Spirowallichiione: A Rearranged Multiflorane from Euphorbia wallichii Hook F. (Euphorbiaceae)
Abstract
:Introduction
Results and Discussion
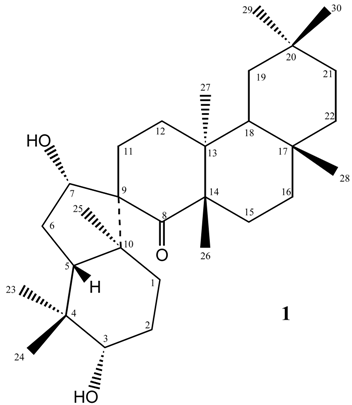
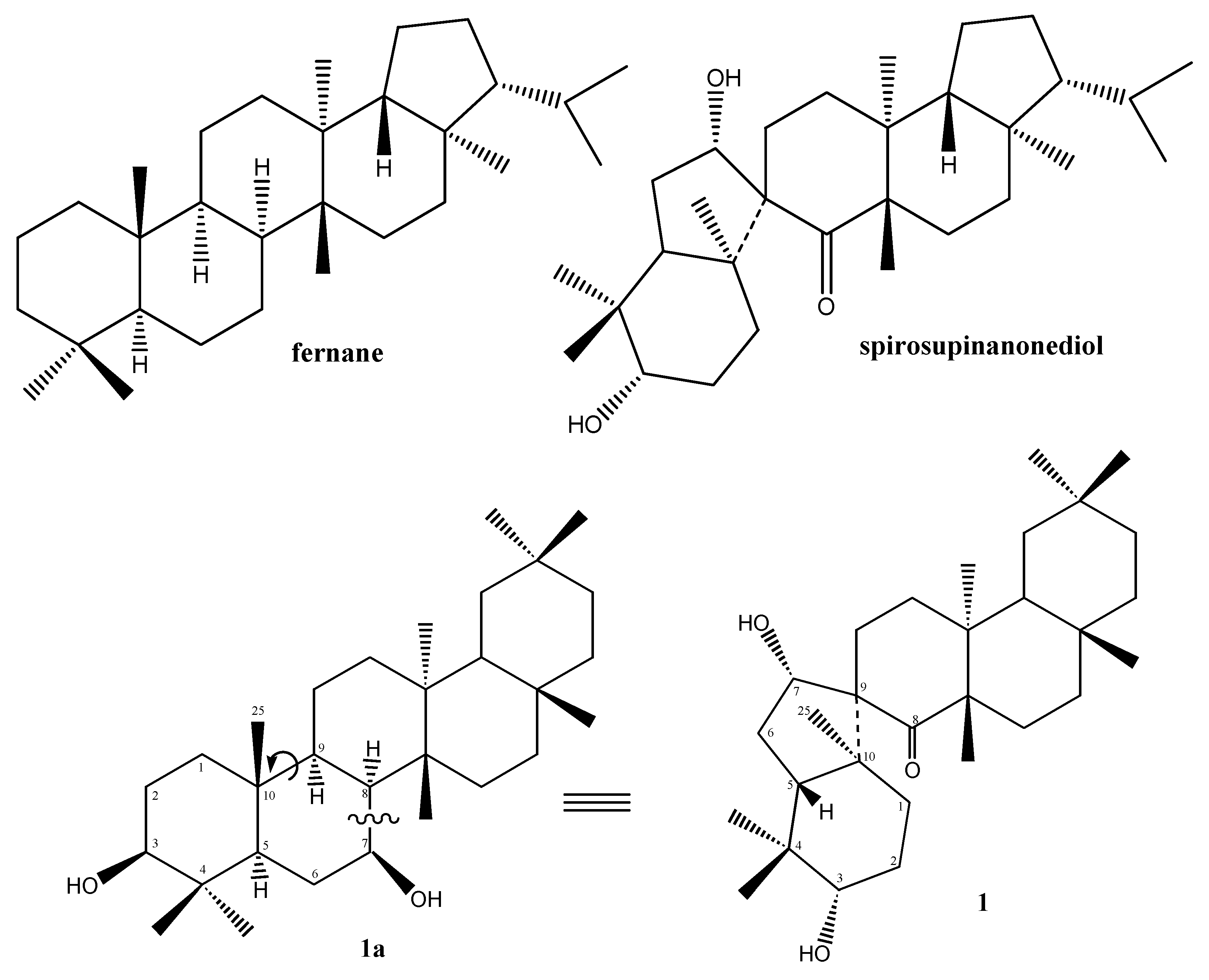
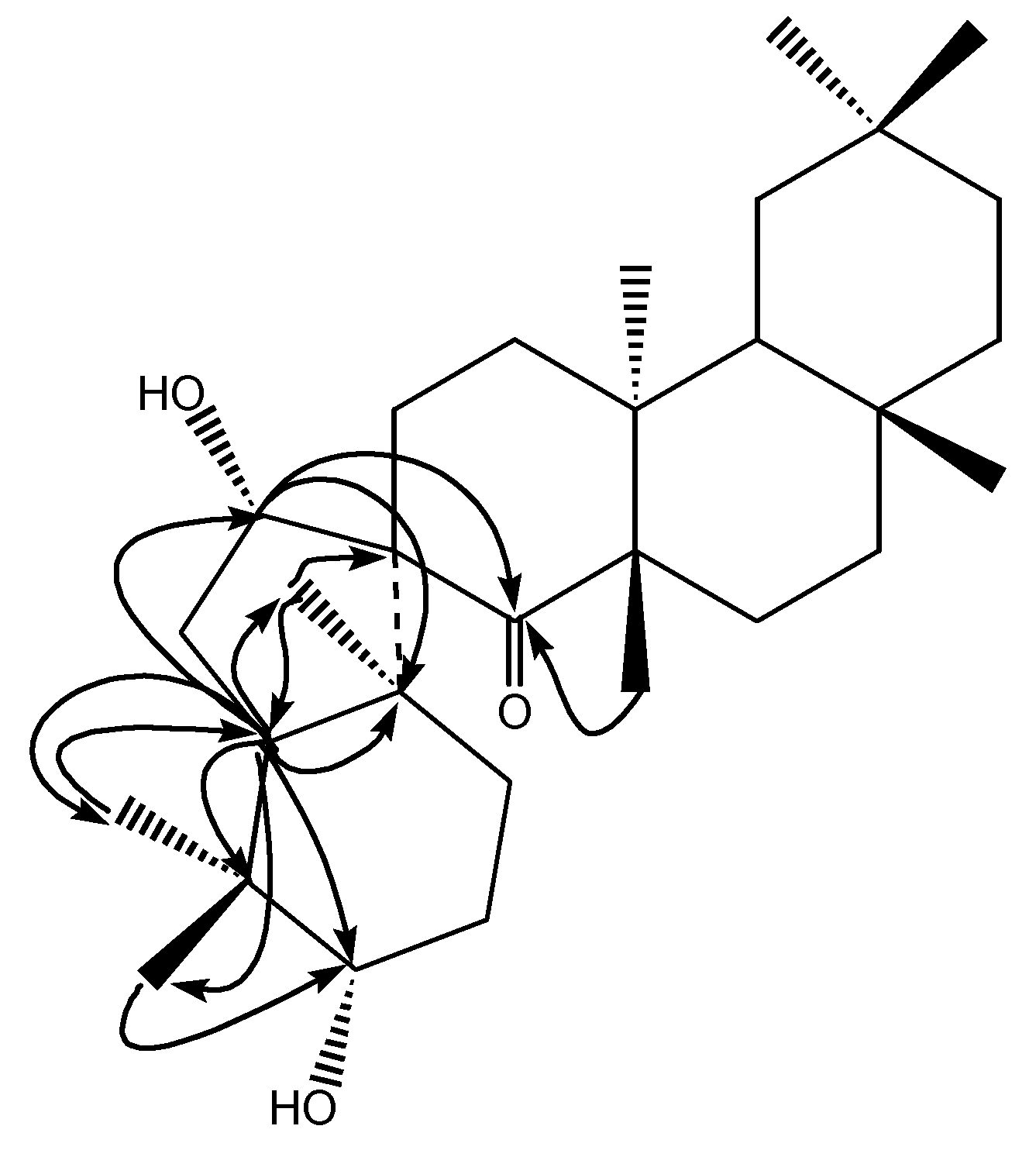
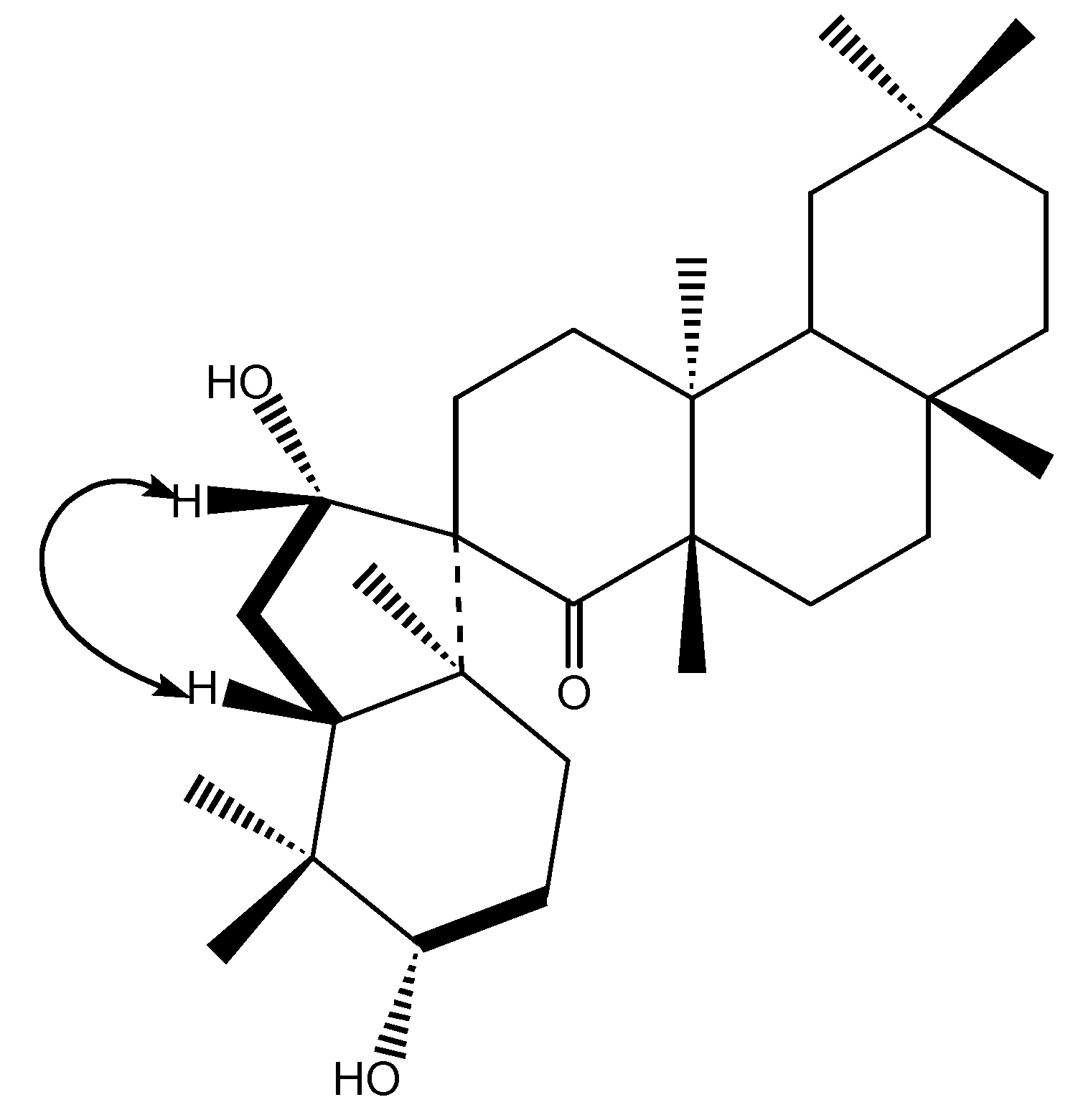
Conclusions
Experimental
General
Collection and Identification
Extraction and Isolation
References
- Smith-Kielland, I.; Dornish, J.M.; Malteruel, K.E.; Hvistendahl, G.; Romming, C.; Bockman, O.C.; Kolsaker, P.; Stenstrom, Y.; Norelal, A. Cytotoxic triterpenoids from the Leaves of Euphorbia pulcherrima. Planta Med. 1966, 62, 322–325. [Google Scholar]
- Singla, A. K.; Pathak, K. Phytoconstituents of Euphorbia species. Fitoterapia 1990, LXI, 483–516. [Google Scholar]
- Saraf, S.; Dixit, V.K. Antihepatotoxic principles of Euphorbia antisyphilitica. Indian Drugs 1994, 31, 28–31. [Google Scholar]
- Singla, A.K.; Pathak, K. Constituents of Banisteriopsis caapi. Fitoterapia 1991, LXII, 453–454. [Google Scholar]
- Wu, L. Preparation of compound ointments for psoriasis. Faming Zhuanli Shenqing Gongkai Shuomingshu 1993. [Chem. Abst. 1994, 120, 331115x]. [Google Scholar]
- Fakunle, C.O.; Connolly, J.D.; Rycroft, D.S. Enukokurin B and C, two other new jatrophane diterpenoide esters from the latex of Euphorbia laterifolia. Fitoterapia 1992, LX111, 329–332. [Google Scholar]
- Satyanarayana, V.; Krupadanam, G.L.D.; Srimannarayana, G. Tetracyclic triterpenes from the latex of Euphorbia nivulia. Fitoterapia 1992, LXII, 82–83. [Google Scholar]
- Lal, A.R.; Cambie, R.C.; Rutledge, P.S.; Woodgta, P.D. ent-Pimarane and ent-abietane diterpenes from Euphorbia fidjiana. Phytochemistry 1990, 29, 2239–2246. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Hussain, H.; Hussain, J.; Jassbi, A.R.; Bukhari, I.A.; Yasin, A.; Choudhary, M.I.; Dar, A. New bioactive diterpenoid from Euphorbia decipiens. Z. Naturforsch. 2002, 57b, 1066–1071. [Google Scholar]
- Jassbi, A.R.; Zamanizadehnajari, S.; Tahara, S. Chemical constituents of Euphorbia marschalliana Boiss. Z. Naturforsch. 2004, 59c, 15–18. [Google Scholar]
- Ahmad, V.U.; Zahid, M.; Khan, T.; Asim, M.; Ahmad, A. Chemical constituents of Euphorbia heteradenia. Proc. Pak. Acad. Sci. 2002, 39, 201–206. [Google Scholar]
- Vasas, A.; Hohmann, J.; Forgo, P.; Szabó, P. New tri- and tetracyclic diterpenes from Euphorbia villosa. Tetrahedron 2004, 60, 5025–5030. [Google Scholar] [CrossRef]
- Parvez, M.; Ahmad, V.U.; Hussain, J.; Farooq, U. 3,7,17-Tri-O-acetyl-5-O-butanoyl-13,15-dihydroxymyrisinol. Acta Crystallogr. 2004, E60, 148–150. [Google Scholar]
- Corea, G.; Fattorusso, C.; Fattorusso, E.; Lanzotti, V. Amygdaloidins A-L, twelve new 13 α-OH jatrophane diterpenes from Euphorbia amygdaloides L. Tetrahedron 2005, 61, 4485–4494. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Hussain, H.; Bukhari, I.A.; Hussain, J.; Jassbi, A.R.; Dar, A. Antinociceptive diterpene from Euphorbia decipiens. Fitoterapia 2005, 76, 230–232. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Hussain, J.; Hussain, H.; Farooq, U.; Ullah, F.; Lodhi, M.A.; Choudhary, M.I. Two new diterpene polyesters from Euphorbia decipiens. Nat. Prod. Res. 2005, 19, 267–274. [Google Scholar] [CrossRef]
- Feizbakhsh, A.; Bighdeli, M.; Tehrani, M.S.; Rustaiyan, A.; Masoudi, S. Chemical constituents of essential oil of Euphorbia teheranica Boiss., a species endemic to Iran. J. Essen. Oil Res. 2004, 16, 40–41. [Google Scholar] [CrossRef]
- Ravikanth, V.; Reddy, V.L.N.; Rao, T.P.; Diwan, P.V.; Ramakrishna, S.; Venkateswarlu, Y. Macrocyclic diterpenes from Euphorbia nivulia. Phytochemistry 2002, 59, 331–335. [Google Scholar] [CrossRef]
- Hohmann, J.; Rédei, D.; Forgo, P.; Molnár, J.; Dombi, G.; Zorig, T. Jatrophane diterpenoids from Euphorbia mangolica as modulators of the multidrug resistance of L5128 mouse lymphoma cells. J. Nat. Prod. 2003, 66, 976–979. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Cai, X.; Ma, Y.; Luo, X. Three new diterpenoids from Euphorbia wallichii. Chin. J. Chem. 2004, 22, 199–202. [Google Scholar]
- Pan, L.; Zhou, P.; Zhou, X.; Peng, S.; Ding, L.; Uiu, S. X. Skeleton-rearranged pentacyclic diterpenoids possessing a cyclobutane ring from Euphorbia wallichii. Org. Lett. 2006, 8, 2775–2778. [Google Scholar] [CrossRef]
- Matsunaga, S.; Morita, R.; Ishida, T.; Inoue, M.; Shigi, M.; Miyamae, A. The structure of spirosupinanonediol, a triterpenoid bearing a novel skeletal system from Euphorbia supine. J. Chem. Soc.Chem. Commun. 1984, 1128–1129. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the corresponding author.
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ali, M.S.; Ahmed, S.; Saleem, M. Spirowallichiione: A Rearranged Multiflorane from Euphorbia wallichii Hook F. (Euphorbiaceae). Molecules 2008, 13, 405-411. https://doi.org/10.3390/molecules13020405
Ali MS, Ahmed S, Saleem M. Spirowallichiione: A Rearranged Multiflorane from Euphorbia wallichii Hook F. (Euphorbiaceae). Molecules. 2008; 13(2):405-411. https://doi.org/10.3390/molecules13020405
Chicago/Turabian StyleAli, Muhammad Shaiq, Shakeel Ahmed, and Muhammad Saleem. 2008. "Spirowallichiione: A Rearranged Multiflorane from Euphorbia wallichii Hook F. (Euphorbiaceae)" Molecules 13, no. 2: 405-411. https://doi.org/10.3390/molecules13020405
APA StyleAli, M. S., Ahmed, S., & Saleem, M. (2008). Spirowallichiione: A Rearranged Multiflorane from Euphorbia wallichii Hook F. (Euphorbiaceae). Molecules, 13(2), 405-411. https://doi.org/10.3390/molecules13020405



