Microextraction Techniques Coupled to Liquid Chromatography with Mass Spectrometry for the Determination of Organic Micropollutants in Environmental Water Samples
Abstract
:1. Introduction
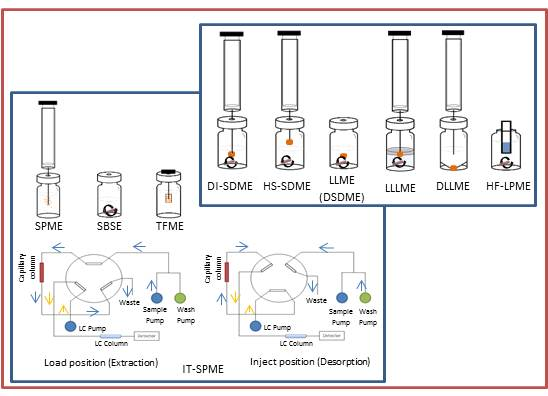
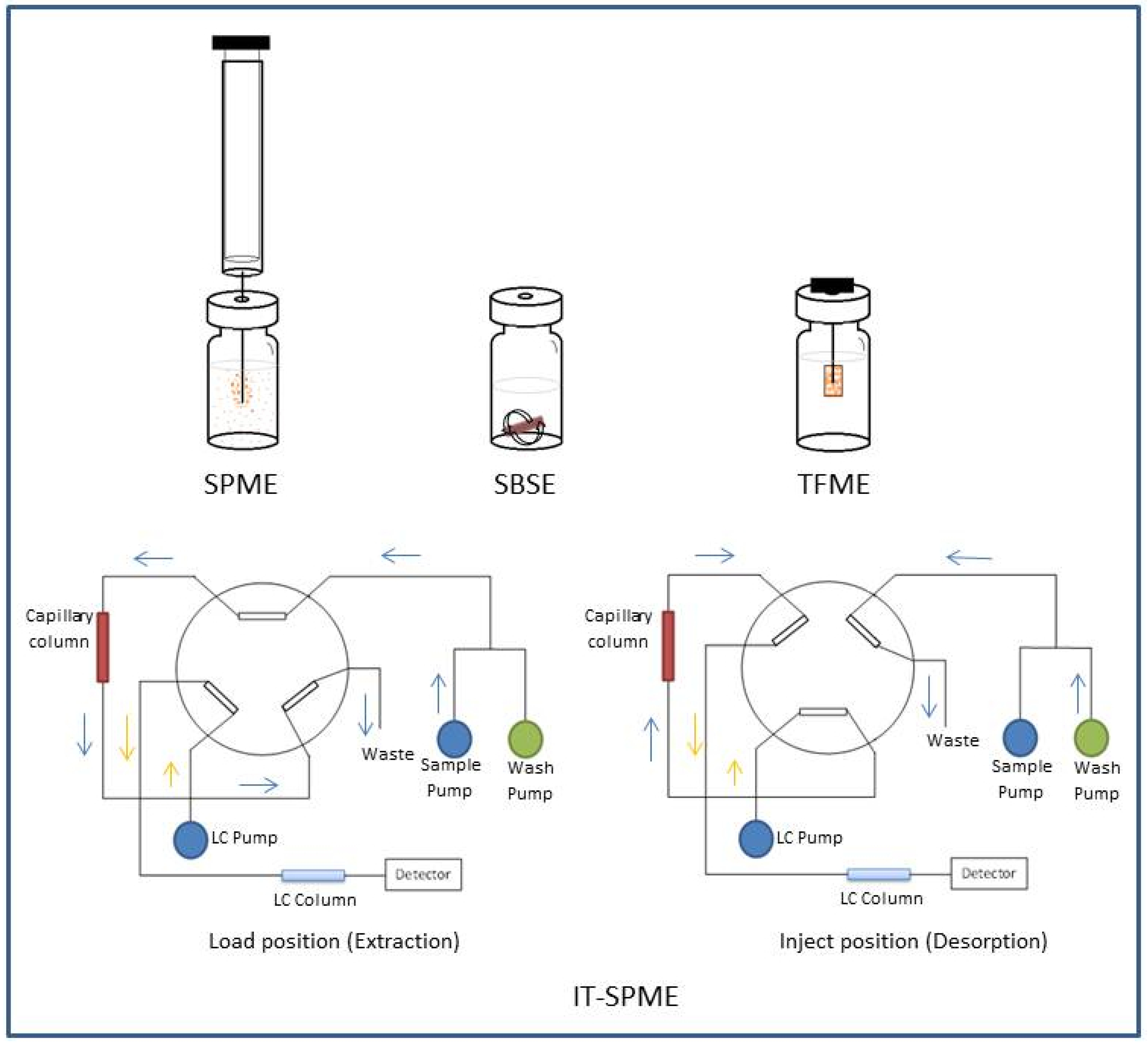
2. Solid-Phase Microextraction
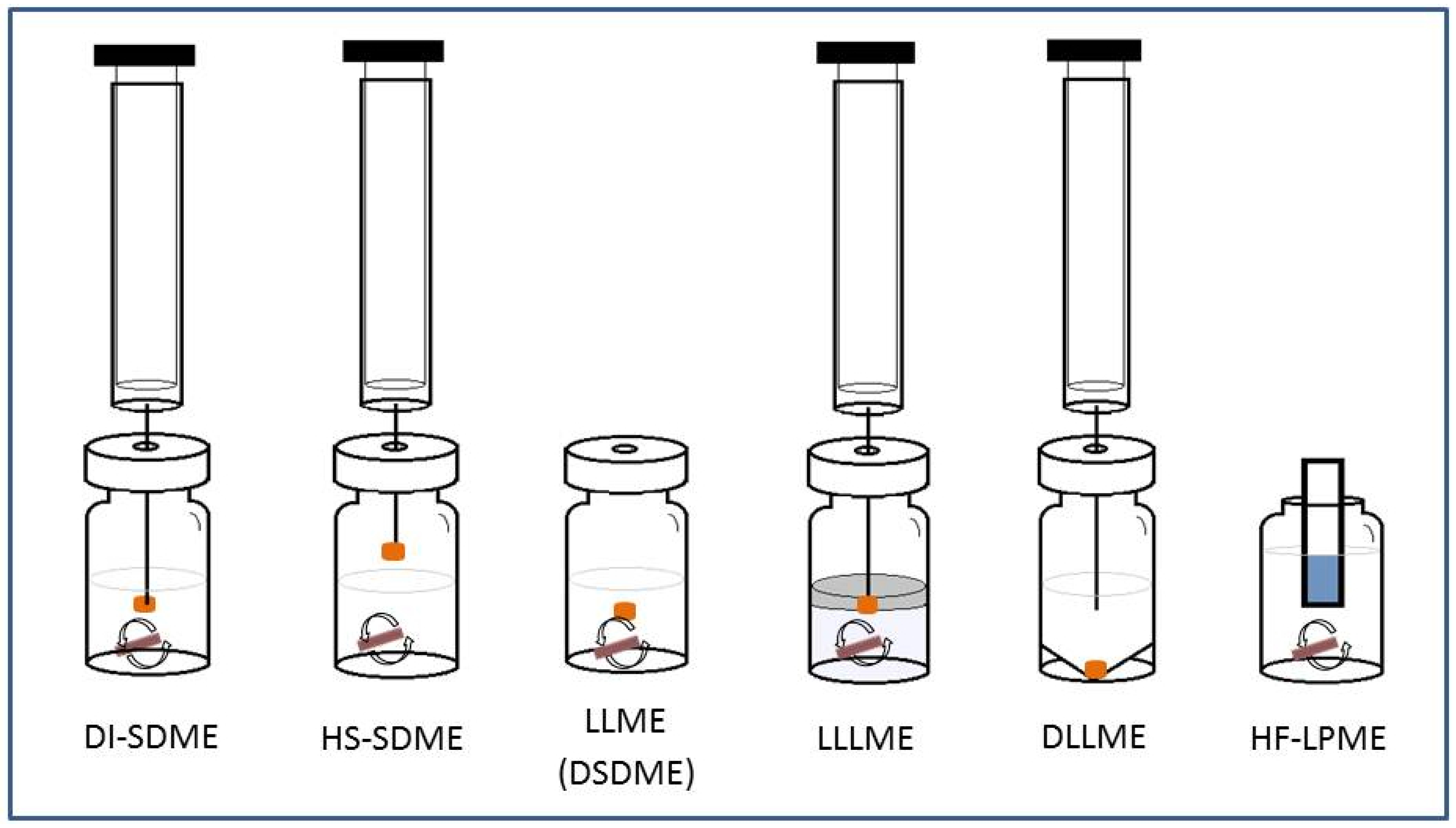
3. Solvent Microextraction
4. Applications of Microextraction Techniques to the Determination of Organic Micropollutants
4.1. Pesticides
4.2. UV Filters Including Benzotriazoles
4.3. Alkylphenols and Bisphenol A
4.4. Perfluorinated Compounds
4.5. Hormones
4.6. Pharmaceuticals
| Compounds | Matrix | Extraction Technique | Optimal Times | Handling | Recovery Accuracy (%) | LOD (ng·L−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Organic tin compounds (trimethyltin chloride, tripropyltin chloride, tri-phenyltin hydroxide, tributyltin chloride) | Freshwater and seawater | SPME | Extraction: 45 min Desorption: 5 min | Easy to use | 71–104 | 6–185 | [59] |
| Benzylic and aliphatic quaternary ammonium compounds | Tap water and surface water | SPME | Extraction: 45 min Desorption: 15 min | 96 well system | 97–143 | 10–500 | [60] |
| Polar pesticides (diuron, fluometuron, linuron, monuron, neburon, siduron, barban, carbaryl, chlorpropham, methiocarb, promecarb, propham) | Tap water, surface water and well water. | IT-SPME | 15 draw/eject cycle 12 min | Lower handling | 77–104 | 10–1200 | [61] |
| Multiresidue (atrazine, chlorfenvinphos, chlorpyriphos, di(2-ethylhexyl)phthalate, diuron, isoproturon, simazine, terbuthylazine, trifluralin) | Wastewater, superficial and coastal water | IT-SPME | 18 min | Lower handling | 8–166 | 25–2500 | [62] |
| Pesticides (alachlor, buprofezin, chlorpyriphos, chlorfenvinphos, diuron, fenthion, hexythiazox, isoproturon, malathion, tolclofos methyl, prochlora, imazalil, abamectin, diazinon, atrazine, simazine) | Surface water | SBSE | Extraction: 60 min Desorption: 30 min | Practical | 3–62 | 10–1000 | [63] |
| Antimicrobial compounds (triclosan, triclocarban) | River water and wastewater | SBSE | Extraction: 180 min Desorption: 15 min | Practical | 25–89 | 2.5–10 | [64] |
| Pesticides (carbofuran, clomazone, tebuconazole) | Tap water | DLLME | Extraction: seconds | Fast. Ease of operation | 62.7–120 | 20 | [65] |
| Triclosan and 2,4-dichlorophenol | Tap water and surface water | DLLME-SFO | Extraction: 1 min | Easy extraction-solidification | 83–119 | 2–20 | [66] |
| Triazine herbicides (cyanazine, simazine, atrazine) | Wastewater, river water underground water and drainage water | IL-DLPME | Extraction: 30 min Centrifugation: 15 min | Simple | 85.1–100 | 50–60 | [67] |
| Triclosan and triclocarban | Wastewater and tap water | IL-DLPME | Extraction: short time Centrifugation: 10 min | Simple | 70.0–103.5 | 40–580 | [68] |
| Compounds | Matrix | Extraction Technique | Optimal Times | Handling | Recovery Accuracy (%) | LOD (ng·L−1) | Ref. |
|---|---|---|---|---|---|---|---|
| UV filters (2,2-dihydroxy-4-methoxybenzophenone, benzophenone-3, octocrylene, and octyldimethyl- p-aminobenzoic acid) | River water and wastewater | SBSE | Extraction: 180 min Desorption: 15 min | Practical | 25–89 | 5–10 | [64] |
| Benzotriazole UV stabilizers (UV P, UV 329, UV 326, UV 328, UV 327, UV 571, UV 360) | Seawater and wastewater | SBSE | Extraction: 120 min Desorption: 20 min | Practical | 68.4–92.2 | 18.4–55.1 | [71] |
| Personal care products (benzotriazole, 2,4-dihydroxybenzophenona, benzylparaben, 2,4-dihydroxy-4-methoxybenzophenone, benzophenone-3) | Wastewater | SBSE | Extraction: 240 min Desorption: 15 min (60 min for PA) | Optimal times depend on coatings | <1–80 | 5.0–10.0 | [72] |
| BPA, APs | Seawater | DLLME | Extraction: 5 min Centrifugation: 3 min | Without any dispersant agent simplifies the process | 84–104 | 5–30 (LOQ) | [74] |
| APs | Wastewater | HF-LPME | Extraction: 30 min | 97–109 | 100 (LOQ) | [75] | |
| PFOS and PFOA | Surface water and wastewater | IT-SPME | 25 min | Lower handling 40 samples/day | 81.1–85.4 | 1.5–3.2 | [80] |
| PFOS and PFOA | River water | SPME | Extraction: 60 min Desorption: 15 min | 88–120 | 2.5–7.5 | [81] | |
| PFOS | Tap, river and well water | VALLME | Extraction: 2 min Centrifugation: 2 min | Not require the use of certain sample preparation apparatus | 90.8–105.1 | 1.6 | [82] |
| Compounds | Matrix | Extraction Technique | Optimal Times | Handling | Recovery Accuracy (%) | LOD (ng·L−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Estrogens (estrone, 17β-estradiol, estriol, ethynil estradiol, diethylstilbestrol) | Wastewater, river water | IT-SPME | 20 draw/eject cycle 30 min | Lower handling 48 samples/day | 86.1–106.8 | 2.7–11.7 | [85] |
| Sulfonamide antibiotics (sulfaguanidine, sulfacetamide, sulfadiazine, sulfathiazine, sulfapyridine, sulfamerazine, sulfamethazine, sulfamethoxazole, sulfadimethoxine, sulfasalazine) | Wastewater | SPME | Extraction: 20 min Desorption: 30 min | Easy to use | 29–229 | 9000–55300 | [88] |
| Antibiotics (sulfamethazine, sulfisoxazole, sulfamethoxazole, sulfadimethoxine, sulfapyridine, trimethoprim, roxithromycin, erythromycin, clarithromycin) | Wastewater | SPME | Extraction: 30 min Desorption: 10 min | Easy to use | – | 2.8–410.0 | [89] |
| Analgesic and anti-inflammatory, antidepressant, antibiotics, lipid regulator, β-blockers, diuretics, ansiolitics, antiepileptic, antipsychotic | Wastewater | dSPME | Extraction: 30 min Desorption: 10 min | Minimizes laborious and complicated sample preparation procedures | 89.2–109.7 | 5.0–50.0 (LOQ) | [90] |
| Pharmaceuticals (carbamazepine) | Wastewater | TFME | - | 96 well-plate | – | – | [91] |
| Fluoroquinolones (enoxacin, ofloxacin, ciprofloxacin, norfloxacin, lomefloxacin) | Surface water and wastewater | IT-SPME | 20 draw/eject cycles 30 min | Lower handling 48 samples/day | 81.8–98 | 7.0–29.0 | [92] |
| Non-steroidal anti-inflammatory drugs (acetaminophen, ibuprofen, naproxen, fenoprofn, flurbiprofen, loxoprofen, ketoprofen, mefenamic acid, flufenamic acid, diclofenac, tolfenamic acid, oxaprozin, phenylbutazone, indomethacin, acemetacin) | Surface water and wastewater | IT-SPME | 20 draw/eject cycles 30 min | Lower handling 48 samples/day | 80.4–100.4 | 5.0–65.0 | [93] |
| Pharmaceuticals (paracetamol, naproxen, diclofenac, caffeine, antipyrine, propanolol, carbamazepine) | River water and wastewater | SBSE | Extraction: 240 min Desorption: 20 min | Practical | 10–92 | 10.0–50.0 | [94] |
| Pharmaceuticals (paracetamol, caffeine, antipyrine, propranolol, carbamazepine, ibuprofen, diclofenac) | River water and wastewater | SBSE | Extraction: 240 min Desorption: 15 min | Practical | 9–110 | 10–50 | [95] |
| Pharmaceuticals (paracetamol, caffeine, antipyrine, propranolol hydrochloride, pridinol methanesulfonate, carbamazepine, diclofenac) | Wastewater | SBSE | Extraction: 60 min Desorption 10 min | Better than commercial coatings | 1–50 | 15–50 | [96] |
| Statin drugs (atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, simvastatin) | Pure water, wastewater and river water | DLLME | Centrifugation: 10 min (two times) | Faster | 13–92 | 0.09–17.0 | [97] |
| SBSE | Extraction: 72 min Desorption: - | 0–38 | 0.08 | ||||
| Anti-inflammatory (paracetamol, ketoprofen, naproxen, ibuprofen, flufenamic acid, tolfenamic acid) β-blockers (metoprolol, bisoprolol, betaxolol) | Wastewater | US-IL-DLLME | Vortexed: 1 min Sonicated: 4 min Ice-water: 3 min Centrifugation: 8 min | Friendly | 88–111 | 0.2–60.0 | [98] |
| Antiinflammatory (diclofenac, ketoprofen, ibuprofen, naproxen) | River and tap water | DLLME | Sonicated: 1 min Centrifugation: 10 min (two times) | Simple and rapid | 71–85 | 0.1–3.0 | [99] |
| Clotrimazole | River water and wastewater | DLLME | Extraction: 1 min Centrifugation: 10 min | 67.9–99.2 | 0.20–0.21 | [100] | |
| Acidic drugs (peroxicam, ketorolac, clofibric acid, naproxen, bezafibrate, fenoprofen, ibuprofen, diclofenac, indomethacin) | Wastewater | HF-LPME | Extraction: 45 min | Poor precision-manual operation | 80–111 | 0.15–12.6 | [101] |
| Antidepressant (amitriptyline, clomipramine, doxepin, mianserine, nortriptyline) | Wastewater | HF-LPME | Extraction: 120 min | Relatively simple | 33–49 | 0.005–0.030 | [102] |
| Antibiotic residue (erythromycin, spiramycin, tilmicosin, sulfathiazole, sulfamethazine, sulfamerazine, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, enrofloxacin) | River water | HF-LPME | Extraction: 60 min | Simple | 79.2–118 | 10.0–250.0 | [103] |
5. Conclusions and Future Trends
Acronyms
| ACN | Acetonitrile |
| AMMWCNT-PDMS | Amino-modified multi-walled carbon nanotube-PDMS |
| APEOs | Alkylphenols ethoxylated |
| APs | Alkylphenols |
| BPA | Bisphenol A |
| BUVSs | Benzotriazole UV stabilizers |
| CCL | Contaminant candidate list |
| CME | Capillary microextraction |
| CNPrTEOS | Cyanopropyltriethoxysilane |
| CNTS | Carbon nanotubes |
| CW/DVB | Carbowax/divinylbenzene |
| CW/TPR | Carbowax/template resin |
| DAD | Diode array detector |
| DESI-MS | Desorption electrospray ionization mass spectrometry |
| DI-SPME | Direct inmersion solid phase microextraction |
| DLLME | Dispersive liquid-liquid microextraction |
| DLLME-SFO | DLLME based on floating organic droplet |
| DLPME | Dispersive liquid phase microextraction |
| DSDME | Directly-suspended droplet microextraction |
| dSPME | dual-SPME |
| EDCs | Endocrine disruptor compounds |
| EG | Ethyleneglycol |
| ESI | Electrospray ionization |
| EU | European Union |
| FD | Fluorescence detector |
| FDA | Food and Drug Administration |
| FQs | Fluoroquinolones |
| GC | Gas chromatography |
| HF(2)ME | Hollow-fibre-protected 2-phase microextraction |
| HF(3)ME | Hollow-fibre-protected 3-phase microextraction |
| HF-LPME | Hollow-fibre liquid phase microextraction |
| HFM-LLLME | Hollow membrane liquid-liquid-liquid microextraction |
| HF-SLPME | Hollow fibre solid-liquid phase microextraction |
| HPLC | High performance liquid chromatography |
| HS-SDME | Headspace single-drop microextraction |
| HS-SPME | Headspace solid phase microextraction |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| IL-DLLME | Ionic liquid-dispersive liquid-liquid microextraction |
| IL-DLPME | Ionic liquid dispersive liquid-phase microextraction |
| ILs | Ionic liquids |
| IT-SPME | In-tube solid phase microextraction |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| LC-MS | Liquid chromatography-mass spectrometry |
| LLE | Liquid-liquid extraction |
| LLLME | Liquid-liquid-liquid microextraction |
| LLME | Liquid-liquid microextraction |
| LODs | Limit of detections |
| LOQs | Limit of quantifications |
| LPME | Liquid-phase microextraction |
| MeOH | Methanol |
| MIPs | Moleculary-imprinted polymers |
| MISPME | Moleculary-imprinted solid phase microextraction |
| MOF | Metal-organic framework |
| MS/MS | Tandem MS |
| MS | Mass spectrometry |
| MWCNTs | Multi-wall carbon nanotubes |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PAHs | Polyciclic aromatic hydrocarbons |
| PCBs | Polychlorinated biphenyls |
| PCPs | Personal care products |
| PDMS/DVB | Polydimethylsiloxane/divinibenzene |
| PDMS | Polydimethylsiloxane |
| PEG | Polyethyleneglycol |
| PFCs | Perfluorinated compounds |
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctane sulfonate |
| PILs | Polymeric ionic liquids |
| POP | Persistent organic pollutants |
| PPCPs | Pharmaceuticals and personal care products |
| PPY | Polypyrrole |
| RDSE | Rotating disk sorptive extraction |
| SBSE | Stir-bar sorptive extraction |
| SDCME | Single-drop coacervative microextraction |
| SDME | Single-drop microextraction |
| SME | Solven microextraction |
| SPE | Solid phase extraction |
| SPME | Solid phase microextraction |
| SWCNTs | Single-wall carbon nanotubes |
| TFME | Thin-film microextraction |
| TF-SPME | Thin-film solid phase microextraction |
| TOF/MS | Time-of-flight mass spectrometry |
| UHPLC | Ultra high performance liquid chromatography |
| UHPLC-MS/MS | Ultra high performance liquid chromatography tandem mass spectrometry |
| UHPLC-MS | Ultra high performance liquid chromatography mass spectrometry |
| USEPA | US Environmental Protection Agency |
| US-IL-DLLME | Ultrasound-assisted ionic liquid dispersive liquid-liquid microextraction |
| VALLME | Vortex-Assisted liquid–liquid Microextraction |
| WFD | Water Framework Directive |
| WWTP | Wastewater treatment plant |
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Farajzadeh, M.A.; Nouri, N.; Khorram, P. Derivatization and Microextraction Methods for Determination of Organic Compounds by Gas Chromatography. TrAC Trends Anal. Chem. 2014, 55, 14–23. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2011, 83, 4614–4648. [Google Scholar] [CrossRef]
- Pawliszyn, J.; Pedersen-Bjergaard, S. Analytical Microextraction: Current Status and Future Trends. J. Chromatogr. Sci. 2006, 44, 291–307. [Google Scholar] [CrossRef]
- Quintana, J.B.; Rodríguez, I. Strategies for the Microextraction of Polar Organic Contaminants in Water Samples. Anal. Bioanal. Chem. 2006, 38, 1447–1461. [Google Scholar]
- Postigo, C.; Lopez de Alda, M.J.; Barceló, D. Analysis of Drugs of Abuse and their Human Metabolites in Water by LC-MS2: A Non-Intrusive Tool for Drug Abuse Estimation at the Community Level. TrAC Trends Anal. Chem. 2008, 27, 1053–1069. [Google Scholar]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of Risk from Potential Emerging Contaminants in UK Groundwater. Sci. Total Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef]
- Petrović, M.; Gonzalez, S.; Barceló, D. Analysis and Removal of Emerging Contaminants in Wastewater and Drinking Water. TrAC Trends Anal. Chem. 2003, 22, 685–696. [Google Scholar]
- Radjenovic, J.; Petrovic, M.; Barceló, D. Analysis of Pharmaceuticals in Wastewater and Removal using a Membrane Bioreactor. Anal. Bioanal. Chem. 2007, 387, 1365–1377. [Google Scholar] [CrossRef]
- Boleda, M.R.; Galceran, M.T.; Ventura, F. Monitoring of Opiates, Cannabinoids and their Metabolites in Wastewater, Surface Water and Finished Water in Catalonia, Spain. Water Res. 2009, 43, 1126–1136. [Google Scholar] [CrossRef]
- Bueno, M.J.M.; Gomez, M.J.; Herrera, S.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Occurrence and Persistence of Organic Emerging Contaminants and Priority Pollutants in Five Sewage Treatment Plants of Spain: Two Years Pilot Survey Monitoring. Environ. Pollut. 2012, 164, 267–273. [Google Scholar] [CrossRef]
- Hernando, M.D.; Fernández-Alba, A.R.; Tauler, R.; Barceló, D. Toxicity Assays Applied to Wastewater Treatment. Talanta 2005, 65, 358–366. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of Human Pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Water: Contaminant Candidate List 3. Available online: http://water.Epa.gov/scitech/drinkingwater/dws/ccl/ccl3.Cfm (accessed on 11 July 2014).
- Directive 2008/105/EC of the European Parliament and the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council, 24 December 2008.
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Ramos, L. Critical Overview of Selected Contemporary Sample Preparation Techniques. J. Chromatogr. A 2012, 1221, 84–98. [Google Scholar] [CrossRef]
- Chong, S.L.; Wang, D.; Hayes, J.D.; Wilhite, B.W.; Malik, A. Sol-Gel Coating Technology for the Preparation of Solid-Phase Microextraction Fibers of Enhanced Thermal Stability. Anal. Chem. 1997, 69, 3889–3898. [Google Scholar]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in Sol-Gel Microextraction Phases for Solvent-Free Sample Preparation in Analytical Chemistry. TrAC Trends Anal. Chem. 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Ibrahim, W.A.W.; Keyon, A.S.A.; Prastomo, N.; Matsuda, A. Synthesis and Characterization of Polydimethylsiloxane-Cyanopropyltriethoxysilane-Derived Hybrid Coating for Stir Bar Sorptive Extraction. J. Sol-Gel Sci. Technol. 2011, 59, 128–134. [Google Scholar]
- Augusto, F.; Carasek, E.; Silva, R.G.C.; Rivellino, S.R.; Batista, A.D.; Martendal, E. New Sorbents for Extraction and Microextraction Techniques. J. Chromatogr. A 2010, 1217, 2533–2542. [Google Scholar] [CrossRef]
- Souza Silva, E.A.; Risticevic, S.; Pawliszyn, J. Recent Trends in SPME Concerning Sorbent Materials, Configurations and in Vivo Applications. TrAC Trends Anal. Chem. 2013, 43, 24–36. [Google Scholar] [CrossRef]
- Hu, C.; Chen, B.; He, M.; Hu, B. Amino Modified Multi-Walled Carbon nanotubes/polydimethylsiloxane Coated Stir Bar Sorptive Extraction Coupled to High Performance Liquid Chromatography-Ultraviolet Detection for the Determination of Phenols in Environmental Samples. J. Chromatogr. A 2013, 1300, 165–172. [Google Scholar] [CrossRef]
- Hu, C.; He, M.; Chen, B.; Zhong, C.; Hu, B. Polydimethylsiloxane/metal-Organic Frameworks Coated Stir Bar Sorptive Extraction Coupled to High Performance Liquid Chromatography-Ultraviolet Detector for the Determination of Estrogens in Environmental Water Samples. J. Chromatogr. A 2013, 1310, 21–30. [Google Scholar] [CrossRef]
- Koster, E.H.M.; Crescenzi, C.; den Hoedt, W.; Ensing, K.; de Jong, G.J. Fibers Coated with Molecularly Imprinted Polymers for Solid-Phase Microextraction. Anal. Chem. 2001, 73, 3140–3145. [Google Scholar]
- Xu, J.; Zheng, J.; Tian, J.; Zhu, F.; Zeng, F.; Su, C.; Ouyang, G. New Materials in Solid-Phase Microextraction. TrAC Trends Anal. Chem. 2013, 47, 68–83. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, J.; Wang, Y.; Chen, X. Developments and Trends of Molecularly Imprinted Solid-Phase Microextraction. J. Chromatogr. Sci. 2013, 51, 577–586. [Google Scholar] [CrossRef]
- Tan, F.; Zhao, H.; Li, X.; Quan, X.; Chen, J.; Xiang, X.; Zhang, X. Preparation and Evaluation of Molecularly Imprinted Solid-Phase Microextraction Fibers for Selective Extraction of Bisphenol A in Complex Samples. J. Chromatogr. A 2009, 1216, 5647–5654. [Google Scholar]
- He, J.; Lv, R.; Zhan, H.; Wang, H.; Cheng, J.; Lu, K.; Wang, F. Preparation and Evaluation of Molecularly Imprinted Solid-Phase Micro-Extraction Fibers for Selective Extraction of Phthalates in an Aqueous Sample. Anal. Chim. Acta 2010, 674, 53–58. [Google Scholar] [CrossRef]
- Djozan, D.; Ebrahimi, B.; Mahkam, M.; Farajzadeh, M.A. Evaluation of a New Method for Chemical Coating of Aluminum Wire with Molecularly Imprinted Polymer Layer. Application for the Fabrication of Triazines Selective Solid-Phase Microextraction Fiber. Anal. Chim. Acta 2010, 674, 40–48. [Google Scholar] [CrossRef]
- Yu, H.; Ho, T.D.; Anderson, J.L. Ionic Liquid and Polymeric Ionic Liquid Coatings in Solid-Phase Microextraction. TrAC Trends Anal. Chem. 2013, 45, 219–232. [Google Scholar] [CrossRef]
- Kataoka, H.; Saito, K. Recent Advances in SPME Techniques in Biomedical Analysis. J. Pharm. Biomed. Anal. 2011, 54, 926–950. [Google Scholar] [CrossRef]
- Aufartová, J.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Solich, P.; Santana-Rodríguez, J.J. Optimisation of an in-Tube Solid Phase Microextraction Method Coupled with HPLC for Determination of some Oestrogens in Environmental Liquid Samples using Different Capillary Columns. Int. J. Environ. Anal. Chem. 2012, 92, 382–396. [Google Scholar] [CrossRef]
- Aufartová, J.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Nováková, L.; Solich, P.; Santana-Rodríguez, J.J. Development of a Novel in-Tube Solid Phase Microextraction Based on Micellar Desorption Followed by LC-DAD-FD for the Determination of some Endocrine Disruptor Compounds in Environmental Liquid Samples. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1654–1672. [Google Scholar] [CrossRef]
- Silva, A.R.M.; Nogueira, J.M.F. Stir-Bar-Sorptive Extraction and Liquid Desorption Combined with Large-Volume Injection Gas chromatography–mass Spectrometry for Ultra-Trace Analysis of Musk Compounds in Environmental Water Matrices. Anal. Bioanal. Chem. 2010, 396, 1853–1862. [Google Scholar] [CrossRef]
- Lancas, F.M.; Queiroz, M.E.C.; Grossi, P.; Olivares, I.R.B. Recent Developments and Applications of Stir Bar Sorptive Extraction. J. Sep. Sci. 2009, 32, 813–824. [Google Scholar] [CrossRef]
- Chaves, A.; Quieroz, M.E. Stir-Bar Sorptive Extraction for Drugs Analysis in Biological Fluids. Quim. Nova 2008, 31, 1814–1819. [Google Scholar] [CrossRef]
- David, F.; Sandra, P. Stir Bar Sorptive Extraction for Trace Analysis. J. Chromatogr. A 2007, 1152, 54–69. [Google Scholar] [CrossRef]
- Gilart, N.; Marcé, R.M.; Borrull, F.; Fontanals, N. New Coatings for Stir-Bar Sorptive Extraction of Polar Emerging Organic Contaminants. TrAC Trends Anal. Chem. 2014, 54, 11–23. [Google Scholar] [CrossRef]
- Neng, N.R.; Silva, A.R.M.; Nogueira, J.M.F. Adsorptive Micro-Extraction techniques—Novel Analytical Tools for Trace Levels of Polar Solutes in Aqueous Media. J. Chromatogr. A 2010, 1217, 7303–7310. [Google Scholar] [CrossRef]
- Neng, N.R.; Nogueira, J.M.F. Development of a Bar Adsorptive Micro-extraction–large-Volume injection–gas chromatography–mass Spectrometric Method for Pharmaceuticals and Personal Care Products in Environmental Water Matrices. Anal. Bioanal. Chem. 2012, 402, 1355–1364. [Google Scholar] [CrossRef]
- Almeida, C.; Nogueira, J.M.F. Comparison of the Selectivity of Different Sorbent Phases for Bar Adsorptive microextraction—Application to Trace Level Analysis of Fungicides in Real Matrices. J. Chromatogr. A 2012, 1265, 7–16. [Google Scholar] [CrossRef]
- Almeida, C.; Stępkowska, A.; Alegre, A.; Nogueira, J.M.F. Determination of Trace Levels of Benzophenone-Type Ultra-Violet Filters in Real Matrices by Bar Adsorptive Micro-Extraction using Selective Sorbent Phases. J. Chromatogr. A 2013, 1311, 1–10. [Google Scholar] [CrossRef]
- Mahugo-Santana, C.; Sosa-Ferrera, Z.; Torres-Padrón, M.E.; Santana-Rodríguez, J.J. Application of New Approaches to Liquid-Phase Microextraction for the Determination of Emerging Pollutants. TrAC Trends Anal. Chem. 2011, 30, 731–748. [Google Scholar] [CrossRef]
- Liu, H.; Dasgupta, P.K. Analytical Chemistry in a Drop. Solvent Extraction in a Microdrop. Anal. Chem. 1996, 68, 1817–1821. [Google Scholar] [CrossRef]
- Kokosa, J.M. Advances in Solvent-Microextraction Techniques. TrAC Trends Anal. Chem. 2013, 43, 2–13. [Google Scholar] [CrossRef]
- Williams, D.B.; George, M.J.; Meyer, R.; Marjanovic, L. Bubbles in Solvent Microextraction: The Influence of Intentionally Introduced Bubbles on Extraction Efficiency. Anal. Chem. 2011, 83, 6713–6716. [Google Scholar] [CrossRef]
- Spietelun, A.; Marcinkowski, Ł.; de la Guardia, M.; Namieśnik, J. Green Aspects, Developments and Perspectives of Liquid Phase Microextraction Techniques. Talanta 2014, 119, 34–45. [Google Scholar] [CrossRef]
- López-Jiménez, F.J.; Rubio, S.; Pérez-Bendito, D. Single-Drop Coacervative Microextraction of Organic Compounds Prior to Liquid Chromatography: Theoretical and Practical Considerations. J. Chromatogr. A 2008, 1195, 25–33. [Google Scholar] [CrossRef]
- Lin, C.; Fuh, M.; Huang, S. Application of Liquid-Liquid-Liquid Microextraction and High-Performance Liquid Chromatography for the Determination of Alkylphenols and Bisphenol-A in Water. J. Sep. Sci. 2011, 34, 428–435. [Google Scholar] [CrossRef]
- Saraji, M.; Boroujeni, M.K. Recent Developments in Dispersive liquid–liquid Microextraction. Anal. Bioanal. Chem. 2014, 406, 2027–2066. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Djozan, D.; Bakhtiyari, R.F. Use of a Capillary Tube for Collecting an Extraction Solvent Lighter than Water After Dispersive liquid–liquid Microextraction and its Application in the Determination of Parabens in Different Samples by Gas chromatography—Flame Ionization Detection. Talanta 2010, 81, 1360–1367. [Google Scholar] [CrossRef]
- Tolcha, T.; Merdassa, Y.; Megersa, N. Low-Density Extraction Solvent Based Solvent-Terminated Dispersive Liquid-Liquid Microextraction for Quantitative Determination of Ionizable Pesticides in Environmental Waters. J. Sep. Sci. 2013, 36, 1119–1127. [Google Scholar] [CrossRef]
- Sun, J.; Chen, J.; Shi, Y. Multiple Functional Ionic Liquids Based Dispersive liquid–liquid Microextraction Combined with High Performance Chromatography for the Determination of Phenolic Compounds in Water Samples. Talanta 2014, 125, 329–335. [Google Scholar] [CrossRef]
- Gure, A.; Lara, F.J.; Megersa, N.; García-Campaña, A.M.; del Olmo-Iruela, M. Hollow-Fiber Liquid-Phase Microextraction Combined with Capillary HPLC for the Selective Determination of Six Sulfonylurea Herbicides in Environmental Waters. J. Sep. Sci. 2013, 36, 3395–3401. [Google Scholar]
- Han, D.; Tang, B.; Ri Lee, Y.; Ho Row, K. Application of Ionic Liquid in Liquid Phase Microextraction Technology. J. Sep. Sci. 2012, 35, 2949–2961. [Google Scholar] [CrossRef]
- Köck-Schulmeyer, M.; Villagrasa, M.; López de Alda, M.; Céspedes-Sánchez, R.; Ventura, F.; Barceló, D. Occurrence and Behavior of Pesticides in Wastewater Treatment Plants and their Environmental Impact. Sci. Total Environ. 2013, 458–460, 466–476. [Google Scholar] [CrossRef]
- Reemtsma, T.; Alder, L.; Banasiak, U. Emerging Pesticide Metabolites in Groundwater and Surface Water as Determined by the Application of a Multimethod for 150 Pesticide Metabolites. Water Res. 2013, 47, 5535–5545. [Google Scholar] [CrossRef]
- Moreno-González, R.; Campillo, J.A.; León, V.M. Influence of an Intensive Agricultural Drainage Basin on the Seasonal Distribution of Organic Pollutants in Seawater from a Mediterranean Coastal Lagoon (Mar Menor, SE Spain). Mar. Pollut. Bull. 2013, 77, 400–411. [Google Scholar] [CrossRef]
- Ugarte, A.; Unceta, N.; Sampedro, M.C.; Goicolea, M.A.; Gomez-Caballero, A.; Barrio, R.J. Solid Phase Microextraction Coupled to Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry for the Speciation of Organotin Compounds in Water Samples. J. Anal. At. Spectrom. 2009, 24, 347–351. [Google Scholar] [CrossRef]
- Boyacı, E.; Sparham, C.; Pawliszyn, J. Thin-Film Microextraction Coupled to LC-ESI-MS/MS for Determination of Quaternary Ammonium Compounds in Water Samples. Anal. Bioanal. Chem. 2014, 406, 409–420. [Google Scholar] [CrossRef]
- Wu, J.; Tragas, C.; Lord, H.; Pawliszyn, J. Analysis of Polar Pesticides in Water and Wine Samples by Automated in-Tube Solid-Phase Microextraction Coupled with High-Performance Liquid chromatography–mass Spectrometry. J. Chromatogr. A 2002, 976, 357–367. [Google Scholar] [CrossRef]
- Masiá, A.; Moliner-Martinez, Y.; Muñoz-Ortuño, M.; Pico, Y.; Campíns-Falcó, P. Multiresidue Analysis of Organic Pollutants by in-Tube Solid Phase Microextraction Coupled to Ultra-High Performance Liquid chromatography–electrospray-Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1306, 1–11. [Google Scholar] [CrossRef]
- Giordano, A.; Fernández-Franzón, M.; Ruiz, M.J.; Font, G.; Picó, Y. Pesticide Residue Determination in Surface Waters by Stir Bar Sorptive Extraction and Liquid chromatography/tandem Mass Spectrometry. Anal. Bioanal. Chem. 2009, 393, 1733–1743. [Google Scholar] [CrossRef]
- Pedrouzo, M.; Borrull, F.; Marcé, R.M.; Pocurull, E. Stir-Bar-Sorptive Extraction and Ultra-High-Performance Liquid chromatography–tandem Mass Spectrometry for Simultaneous Analysis of UV Filters and Antimicrobial Agents in Water Samples. Anal. Bioanal. Chem. 2010, 397, 2833–2839. [Google Scholar] [CrossRef]
- Caldas, S.S.; Costa, F.P.; Primel, E.G. Validation of Method for Determination of Different Classes of Pesticides in Aqueous Samples by Dispersive liquid–liquid Microextraction with Liquid chromatography–tandem Mass Spectrometric Detection. Anal. Chim. Acta 2010, 665, 55–62. [Google Scholar] [CrossRef]
- Zheng, C.; Zhao, J.; Bao, P.; Gao, J.; He, J. Dispersive liquid–liquid Microextraction Based on Solidification of Floating Organic Droplet Followed by High-Performance Liquid Chromatography with Ultraviolet Detection and Liquid chromatography–tandem Mass Spectrometry for the Determination of Triclosan and 2,4-Dichlorophenol in Water Samples. J. Chromatogr. A 2011, 1218, 3830–3836. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, Y. Combination of Ionic Liquid Dispersive Liquid-Phase Microextraction and High Performance Liquid Chromatography for the Determination of Triazine Herbicides in Water Samples. Chin. Chem. Lett. 2014, 25, 745–748. [Google Scholar] [CrossRef]
- Zhao, R.S.; Wang, X.; Sun, J.; Wang, S.S.; Yuan, J.P.; Wang, X.K. Trace Determination of Triclosan and Triclocarban in Environmental Water Samples with Ionic Liquid Dispersive Liquid-Phase Microextraction Prior to HPLC–ESI-MS–MS. Anal. Bioanal. Chem. 2010, 397, 1627–1633. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Vega-Morales, T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Extraction and Determination Methodologies for Benzotriazole UV Stabilizers in Personal-Care Products in Environmental and Biological Samples. TrAC Trends Anal. Chem. 2013, 51, 23–32. [Google Scholar] [CrossRef]
- Inert (other) Pesticide Ingredients in Pesticide Products – Categorized List of Inert (other) Pesticide Ingredients. Available online: http://www.Epa.gov/opprd001/inerts/oldlists.html (accessed on 11 July 2014).
- Montesdeoca-Esponda, S.; del Toro-Moreno, A.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Development of a Sensitive Determination Method for Benzotriazole UV Stabilizers in Enviromental Water Samples with Stir Bar Sorption Extraction and Liquid Desorption Prior to Ultra-High Performance Liquid Chromatography with Tandem Mass Spectrometry. J. Sep. Sci. 2013, 36, 2168–2175. [Google Scholar] [CrossRef]
- Gilart, N.; Miralles, N.; Marcé, R.M.; Borrull, F.; Fontanals, N. Novel Coatings for Stir Bar Sorptive Extraction to Determine Pharmaceuticals and Personal Care Products in Environmental Waters by Liquid Chromatography and Tandem Mass Spectrometry. Anal. Chim. Acta 2013, 774, 51–60. [Google Scholar] [CrossRef]
- Vega Morales, T.; Torres Padrón, M.E.; Sosa Ferrera, Z.; Santana Rodríguez, J.J. Determination of Alkylphenol Ethoxylates and their Degradation Products in Liquid and Solid Samples. TrAC Trends Anal. Chem. 2009, 28, 1186–1200. [Google Scholar] [CrossRef]
- Salgueiro-González, N.; Concha-Graña, E.; Turnes-Carou, I.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Determination of Alkylphenols and Bisphenol A in Seawater Samples by Dispersive liquid–liquid Microextraction and Liquid Chromatography Tandem Mass Spectrometry for Compliance with Environmental Quality Standards (Directive 2008/105/EC). J. Chromatogr. A 2012, 1223, 1–8. [Google Scholar] [CrossRef]
- Fabregat-Cabello, N.; Sancho, J.V.; Vidal, A.; González, F.V.; Roig-Navarro, A.F. Development and Validation of a Liquid Chromatography Isotope Dilution Mass Spectrometry Method for the Reliable Quantification of Alkylphenols in Environmental Water Samples by Isotope Pattern Deconvolution. J. Chromatogr. A 2014, 1328, 43–51. [Google Scholar] [CrossRef]
- Houde, M.; de Silva, A.O.; Muir, D.C.; Letcher, R.J. Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review. Environ. Sci. Technol. 2011, 45, 7962–1973. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Ventouri, E.I.; Stasinakis, A.S.; Thomaidis, N.S. Occurrence of Different Classes of Perfluorinated Compounds in Greek Wastewater Treatment Plants and Determination of their solid–water Distribution Coefficients. J. Hazard. Mater. 2012, 239–240, 24–31. [Google Scholar] [CrossRef]
- Theobald, N.; Caliebe, C.; Gerwinski, W.; Hühnerfuss, H.; Lepom, P. Occurrence of Perfluorinated Organic Acids in the North and Baltic Seas. Part 1: Distribution in Sea Water. Environ. Sci. Pollut. Res. Int. 2011, 18, 1057–1069. [Google Scholar] [CrossRef]
- Voogt, P.D.; Sáez, M. Analytical Chemistry of Perfluoroalkylated Substances. TrAC Trends Anal. Chem. 2006, 25, 326–342. [Google Scholar] [CrossRef]
- Saito, K.; Uemura, E.; Ishizaki, A.; Kataoka, H. Determination of Perfluorooctanoic Acid and Perfluorooctane Sulfonate by Automated in-Tube Solid-Phase Microextraction Coupled with Liquid chromatography–mass Spectrometry. Anal. Chim. Acta 2010, 658, 141–146. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Yang, S.; Yan, Z.; Cai, Q.; Yao, S. Analysis of Perfluorooctane Sulfonate and Perfluorooctanoic Acid with a Mixed-Mode Coating-Based Solid-Phase Microextraction Fiber. Talanta 2013, 114, 11–16. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Román, I.P.; Canals, A.; Tyrovola, K.; Psillakis, E. Fast Screening of Perfluorooctane Sulfonate in Water using Vortex-Assisted liquid–liquid Microextraction Coupled to Liquid chromatography–mass Spectrometry. Anal. Chim. Acta 2011, 691, 56–61. [Google Scholar] [CrossRef]
- Sosa-Ferrera, Z.; Mahugo-Santana, C.; Santana-Rodríguez, J.J. Steroid hormones in biological and environmental samples: Extraction and determination techniques. In Steroids: Biosynthesis, Functions and Health Implications; Amada Figueiredo, C., Cação Garces, L., Eds.; Nova Science Publishers: New York, NY, USA, 2013; pp. 83–128. [Google Scholar]
- Aufartová, J.; Mahugo-Santana, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Nováková, L.; Solich, P. Determination of Steroid Hormones in Biological and Environmental Samples using Green Microextraction Techniques: An Overview. Anal. Chim. Acta 2011, 704, 33–46. [Google Scholar] [CrossRef]
- Mitani, K.; Fujioka, M.; Kataoka, H. Fully Automated Analysis of Estrogens in Environmental Waters by in-Tube Solid-Phase Microextraction Coupled with Liquid chromatography–tandem Mass Spectrometry. J. Chromatogr. A 2005, 1081, 218–224. [Google Scholar] [CrossRef]
- Vazquez-Roig, P.; Blasco, C.; Picó, Y. Advances in the Analysis of Legal and Illegal Drugs in the Aquatic Environment. TrAC Trends Anal. Chem. 2013, 50, 65–77. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Potential Ecological and Human Health Risks Associated with the Presence of Pharmaceutically Active Compounds in the Aquatic Environment. Crit. Rev. Toxicol. 2004, 34, 335–350. [Google Scholar] [CrossRef]
- Balakrishnan, V.K.; Terry, K.A.; Toito, J. Determination of Sulfonamide Antibiotics in Wastewater: A Comparison of Solid Phase Microextraction and Solid Phase Extraction Methods. J. Chromatogr. A 2006, 1131, 1–10. [Google Scholar] [CrossRef]
- McClure, E.L.; Wong, C.S. Solid Phase Microextraction of Macrolide, Trimethoprim, and Sulfonamide Antibiotics in Wastewater. J. Chromatogr. A 2007, 1169, 53–62. [Google Scholar] [CrossRef]
- Unceta, N.; Sampedro, M.C.; Bakar, N.K.A.; Gómez-Caballero, A.; Goicolea, M.A.; Barrio, R.J. Multi-Residue Analysis of Pharmaceutical Compounds in Wastewaters by Dual Solid-Phase Microextraction Coupled to Liquid Chromatography Electrospray Ionization Ion Trap Mass Spectrometry. J. Chromatogr. A 2010, 1217, 3392–3399. [Google Scholar] [CrossRef]
- Strittmatter, N.; During, R.; Takats, Z. Analysis of Wastewater Samples by Direct Combination of Thin-Film Microextraction and Desorption Electrospray Ionization Mass Spectrometry. Analyst 2012, 137, 4037–4044. [Google Scholar] [CrossRef]
- Mitani, K.; Kataoka, H. Determination of Fluoroquinolones in Environmental Waters by in-Tube Solid-Phase Microextraction Coupled with Liquid chromatography–tandem Mass Spectrometry. Anal. Chim. Acta 2006, 562, 16–22. [Google Scholar] [CrossRef]
- Ohcho, K.; Saito, K.; Kataoka, K. Automated Analysis of Non-Steroidal Anti-Inflammatory Drugs in Environmental Water by on-Line in-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry. J. Environ. Chem. 2008, 18, 511–520. [Google Scholar] [CrossRef]
- Bratkowska, D.; Fontanals, N.; Cormack, P.A.G.; Borrull, F.; Marcé, R.M. Preparation of a Polar Monolithic Stir Bar Based on Methacrylic Acid and Divinylbenzene for the Sorptive Extraction of Polar Pharmaceuticals from Complex Water Samples. J. Chromatogr. A 2012, 1225, 1–7. [Google Scholar]
- Bratkowska, D.; Marcé, R.M.; Cormack, P.A.G.; Borrull, F.; Fontanals, N. Development and Application of a Polar Coating for Stir Bar Sorptive Extraction of Emerging Pollutants from Environmental Water Samples. Anal. Chim. Acta 2011, 706, 135–142. [Google Scholar] [CrossRef]
- Gilart, N.; Cormack, P.A.G.; Marcé, R.M.; Borrull, F.; Fontanals, N. Preparation of a Polar Monolithic Coating for Stir Bar Sorptive Extraction of Emerging Contaminants from Wastewaters. J. Chromatogr. A 2013, 1295, 42–47. [Google Scholar] [CrossRef]
- Martín, J.; Buchberger, W.; Alonso, E.; Himmelsbach, M.; Aparicio, I. Comparison of Different Extraction Methods for the Determination of Statin Drugs in Wastewater and River Water by HPLC/Q-TOF-MS. Talanta 2011, 85, 607–615. [Google Scholar] [CrossRef]
- Parrilla Vázquez, M.M.; Parrilla Vázquez, P.; Martínez Galera, M.; Gil García, M.D.; Uclés, A. Ultrasound-Assisted Ionic Liquid Dispersive liquid–liquid Microextraction Coupled with Liquid Chromatography-Quadrupole-Linear Ion Trap-Mass Spectrometry for Simultaneous Analysis of Pharmaceuticals in Wastewaters. J. Chromatogr. A 2013, 1291, 19–26. [Google Scholar] [CrossRef]
- Zgoła-Grześkowiak, A. Application of DLLME to Isolation and Concentration of Non-Steroidal Anti-Inflammatory Drugs in Environmental Water Samples. Chromatographia 2010, 72, 671–678. [Google Scholar] [CrossRef]
- Zgola-Grześkowiak, A.; Grzeskowiak, T. Application of Dispersive Liquid-Liquid Microextraction Followed by HPLC-MS/MS for the Trace Determination of Clotrimazole in Environmental Water Samples. J. Sep. Sci. 2013, 36, 2514–2521. [Google Scholar] [CrossRef]
- Quintana, J.B.; Rodil, R.; Reemtsma, T. Suitability of Hollow Fibre Liquid-Phase Microextraction for the Determination of Acidic Pharmaceuticals in Wastewater by Liquid chromatography–electrospray Tandem Mass Spectrometry without Matrix Effects. J. Chromatogr. A 2004, 1061, 19–26. [Google Scholar] [CrossRef]
- Ho, T.S.; Vasskog, T.; Anderssen, T.; Jensen, E.; Rasmussen, K.E.; Pedersen-Bjergaard, S. 25,000-Fold Pre-Concentration in a Single Step with Liquid-Phase Microextraction. Anal. Chim. Acta 2007, 592, 1–8. [Google Scholar]
- Yudthavorasit, S.; Chiaochan, C.; Leepipatpiboon, N. Simultaneous Determination of Multi-ClassAntibiotic Residues in Water using Carrier-Mediated Hollow-Fiber Liquid-Phase Microextraction Coupled with Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Microchim. Acta 2011, 172, 39–49. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padrón, M.E.T.; Afonso-Olivares, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Microextraction Techniques Coupled to Liquid Chromatography with Mass Spectrometry for the Determination of Organic Micropollutants in Environmental Water Samples. Molecules 2014, 19, 10320-10349. https://doi.org/10.3390/molecules190710320
Padrón MET, Afonso-Olivares C, Sosa-Ferrera Z, Santana-Rodríguez JJ. Microextraction Techniques Coupled to Liquid Chromatography with Mass Spectrometry for the Determination of Organic Micropollutants in Environmental Water Samples. Molecules. 2014; 19(7):10320-10349. https://doi.org/10.3390/molecules190710320
Chicago/Turabian StylePadrón, Mª Esther Torres, Cristina Afonso-Olivares, Zoraida Sosa-Ferrera, and José Juan Santana-Rodríguez. 2014. "Microextraction Techniques Coupled to Liquid Chromatography with Mass Spectrometry for the Determination of Organic Micropollutants in Environmental Water Samples" Molecules 19, no. 7: 10320-10349. https://doi.org/10.3390/molecules190710320
APA StylePadrón, M. E. T., Afonso-Olivares, C., Sosa-Ferrera, Z., & Santana-Rodríguez, J. J. (2014). Microextraction Techniques Coupled to Liquid Chromatography with Mass Spectrometry for the Determination of Organic Micropollutants in Environmental Water Samples. Molecules, 19(7), 10320-10349. https://doi.org/10.3390/molecules190710320