elicitation conditions, specifically in treatments with MeJA [80,118,119,120,121], CDs [80], and fungal homogenates [83,122,123] alone or in combination [80]. In fact, the transcript levels of TDC and STR were induced by MeJA treatments [80,119,120,121]. In contrast to these results, the expression levels of SLS and G10H were not increased [80] or increased slightly at short periods of time analyzed and then, these transcripts levels returned to the control levels at long treatment times [118,119,120]. This could be due to the fact that the expression profile of transcripts induced by MeJA in C. roseus depends on factors such as the developmental stage and type of the plant material, the cultivar of C. roseus and MeJA concentration used in the experiments. It is also important to note that the expression levels of these transcripts were always well-correlated with the levels of TIAs accumulated in MeJA-treated cells [80].
The expression of genes encoding enzymes from TIA biosynthesis can also be stimulated by adding biotic elicitors such as fungal preparations. Pasquali et al. [121] and Menke et al. [90] observed that TDC and STR genes in C. roseus cell cultures were strongly up-regulated after treatment with a filtrate of Pythium aphanidermatum and yeast extract. Similarly, Chen et al. [83] also demonstrated that C. roseus cells treated with PB90, displayed a time-dependent increase in catharanthine production involving a high level expression of TDC and STR. Similarly, CDs which have oligosaccharide structure and mimick fungal attack acting as elicitors [123], activated the expression of G10H, SLS, TDC and STR at all time points analyzed [80].
On the other hand, the combined effect of elicitors on the production of TIAs is a widely used strategy to increase TIAs, however there is few studies related with the joint action of elicitors on the expression of genes codifying enzymes from TIA biosynthesis. Almagro et al. [80] showed that the joint action of CDs and MeJA induced in C. roseus cell cultures, the highest expression levels of G10H, SLS, TDC and STR, observing a synergistic effect on both gene expression and TIA production. Therefore, it is evident that CDs activated a distinct pathway or played a different role in regulating TIA metabolism of that seen with MeJA in C. roseus cell cultures. Also the joint use of CDs and MeJA induced an important synergistic reprogramming of gene expression in C. roseus suspension cultured cells of C. roseus, which is probably the reason for the high accumulation levels of ajmalicine and catharanthine in those conditions. The molecular mechanism underlying the synergy between these two elicitors needs to be explored further.
5.2. Metabolic Engineering to Improve the Production of TIAs
Although there are only a few reports about the genetic transformation of C. roseus, metabolic engineering may be a potent tool for increasing TIA production in C. roseus cell platforms. Usually, enzymes of the biosynthetic pathway are selected as targets for gene cloning and then, manipulated by genetic engineering e.g., to raise metabolic flow rate toward the compound of interest and hence, enhance its levels. This approach has a limited value since a more predictable control of metabolic flux could be achieved using transcription factors because they can regulate one or more catalytic steps from TIA biosynthesis [124].
One of the major limitations to metabolic engineering of these systems is the lack of fully elucidated plant biosynthetic pathways. Although the “omics” era has led to an enormous increase in the available data, correct annotation and functional characterization of enzymes is crucial to the successful implementation of metabolic engineering strategies. In addition, the complex interactions amongst biosynthetic pathways and sophisticated regulatory mechanisms further complicate engineering efforts [125]. Therefore, amongst the strategies used to enhance TIA biosynthesis in C. roseus in vitro cultures, could be highlighted the overexpression of transcription factors and genes of their biosynthetic pathways as well as the overexpression of genes from the TIA biosynthesis in other organisms.
5.2.1. Overexpression of Genes which Regulate TIA Biosynthesis
Some studies were focussed on cell and tissue genetic engineering in C. roseus, resulting in higher yields of some TIAs [6,126] being TDC and STR the most overexpressed genes. In fact, STR overexpression in a transgenic C. roseus culture resulted in a significant increase of TIAs, reaching levels of 123 mg/L [86] (Table 1). Moreover, loganin feeding experiments provoked an increase in total TIAs of 300 mg/L [86]. Similarly, TDC overexpression did not increase [87] or slightly enhanced total TIAs levels (75 mg/L, [86]). However, when the precursors loganin and secologanin, were added to cell lines overexpressing TDC, the production of serpentine, catharanthine and strictosidine increased until 125-fold, resulting in a total TIA production of 625 mg/L [87] (Table 1).
In a similar way, C. roseus hairy root cultures overexpressing DAT genes accumulated hörhammericine until 4-fold more than the wild-type (Table 1, [88]) while transgenic hairy roots overexpressing the gene encoding a Prx, accumulated 5- and 3-fold more serpentine and ajmalicine than their respective controls (Table 1, [89]). Moreover, as a result of overexpressing geranyl diphosphate synthase (gene encoding an enzyme that catalyzes the first reaction of terpenoid pathway) in C. roseus leaves, an increase in vindoline (Table 1) was observed [91]. Other study focused on the co-overexpression of several key genes including those monoterpenoid and indole pathway, as well as G10H, alone or in combination in hairy root cultures of C. roseus [115], resulted in a significant increase in ajmalicine, lochnericine, and tabersonine (Table 1). This experiment pointed out the need for overexpressing multiple genes within the TIA pathway to obtain significant results.
On the other hand, two genes involved in early steps of TIA biosynthethic pathway, TDC and STR, have been introduced in other cell cultures, specifically tobacco and M. citrifolia [92]. In these conditions, an increase of strictosidine was observed in both tobacco and M. citrifolia cell cultures fed with tryptamine and secologanin reaching levels of 21.2 and 5.3 mg/L, respectively (Table 1). Similarly, these two genes were overexpressed in C. officinalis hairy root cultures resulting in high levels of strictosidine (1.95 mg/g FW) [93]. Another strategy is the bioproduction of TIA using microorganisms like S. cerevisiae and Escherichia coli [94]. Thus, transgenic yeasts overexpressing STR and SGD were able to produce a high amount of strictosidine (2000 mg/L) when these cultures were fed with tryptamine and secologanin (Table 1) while a transgenic E. coli overexpressing STR was able to transform strictosidine in vallesiachotamine and isovallesiachotamine, two TIAs found in plants which belong to the family Apocynaceae [127].
5.2.2. Overexpression of Transcription Factors which Regulate TIA Biosynthesis
Most of genes codifying enzymes from TIA biosynthetic pathway are tightly regulated by specific transcription factors in a coordinate manner together with developmental and environmental factors. In C. roseus, only a few transcription factors have been isolated and characterized, highlighting the Octadecanoid-Responsive Catharanthus AP2/ERF-domain proteins (ORCA2 and ORCA3), CrBPF1, CrWRKY1, CrMYC1 and CrMYC2 [73,78,79,80,81,82,83,84,86,87,88,89,90,91,92,93,94,95,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127]. These transcription factors respond to jasmonic acid, MeJA, and/or elicitors. Two bHLH (a basic helix-loop-helix) transcription factors, CrMYC1 and CrMYC2, were isolated and characterized from C. roseus. Both CrMYC1 and CrMYC2 were involved in the response to MeJA while CrMYC1 also responded to fungal elicitors [128,129], suggesting that these transcription factors may be involved in the regulation of TIA biosynthesis in response to these signals. Moreover, CrMYC2 activate the expression of two transcription factors, ORCA2 and ORCA3, which bind to the JERE (a cis-acting element involved in jasmonic acid and elicitor responses) element of the STR promoter. However, while ORCA2 respond to MeJA and elicitors, ORCA3 only respond to jasmonic acid. Likewise, the isolation of CrBPF1 transcription factor which is induced by elicitors but not by MeJA, suggest that elicitors induce STR gene expression via jasmonic acid-dependent and independent pathways [130].
On the other hand, ORCA3 overexpression resulted in increasing levels of some genes involved in TIA biosynthetic pathway, specially TDC, STR and D4H [118,130] which in turn, provokes the accumulation of TIAs [97,99,131]. In fact, the overexpression of ORCA3 in hairy root cultures increased the content of catharanthine up to 2.5-fold in comparison with the control cultures [97] (Table 1). Moreover, ORCA2 is a transcription factor key in regulating genes encoding the enzymes STR, TDC and SGD involved in the first two branches of TIA biosynthetic pathway [98,132]. Liu et al. [98] also observed that the overexpression of ORCA2 in C. roseus hairy root cultures provoked an increase of 2.0-fold in catharanthine (Table 1). Likewise, the combined overexpression of G10H and ORCA3 in hairy roots provoked an increase of catharanthine (6.5-fold) [131]. The same overexpression was carried out in plants of C. roseus resulting in an important increase of vindoline, catharanthine, ajmalicine, anhydrovinblastine and vinblastine (Table 1, [99]).
Other transcription factors involved in the biosynthesis of TIAs is the WRKY family. Overexpression of CrWRKY1 in hairy roots increased the expression of genes encoding AS, SLS, SDG and TDC and repressed ORCA2, ORCA3, and CrMYC2 resulting in an increase of ajmalicine and serpentine (Table 1, [95]).
Finally, CrMPK3 is also an important component of intracellular signalling pathway triggered by different stresses in C. roseus. In fact, the overexpression of CrMPK3 in C. roseus leaves raised both the expression of ORCA3 and genes involved in TIA biosynthetic pathway as well as serpentine, vindoline, catharanthine and vincristine levels (Table 1, [96]).
6. Conclusions
C. roseus is an amazing chemical factory, producing more than 130 TIAs, some of which exhibit strong pharmacological activities. The most striking biological activity investigated during recent years has been the antitumour effect of the dimeric alkaloids vinblastine and vincristine, as well as anhydrovinblastine, together with a number of semi-synthetic derivatives, known as the Vinca alkaloids. However, the scarcity of antitumoral TIAs found in C. roseus plants has stimulated an intense research effort aiming to obtain C. roseus in vitro cultures with higher production of these antitumour TIAs. To increase the production of TIAs, empirical strategies have been developed using plants, shoot, hairy root and cell in vitro cultures. Special attention has also been focused on experimental designs enhancing TIA production, which are the most promising strategies to improve TIA production in the future.
Metabolic engineering may be a potent tool for increasing TIA production in C. roseus platforms. Usually, enzymes of the biosynthetic pathway are selected as targets for gene cloning and then, manipulated by genetic engineering to raise metabolic flow rate toward the compound of interest and hence enhance its level. Indeed, the overexpression of genes encoding different transcription factors alone or in combination with key enzymes from the metabolic pathway especially in cells and hairy root cultures as well as in plants resulted in the developing of new improved biotechnological systems for producing important dimeric indole alkaloids such as anhydrovinblastine, vinblastine and vincristine, as well as the monoterpenic indole alkaloids vindoline, serpentine and lochnericine. A clear relationship is observed between the expression profile of the genes and the production of TIA.
The use of elicitors to activate genes involved in TIA metabolic pathways is an effective strategy to increase the biotechnological production of these compounds. Supplementing the medium with elicitors such as MeJA and CDs, individually or even in combination with UV light, induces an important reprogramming of gene expression in C. roseus cell cultures, which likely accounts for the observed enhanced production of some TIAs, specially tabersonine, ajmaline, ajmalicine and catharanthine. Although our knowledge increases, various aspects on the molecular mechanisms behind the action of elicitors and the possible synergy amongst them remain unknown. The ability of some elicitors like CDs to excrete these compounds to the culture medium is crucial for their biotechnological production. The use of high-producing C. roseus cultures elicited with CDs individually or in combination with other elicitors that excrete the bioactive compounds into the culture medium would allow the establishment of continuous systems in bioreactors without destroying the cell biomass, since the processes required for extracting and purifying of the target compounds would be simpler, more sustainable and economically more viable.
Acknowledgments
We are also grateful to Estefanía Pedreño for helping us to revise the English style of the manuscript. We are grateful to the European Cooperation in the field of Scientific and Technical Research (Cost Action FA1006).
Author Contributions
Each author has written a section of this review and all authors have read and approved the final manuscript. LA, FFP and MAP discussed the content of the paper, LA and FFP wrote the paper with the help of MAP.
Conflicts of Interest
The authors declare no conflict of interest
References
- Van der Heijden, R.; Jacobs, D.I.; Snoeijer, W.; Hallard, D.; Verpoorte, R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr. Med. Chem. 2004, 11, 1241–1253. [Google Scholar]
- Rowinsky, E. The Vinca alkaloids. In Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Gansler, T.S., Holland, J.F., Frei, E., Eds.; BC Decker Publisher: Hamilton, ON, Canada, 2003; Volume 2. [Google Scholar]
- Schutz, F.A.; Bellmunt, J.; Rosenberg, J.E.; Choueiri, T.K. Vinflunine: Drug safety evaluation of this novel synthetic vinca alkaloid. Expert Opin. Drug Saf. 2011, 10, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Delord, J.P.; Campone, M.; Nguyen, L. Vinflunine: A new microtubule inhibitor agent. Clin. Cancer Res. 2008, 14, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Grellier, P.; Sinou, V.; Garrea de Loubresse, N.; Bylen, E.; Boulard, Y.; Schrevel, J. Selective and reversible effects of vinca alkaloids on Trypanosoma cruzi epimastigote forms: Blockage of cytokinesis without inhibition of the organelle duplication. Cell Motil. Cytoskeleton. 1999, 42, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; de Luca, V. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc. Natl. Acad. Sci. USA 2013, 110, 15830–15835. [Google Scholar] [CrossRef] [PubMed]
- Roepke, J.; Salim, V.; Wu, M.; Thamm, A.M.; Murata, J.; Ploss, K.; Boland, W.; de Luca, V. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc. Natl. Acad. Sci. USA 2010, 107, 15287–15292. [Google Scholar] [CrossRef] [PubMed]
- Naaranlahti, T.; Auriola, S.; Lapinjoki, S.P. Growth related dimerization of vindoline and catharanthine in Catharanthus roseus and effect of wounding on the process. Phytochemistry 1991, 30, 1451–1453. [Google Scholar] [CrossRef]
- Balsevich, J.; Bishop, G. Distribution of catharanthine, vindoline and 3',4'-anhydrovinblastine in the aerial parts of some Catharanthus roseus plants and the significance thereof in relation to alkaloid production in cultured cells. In Primary and Secondary Metabolism of Plant Cell Cultures II, 1st ed.; Kurz, W.G.W., Ed.; Springer-Verlag: Berlin, Germany, 1989; pp. 149–153. [Google Scholar]
- Goodbody, A.E.; Watson, C.D.; Chapple, C.C.S.; Vukovic, J.; Misawa, M. Extraction of 3',4'-anhydrovinblastine from Catharanthus roseus. Phytochemistry 1988, 27, 1713–1717. [Google Scholar] [CrossRef]
- Sottomayor, M.; Lopéz-Serrano, M.; DiCosmo, F.; Ros Barceló, A. Purification and characterization of α-3',4'-anhydrovinblastine synthase (peroxidase-like) from Catharanthus roseus (L.) G. Don. FEBS Lett. 1998, 428, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Carqueijeiro, I.; Noronha, H.; Duarte, P.; Geros, H.; Sottomayor, M. Vacuolar transport of the medicinal alkaloids from Catharanthus roseus is mediated by a proton-driven antiport. Plant Physiol. 2013, 162, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Verpoorte, R. Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: From biochemical processing to metabolic engineering. Phytochem. Rev. 2007, 6, 435–457. [Google Scholar] [CrossRef]
- Koul, M.; Lakra, N.S.; Chandra, R.; Chandra, S. Catharanthus roseus and prospects of its endophytes: A new avenue for production of bioactive metabolites. Int. J. Pharm. Sci. Res. 2013, 4, 2705–2716. [Google Scholar]
- Nayak, B.S.; Pereira, L.M.P. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complement. Altern. Med. 2006, 6, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Millon, M.J.; Newman, T.A.; Audinot, V.; Cussac, D.; Lejeune, F.; Nicolas, J.P.; Cogé, F.; Galizzi, J.P.; Boutin, J.A. Agonist and antagonist actions of Yohimbine as compared to fluparoxan at alpha (2)-adrenergic receptors (AR). Synapse 2002, 35, 79–95. [Google Scholar] [CrossRef]
- Briones-Martin-Del-Campo, M.; Orta-Zavalza, E.; Juarez-Cepeda, J.; Gutierrez-Escobedo, G.; Cañas-Villamar, I.; Castaño, I.; de Las Peñas, A. The oxidative stress response of the opportunistic fungal pathogen Candida glabrata. Rev. Iberoam. Micol. 2014, 31, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, H.P.; Di, Y.T.; Zhang, Y.; Hao, X.J. Catharoseumine, a new monoterpenoid indole alkaloid possessing a peroxy bridge from Catharanthus roseus. Tetrahedron Lett. 2012, 53, 1576–1578. [Google Scholar] [CrossRef]
- Vats, V.; Yadav, S.P.; Biswas, N.R.; Grover, J.K. Anti-cataract activity of Pterocarpus marsupium bark and Trigonella foenum-graecum seeds extract in alloxan diabetic rats. J. Ethnopharmacol. 2004, 93, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Hunag, Y.; Glinka, Y.; Prud’Homme, G.J.; Wang, Q. Gene therapy of diabetes using a novel GLP-1/IgG1-Fc fusion construct normalizes glucose levels in db/db mice. Gene Ther. 2006, 14, 162–172. [Google Scholar] [PubMed]
- Ghosh, S.; Suryawanshi, S.A. Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. Indian J. Exp. Biol. 2001, 39, 748–759. [Google Scholar] [PubMed]
- Tiong, S.H.; Looi, C.Y.; Hazni, H.; Arya, A.; Paydar, M.; Wong, W.F.; Cheah, S.C.; Mohd Rais, M.; Awang, K. Antidiabetic and antioxidant properties of alkaloids from Catharanthus roseus (L.) G. Don. Molecules 2013, 18, 9770–9784. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.W.; Worcel, M.; Wyllie, M. Yohimbine: A clinical review. Pharmacol. Ther. 2001, 91, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Lebret, T.; Hervé, J.M.; Gorny, P.; Worcel, M.; Botto, H. Efficacy and safety of a novel combination of L-arginine glutamate and yohimbine hydrochloride: A new oral therapy for erectile dysfunction. Eur. Urol. 2002, 41, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.M.; Rothwell, N.J. Inflammation in central nervous system injury. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; O’Banion, M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Scatena, R.; Martorona, G.E.; Bottani, P.; Botta, G.; Pastove, P.; Giardina, B. An update of pharmacological approaches to neurodegenerative diseases. Expert Opin. Investig. Drugs 2007, 16, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Manigandan, V.; Gurudeeban, S.; Satyavani, K.; Ramanathan, T. Molecular docking studies of Rhizophora mucronata alkaloids against neuroinflammatory marker cyclooxygenase 2. Int. J. Biol. Chem. 2014, 8, 91–99. [Google Scholar] [CrossRef]
- Wink, M.; Schmeller, T.; Latz-Brüning, B. Modes of action of allelochemical alkaloids: Interaction with neuroreceptors, DNA, and other molecular targets. J. Chem. Ecol. 1998, 24, 1881–1937. [Google Scholar] [CrossRef]
- Vakil, R.J. Rauwolfia serpentina in the treatment of high blood pressure. A review of the literature. Circulation 1955, 12, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Nirmala, M.J.; Samundeeswari, A.; Sankar, P.D. Natural plant resources in anti-cancer therapy-A review. Res. Plant Biol. 2011, 1, 1–14. [Google Scholar]
- Junaid, A.; Sheba, H.K.; Zahid, H.S.; Zohra, F.; Mehpara, M.; Mukthar, A.B.; Sekh, A.N.; Ilah, A.; Iffat, Z.A.; Saeed, A.K.; et al. Catharanthus roseus (L.) G. Don. An important drug: It’s applications and production. Pharm. Glob. IJCP 2010, 4, 1–16. [Google Scholar]
- Rai, V.; Tandon, P.K.; Khatoon, S. Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: Vincristine and vinblastine. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kutney, J.; Mayer, L.; Schmidt, B. Anhydrovinblastine for the Treatment of Cervical and Lung Cancer. EP0969839 A1.
- Zhu, R.H.; Li, H.D.; Cai, H.L.; Jiang, Z.P.; Xu, P.; Dai, L.B.; Peng, W.X. Validated HILIC–MS/MS assay for determination of vindesine in human plasma: Application to a population pharmacokinetic study. J. Pharm. Biomed. Anal. 2014, 96, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Rhomberg, W.; Eiter, H.; Schmid, F.; Saely, C. Razoxane and vindesine in advanced soft tissue sarcomas: Impact on metastasis, survival and radiation response. Anticancer Res. 2007, 27, 3609–3614. [Google Scholar] [PubMed]
- Gregory, R.K.; Smith, I.E. Vinorelbine—A clinical review. Br. J. Cancer 2000, 82, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Finek, J. Oral vinorelbine in metastatic breast cancer: A review of current clinical trial results. Cancer Treat. Rev. 2012, 38, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Fougeray, R.; Rosenberg, J.E.; von der Maase, H.; Schutz, F.A.; Salhi, Y.; Culine, S.; Choueiri, T.K. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann. Oncol. 2013, 24, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.A.; Lawrence, N.J.; McGown, A.T. Tubulin as a target for anticancer drugs: Agents which interact with the mitotic spindle. Med. Res. Rev. 1998, 18, 259–296. [Google Scholar] [CrossRef] [PubMed]
- Gigant, B.; Wang, C.; Ravelli, R.B.; Roussi, F.; Steinmetz, M.O.; Curmi, P.A.; Sobel, A.; Knossow, M. Structural basis for the regulation of tubulin by vinblastine. Nature 2005, 435, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Okouneva, T.; Hill, B.T.; Wilson, L.; Jordan, M.A. The effects of vinflunine, vinorelbine, and vinblastine on centromere dynamics. Mol. Cancer Ther. 2003, 2, 427–436. [Google Scholar] [PubMed]
- Gidding, C.E.; Kellie, S.J.; Kamps, W.A.; de Graaf, S.S. Vincristine revisited. Crit. Rev. Oncol. Hematol. 1999, 29, 267–287. [Google Scholar] [CrossRef] [PubMed]
- Takanari, H.; Yosida, T.; Morita, J.; Izutsu, K.; Ito, T. Instability of pleomorphic tubulin paracrystals artificially induced by Vinca alkaloids in tissue-cultured cells. Biol. Cell 1990, 70, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Daly, E.M.; Taylor, R.E. Entropy and enthalpy in the activity of tubulin-based antimitotic agents. Curr. Chem. Biol. 2009, 3, 47–59. [Google Scholar] [CrossRef]
- Sertel, S.; Fu, Y.; Zu, Y.; Rebacz, B.; Konkimalla, B.; Plinkert, P.K.; Krämer, A.; Gertsch, J.; Efferth, T. Molecular docking and pharmacogenomics of Vinca alkaloid and their monomeric precursor, vindoline and catharanthine. Biochem. Pharmacol. 2011, 81, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Coderch, C.; Morreale, A.; Gago, F. Tubulin-based structure-affinity relationship for antimitotic Vinca alkaloid. Anticancer Agents Med. Chem. 2012, 12, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Makarov, A.A.; Tsvetkov, P.O.; Villard, C.; Esquieu, D.; Pourroy, B.; Fahy, J.; Braguer, D.; Peyrot, V.; Lafitte, D. Vinflunine, a novel microtubule inhibitor, suppresses calmodulin interaction with the microtubule-associated protein STOP. Biochemistry 2007, 46, 14899–14906. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, F.O.; Kulikova, A.A.; Devred, F.; Zernii, E.; Lafitte, D.; Makarov, A.A. Thermodynamics of calmodulin and tubulin binding to the vinca-alkaloid vinorelbine. Mol. Biol. 2011, 45, 697–702. [Google Scholar] [CrossRef]
- Barbier, P.; Tsvetkov, P.O.; Breuzard, G.; Devred, F. Deciphering the molecular mechanism of anti-tubulin plant derived drugs. Phytochem. Rev. 2013, 13, 157–169. [Google Scholar] [CrossRef]
- Deus, B.; Zenk, M.H. Exploitation of plant cells for the production of natural compounds. Biotechnol. Bioeng. 1982, 24, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Kurz, W.G.W.; Chatson, K.B.; Constabel, F. Biosynthesis and accumulation of indole alkaloids in cell suspension cultures of Catharanthus roseus cultivars. In Primary and Secondary Metabolism of Plant Cell Cultures, 1st ed.; Neumann, K.H., Barz, W., Reinhard, E., Eds.; Springer-Verlag: Berlin, Germany, 1985; Volume 5, pp. 143–153. [Google Scholar]
- Ganapathi, B.; Kargi, F. Recent Advances in Indole Alkaloid Production by Catharanthus roseus (Periwinkle). J. Exp. Bot. 1990, 41, 259–267. [Google Scholar] [CrossRef]
- Mannonen, L.; Toivonen, L.; Kauppinen, V.C. Effects of long term preservation on growth and productivity of Panax ginseng and Catharanthus roseus cell culture. Plant Cell Rep. 1990, 9, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Bachiri, Y.; Gazeau, C.; Hansz, J.; Morisset, C.; Dereuddre, J. Successful cryopreservation of suspension cells by encapsulation dehydration. Plant Cell Tissue Organ Cult. 1995, 43, 241–248. [Google Scholar]
- El-Sayed, M.; Verpoorte, R. Catharanthus terpenoid indole alkaloids: Biosynthesis and regulation. Phytochem. Rev. 2007, 6, 277–305. [Google Scholar] [CrossRef]
- Endo, T.; Goodbody, A.E.; Misawa, M. Alkaloid production in root and shoot cultures of Catharanthus roseus. Planta Med. 1987, 53, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Shasany, A.K.; Verma, R.K.; Gupta, M.M.; Mathur, A.K.; Khanuja, P.S.P. Influence of cellular differentiation and elicitation on intermediate and late steps of terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Protoplasma 2010, 24, 35–47. [Google Scholar] [CrossRef]
- Bhadra, R.; Vani, S.; Shanks, J.V. Production of indole alkaloids by selected hairy root lines of Catharanthus roseus. Biotechnol. Bioeng. 1993, 41, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Kwak, S.S.; Choi, C.Y.; Liu, J.R. An interchangeable system of hairy root and cell suspension cultures of Catharanthus roseus for indole alkaloid production. Plant Cell Rep. 1995, 15, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Palazón, J.; Cusidó, R.M.; Gonzalo, J.; Bonfill, M.; Morales, C.; Pinol, T. Relation between the amount of rolC gene product and indole alkaloid accumulation in Catharanthus roseus transformed root cultures. J. Plant Physiol. 1998, 153, 712–718. [Google Scholar] [CrossRef]
- Van der Heijden, R.; Verpoorte, R.; ten Hoopen, H.J.G. Cell and tissue cultures of Catharanthus roseus (L.) G. Don: A literature survey. Plant Cell Tissue Organ Cult. 1989, 18, 231–280. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Q.; Guo, Y.Q.; Zhu, W.H. Effects of stress factors, bioregulators, and synthetic precursors on indole alkaloid production in compact callus clusters cultures of Catharanthus roseus. Appl. Microbiol. Biotechnol. 2001, 55, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Liu, Z.P.; Liu, L.; Liu, C. Effects of NaCl on the growth and alkaloid content of Catharanthus roseus seedlings. J. Appl. Ecol. 2008, 19, 2143–2148. [Google Scholar]
- Schlatmann, J.E.; Koolhaas, C.M.A.; Vinke, J.L.; ten Hoopen, H.J.G.; Heijnen, J.J. The role of glucose in ajmalicine production by Catharanthus roseus cell cultures. Biotechnol. Bioeng. 1995, 47, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, W.H.; Hu, Q.; He, X.W. Enhanced indole alkaloid production in suspension compact callus clusters of Catharanthus roseus: Impacts of plant growth regulators and sucrose. Plant Growth Reg. 2001, 33, 33–41. [Google Scholar] [CrossRef]
- Jung, K.H.; Kwak, S.S.; Kim, S.W.; Lee, H.; Choi, C.Y.; Liu, J.R. Improvement of the catharanthine productivity in hairy root cultures of Catharanthus roseus by using monosaccharides as a carbon source. Biotechnol. Lett. 1992, 14, 695–700. [Google Scholar] [CrossRef]
- Almagro, L.; López-Pérez, A.J.; Pedreño, M.A. New method to enhance ajmalicine production in Catharanthus roseus cell cultures based on the use of cyclodextrins. Biotechnol. Lett. 2010, 33, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.T.; Shuler, M.L. The effect of inoculum density and conditioned medium on the production of ajmalicine and catharanthine from immobilized Catharanthus roseus cells. Biotechnol. Bioeng. 2000, 67, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Shuler, M.L. Stimulation of ajmalicine production and excretion from Catharanthus roseus: Effects of adsorption in situ, elicitors, and alginate immobilization. Appl. Microbiol. Biotechnol. 1989, 30, 475–481. [Google Scholar]
- Almagro, L.; Sabater-Jara, A.B.; Belchi-Navarro, S.; Fernandez-Perez, F.; Bru, R.; Pedreño, M.A. Effect of UV light on secondary metabolite biosynthesis in plant cell cultures elicited with cyclodextrins and methyljasmonate. In Plants and Environment, 1st ed.; Vasanthaiah, H.K.N., Kambiranda, D., Eds.; InTech Janeza Trdine: Rijeka, Croatia, 2011; Volume 1, pp. 115–136. [Google Scholar]
- Satdive, R.K.; Fulzele, D.P.; Eapen, S. Studies on Production of Ajmalicine in Shake Flasks by Multiple Shoot Cultures of Catharanthus roseus. Biotechnol. Prog. 2003, 19, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Lee-Parson, C.W.T.; Shuler, M.L. Sparge gas composition affects biomass and ajmalicine production from immobilized cell cultures of Catharanthus roseus. Enzym. Microb. Technol. 2005, 37, 424–434. [Google Scholar] [CrossRef]
- El-Sayed, M.; Verpoorte, R. Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tissue Organ Cult. 2002, 68, 265–270. [Google Scholar] [CrossRef]
- Morgan, J.A.; Shanks, J.V. Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J. Biotechnol. 2000, 79, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Peebles, C.A.; Shanks, J.V.; San, K.Y. Effect of sodium nitroprusside on growth and terpenoid indole alkaloid production in Catharanthus roseus hairy root cultures. Biotechnol. Prog. 2011, 27, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber-Pasillas, G.D.; Mustafa, N.R.; Verpoorte, R. Jasmonic Acid Effect on the Fatty Acid and Terpenoid Indole Alkaloid Accumulation in Cell Suspension Cultures of Catharanthus roseus. Molecules 2014, 19, 10242–10260. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Gutierrez, J.; Pedreño, M.A.; Sottomayor, M. Synergistic and additive influence of cyclodextrins and methyl jasmonate on the expression of the terpenoid indole alkaloid pathway genes and metabolites in Catharanthus roseus cell cultures. Plant Cell Tissue Organ Cult. 2014, 119, 543–551. [Google Scholar] [CrossRef]
- Ruiz-May, E.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Differential secretion and accumulation of terpene indole alkaloids in hairy roots of Catharanthus roseus treated with methyl jasmonate. Mol. Biotechnol. 2009, 41, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, A.; Patil, S.; Fulzele, D.P. Influence of fungal elicitors on production of ajmalicine by cell cultures of Catharanthus roseus. Biotechnol. Prog. 2002, 18, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, Z.; Lu, L.; Jin, H.; Sun, L.; Yu, Q.; Xu, H.; Yang, F.; Fu, M.; Li, S.; et al. Interaction between abscisic acid and nitric oxide in PB90 induced catharanthine biosynthesis of Catharanthus roseus cell suspension cultures. Biotechnol. Prog. 2013, 29, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Jayabaskaran, C. Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J. Mol. Signal. 2008, 3, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, W.H.; Hu, Q. Enhanced catharanthine production in Catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enzym. Microb. Technol. 2001, 28, 673–681. [Google Scholar] [CrossRef]
- Canel, C.; Lopes-Cardoso, M.I.; Whitmer, S.; van der Fits, L.; Pasquali, G.; van der Heijden, R.; Hoge, J.H.C.; Verpoorte, R. Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 1998, 205, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, S.; van der Heijden, R.; Verpoorte, R. Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus. J. Biotechnol. 2002, 96, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Magnotta, M.; Murata, J.; Chen, J.; de Luca, V. Expression of deacetylvindoline-4-O-acetyltransferase in Catharanthus roseus hairy roots. Phytochemistry 2007, 68, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, M.; Kumar, S.; Sinha, A.K. Overexpression of an apoplastic peroxidase gene CrPrx in transgenic hairy root lines of Catharanthus roseus. Appl. Microbiol. Biotechnol. 2011, 90, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Peebles, C.; Sander, G.W.; Hughes, E.H.; Peacock, R.; Shanks, J.V.; San, K.Y. The expression of 1-deoxy-d-xylulose synthase and geraniol-10-hydroxylase or anthranilate synthase increases terpenoid indole alkaloid accumulation in Catharanthus roseus hairy roots. Metab. Eng. 2011, 13, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Smita, S.S.; Singh, A.K.; Shanker, K.; Nagegowda, D.A. Heteromeric and homomeric geranyl diphosphate synthases from Catharanthus roseus and their role in monoterpene indole alkaloid biosynthesis. Mol. Plant 2013, 6, 1531–1549. [Google Scholar] [CrossRef] [PubMed]
- Hallard, D.; van der Heijden, R.; Verpoorte, R.; Lopez-Cardoso, I.; Pasquali, G.; Memelink, J.; Hoge, J.H.C. Suspension cultured transgenic cells of Nicotiana tabacum expressing tryptophan decarboxylase and strictosidine synthase cDNAs from Catharanthus roseus produce strictosidine upon feeding of secologanin. Plant Cell Rep. 1997, 17, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Hallard, D. Transgenic Plant Cells for the Production of Indole Alkaloids. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2000. [Google Scholar]
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for production of plant secondary metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Suttipanta, N.; Pattanaik, S.; Kulshrestha, M.; Patra, B.; Singh, S.K.; Yuan, L. The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 2011, 157, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.K.; Wankhede, D.P.; Jaggi, M.; Singh, P.; Jalmi, S.K.; Raghuram, B.; Sheikh, A.H.; Sinha, A.K. CrMPK3, a mitogen activated protein kinase from Catharanthus roseus and its possible role in stress induced biosynthesis of monoterpenoid indole alkaloids. BMC Plant Biol. 2012, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.X.; Liu, D.H.; Wang, Y.L.; Cui, L.J.; Ren, W.W.; Sun, F. Overexpression of transcriptional factor ORCA3 increases the accumulation of catharanthine and vindoline in Catharanthus roseus hairy roots. Russ. J. Plant Physiol. 2011, 58, 415–422. [Google Scholar] [CrossRef]
- Liu, D.H.; Ren, W.W.; Cui, L.J.; Zhang, L.D.; Sun, X.F.; Tang, K.X. Enhanced accumulation of catharanthine and vindoline in Catharanthus roseus hairy roots by overexpresion of transcriptional factor ORCA2. Afr. J. Biotechnol. 2011, 10, 3260–3268. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Q.; Yuan, F.; Xing, S.; Zhao, J.; Choi, Y.H.; Verpoorte, R.; Tian, Y.; Wang, G.; Tang, K. Overexpression of ORCA3 and G10H in Catharanthus roseus plants regulated alkaloid biosynthesis and metabolism revealed by NMR-metabolomics. PLoS One 2012, 7, e43038. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, S.; Verpoorte, R.; Canel, C. Influence of auxins on alkaloid accumulation by a transgenic cell line of Catharanthus roseus. Plant Cell Tissue Organ Cult. 1998, 53, 135–141. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.H.; Hu, Q. Effects of light and plant growth regulators on the biosynthesis of vindoline and other indole alkaloids in Catharanthus roseus callus cultures. Plant Growth Reg. 2001, 33, 43–49. [Google Scholar] [CrossRef]
- Lee, C.W.T.; Shuler, M.L. Different shake flask closures alter gas phase composition and ajmalicine production in Catharanthus roseus cell suspensions. Biotechnol. Technol. 1991, 5, 173–178. [Google Scholar] [CrossRef]
- El-Sayed, M.; Choi, Y.H.; Fréderichrich, M.; Roytrakul, S.; Verpoorte, R. Alkaloid accumulation in Catharanthus roseus cell suspension cultures fed with stemmadenine. Biotechnol. Lett. 2004, 26, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, S.; van der Heijden, R.; Verpoorte, R. Effect of precursor feeding on alkaloid accumulation by a strictosidine synthase over-expressing transgenic cell line S1 of Catharanthus roseus. Plant Cell Tissue Organ Cult. 2000, 69, 85–93. [Google Scholar] [CrossRef]
- Moreno, P.R.H.; van der Heijden, R.; Verpoorte, R. Effect of terpenoid precursor feeding and elicitation on formation of indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Rep. 1993, 12, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Krishnamurthy, R. Elicitors in plant tissue culture. J. Pharmacogn. Phytochem. 2013, 2, 60–65. [Google Scholar]
- Seitz, H.U.; Eilert, U.; de Luca, V.; Kurz, W.G.W. Elicitor mediated induction of phenylalanine ammonia lyase and tryptophan decarboxylase; accumulation of phenols and indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Tissue Organ Cult. 1989, 18, 71–78. [Google Scholar] [CrossRef]
- Kargi, F.; Ganapathi, B. Effects of precursor stimulating agents on formation of indole formation by C. roseus in a biofilm reactor. Enzym. Microb. Technol. 1991, 13, 643–647. [Google Scholar] [CrossRef]
- Hirata, K.; Horiuchi, M.; Asada, M.; Ando, T.; Miyamota, K.; Miura, Y. Stimulation of dimeric alkaloid production by near ultraviolet light in multiple shoot cultures of Catharanthus roseus. Ferment Bioeng. 1992, 74, 222–225. [Google Scholar] [CrossRef]
- Lee-Parsons, C.W.; Ertürk, S.; Tengtrakool, J. Enhancement of ajmalicine production in Catharanthus roseus cell cultures with methyl jasmonate is dependent on timing and dosage of elicitation. Biotechnol. Lett. 2004, 26, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Dong, J. Elicitor-induced nitric oxide burst is essential for triggering catharanthine synthesis in Catharanthus roseus suspension cells. Appl. Microbiol. Biotechnol. 2005, 67, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Memelink, J.; Gantet, P. Transcription factors involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Phytochem. Rev. 2007, 6, 353–362. [Google Scholar] [CrossRef]
- Godoy-Hernández, G.C.; Loyola-Vargas, V.M. Effect of acetylsalicylic acid on secondary metabolism of Catharanthus roseus tumor suspension cultures. Plant Cell Rep. 1997, 16, 287–290. [Google Scholar] [CrossRef]
- Binder, B.Y.; Peebles, C.A.; Shanks, J.V.; San, K.Y. The effects of UV-B stress on the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Biotechnol. Prog. 2009, 25, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Guirimand, G.; Courdavault, V.; Lanoue, A.; Mahroug, S.; Guihur, A.; Blanc, N.; Giglioli-Guivarc’h, N.; St-Pierre, B.; Burlat, V. Strictosidine activation in Apocynaceae: Towards a “nuclear time bomb”? BMC Plant Biol. 2010, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Guirimand, G.; Guihur, A.; Poutrain, P.; Héricourt, F.; Mahroug, S.; St-Pierre, B.; Burlat, V.; Courdavault, V. Spatial organization of the vindoline biosynthetic pathway in Catharanthus roseus. J. Plant Physiol. 2011, 168, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.R.; Hilliou, F.; Duarte, P.; Pereira, L.G.; Almeida, I.; Leech, M.; Memelink, J.; Barcelo, A.R.; Sottomayor, M. Molecular cloning and characterization of a vacuolar class III peroxidase involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol. 2008, 146, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Van Der Fits, L.; Memelink, J. The jasmonate-inducible AP2/ERF-domain transcription factor ORCA3 activates gene expression via interaction with a jasmonate-responsive promoter element. Plant J. 2001, 25, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wei, S. Methyl jasmonic acid induced expression pattern of terpenoid indole alkaloid pathway genes in Catharanthus roseus seedlings. Plant Growth Regul. 2010, 61, 243–251. [Google Scholar] [CrossRef]
- Zhou, M.L.; Zhu, X.M.; Shao, J.R.; Wu, Y.M.; Tang, Y.X. Transcriptional response of the catharanthine biosynthesis pathway to methyl jasmonate/nitric oxide elicitation in Catharanthus roseus hairy root culture. Appl. Microbiol. Biotechnol. 2010, 88, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, G.; Goddijn, O.J.; de Waal, A.; Verpoorte, R.; Schilperoort, R.A.; Hoge, J.H.C.; Memelink, J. Coordinated regulation of two indole alkaloid biosynthetic genes from Catharanthus roseus by auxin and elicitors. Plant Mol. Biol. 1992, 18, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Menke, F.L.; Parchmann, S.; Mueller, M.J.; Kijne, J.W.; Memelink, J. Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in Catharanthus roseus. Plant Physiol. 1999, 119, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; Sellés, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Capell, T.; Christou, P. Progress in plant metabolic engineering. Curr. Opin. Biotechnol. 2004, 15, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.A.; Roberts, S.C. Metabolic engineering approaches for production of biochemicals in food and medicinal plants. Curr. Opin. Biotechnol. 2014, 26, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zárate, R.; Verpoorte, R. Strategies for the genetic modification of the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem. Rev. 2007, 6, 475–491. [Google Scholar] [CrossRef]
- Geerlings, A.; Redondo, F.J.; Contin, A.; Memelink, J.; van der Heijden, R.; Verpoorte, R. Biotransformation of tryptamine and secologanin into plant terpenoid indole alkaloids by transgenic yeast. Appl. Microbiol. Biotechnol. 2001, 56, 420–424. [Google Scholar] [CrossRef]
- Chatel, G.; Montiel, G.; Pre, M.; Memelink, J.; Thiersault, M.; Saint-Pierre, B.; Doireau, P.; Gantet, P. CrMYC1, a Catharanthus roseus elicitor- and jasmonate-responsive bHLH transcription factor that binds the G-box element of the strictosidine synthase gene promoter. J. Exp. Bot. 2003, 54, 2587–2588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, H.; Hedhili, S.; Montiel, G.; Zhang, Y.; Chatel, G.; Pre, M.; Gantet, P.; Memelink, J. The basic helix-loop-helix transcription factor CrMYC2 controls the jasmonate-responsive expression of the ORCA genes that regulate alkaloid biosynthesis in Catharanthus roseus. Plant J. 2011, 67, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; Memelink, J. ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 2000, 289, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Liu, H.; Gao, X.S.; Zhang, H.X. Overexpression of G10H and ORCA3 in the hairy roots of Catharanthus roseus improves catharanthine production. Plant Cell Rep. 2010, 29, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Leopold, A.L.; Sander, G.W.; Shanks, J.V.; Zhao, L.; Gibson, S.I. The ORCA2 transcription factor plays a key role in regulation of the terpenoid indole alkaloid pathway. BMC Plant Biol. 2013, 13, 155. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).