Citrus Peel Extracts for Industrial-Scale Production of Bio-Based Active Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selected Commercial Citrus Extracts and Chemicals
2.2. Spray-Drying and Encapsulation Recovery Yields
2.3. Characterization of Commercial Extracts and Spray-Dried Encapsulated Extracts
2.3.1. Moisture Content and Water Activity
2.3.2. Color Analysis
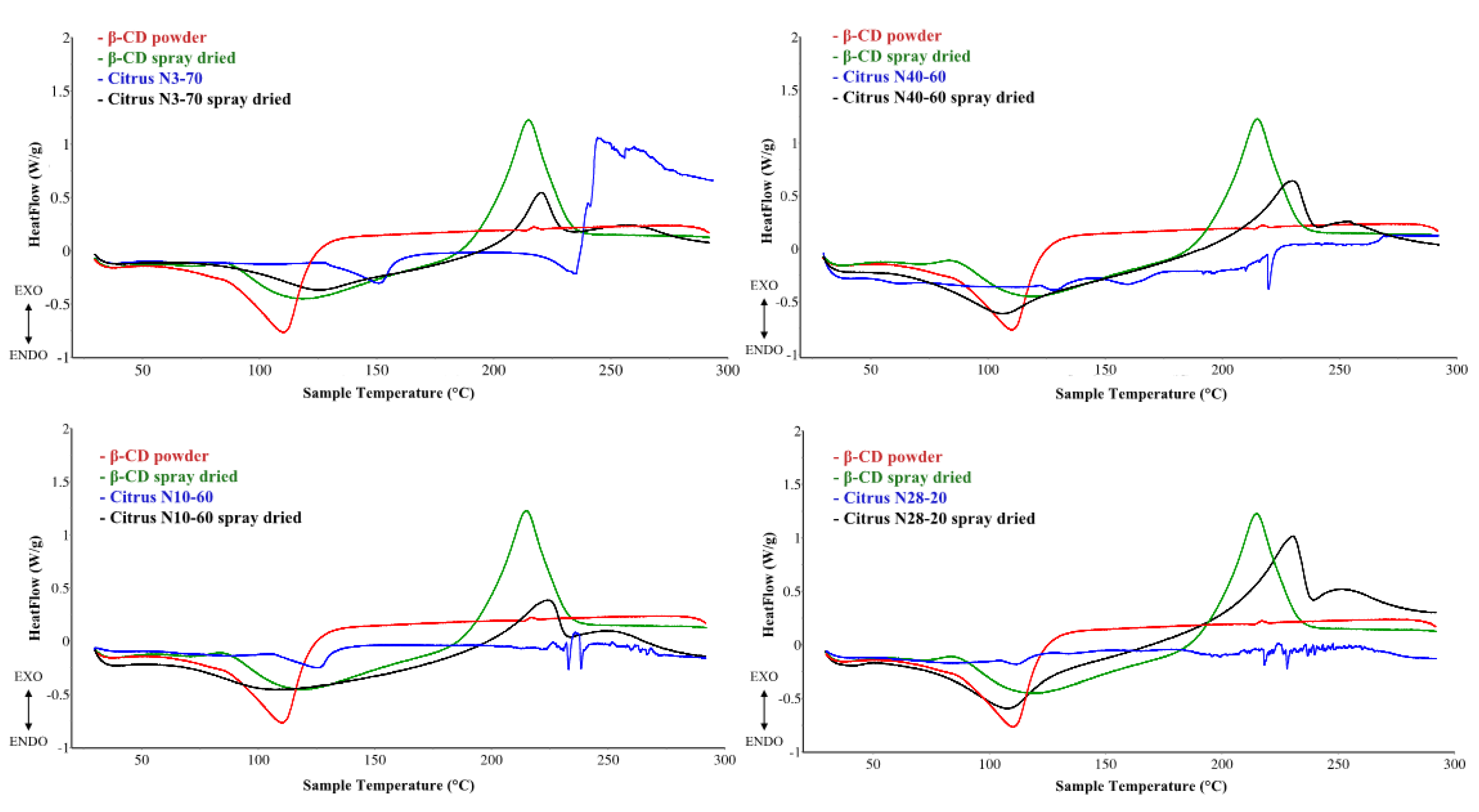
2.3.3. Differential Scanning Calorimetry
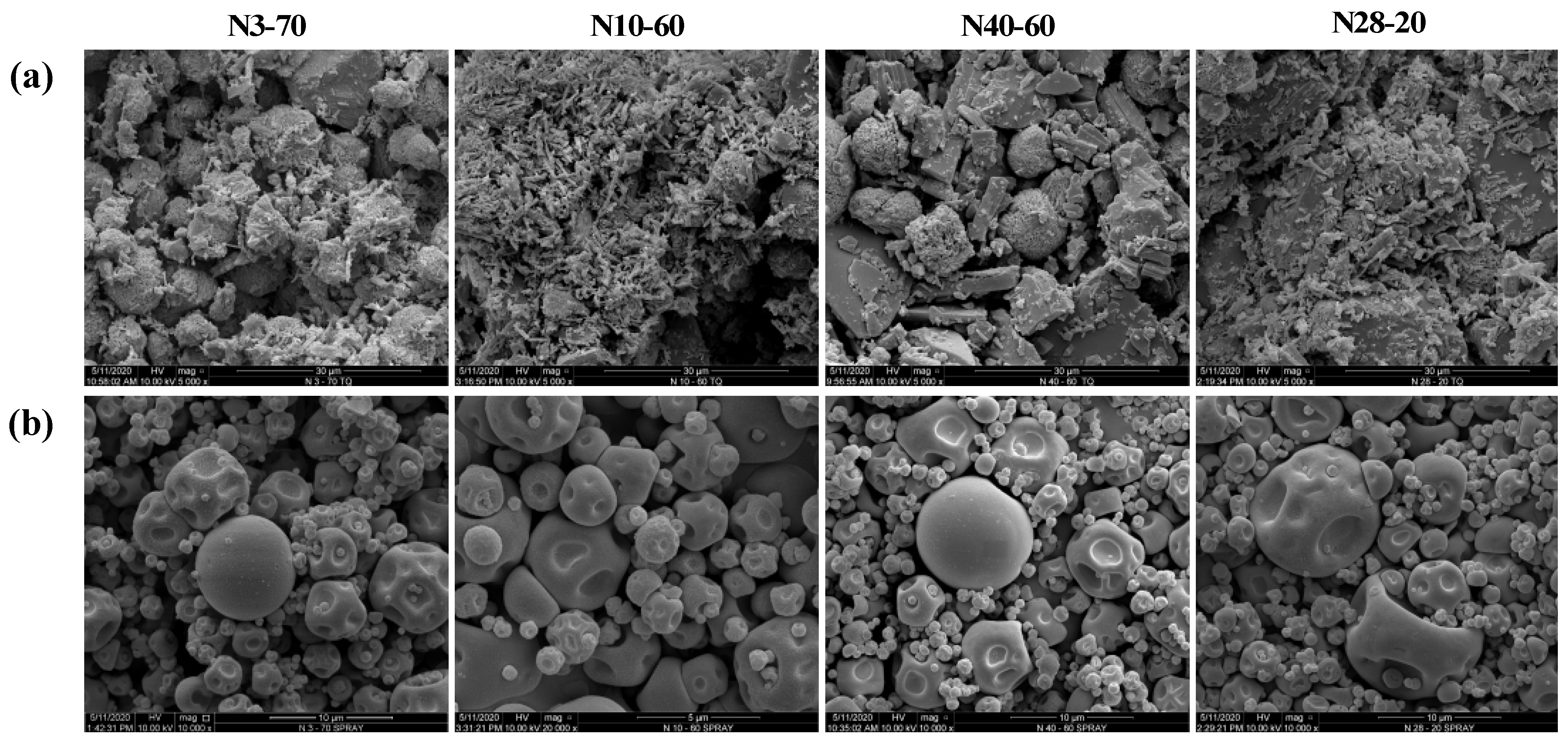
2.3.4. Particle Morphology
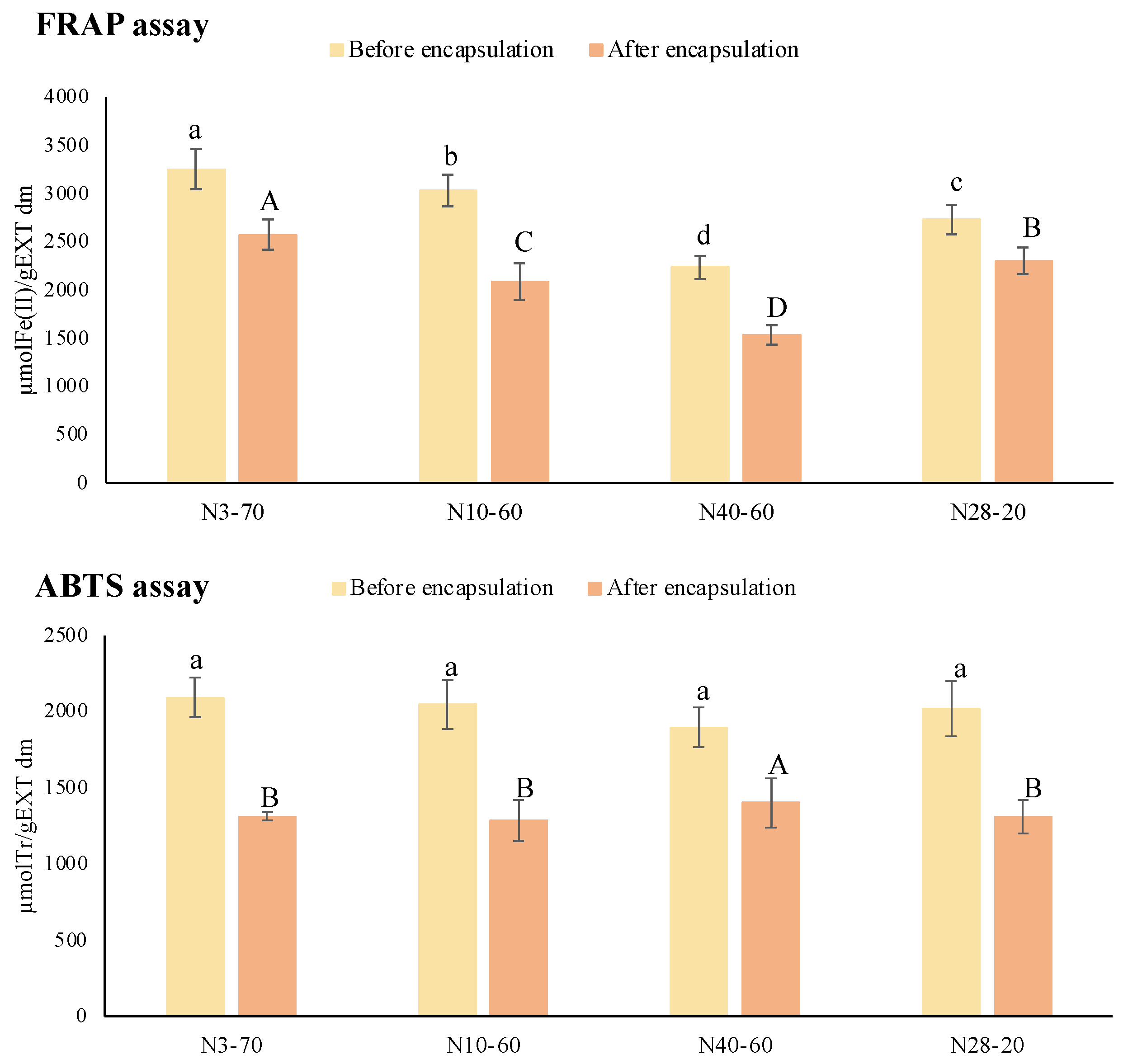
2.3.5. Total Phenolic Content and In Vitro Antioxidant Capacity (FRAP and ABTS Assays)
2.3.6. Phenolic Profiling by Ultra-High-Pressure Liquid Chromatography (UHPLC) Coupled with High-Resolution Mass Spectrometry (HRMS)
2.3.7. Antimicrobial Activity
2.4. Film Production
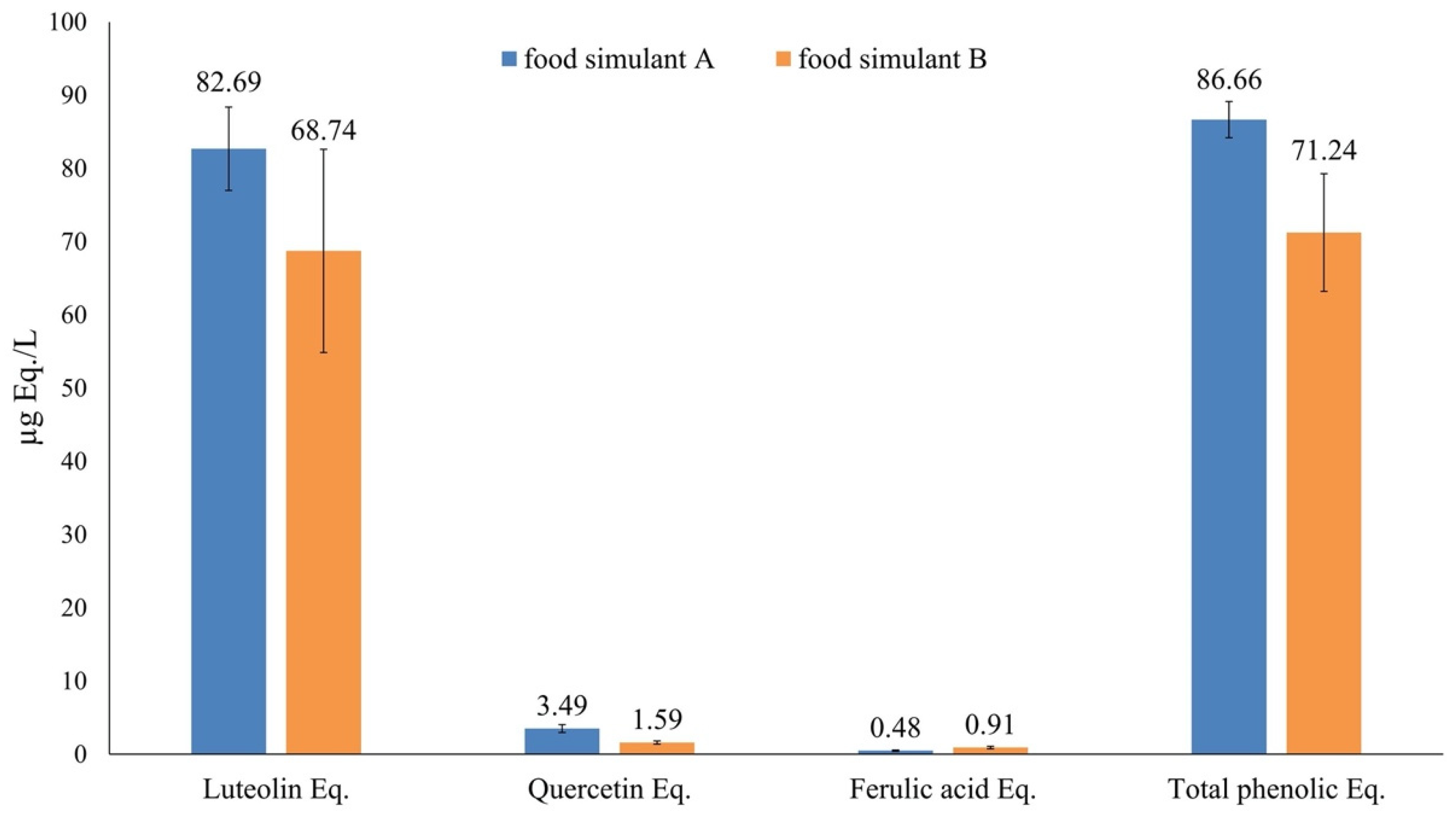
2.5. Overall Migration Test
- M: overall migration of the simulant,
- ma: mass of the residue from the specimen (reverse pouch) after evaporation of the simulant in which it was immersed (g),
- mb: mass of the simulant residue (blank) (g),
- S: surface area of the pouch intended to come into contact with food (dm2).
- M: overall migration in olive oil,
- ma: initial mass of the specimen (reverse pouch) before contact with the simulant (g),
- mb: mass of the specimen (reverse pouch) after contact with simulant (olive oil) (g),
- mc: mass of oil absorbed by the specimen (reverse pouch) (g),
- S: surface area of the specimen (reverse pouch) intended to come into contact with food (dm2).
2.6. Release of Bioactive Compounds
2.7. Antimicrobial Activity of the Film
- N: number of viable bacteria recovered per cm2 per test specimen;
- C: average plate count for the duplicate plates;
- D: dilution factor for the plate counted;
- V: volume of SCFLP added to the specimen (mL);
- S: surface area of the covered film (mm2).
- U0 = average of the common logarithm of the number of viable bacteria (cells/cm2), recovered from the untreated test specimens immediately after inoculum;
- Ut = average of the common logarithm of the number of viable bacteria (cells/cm2), recovered from the untreated test specimens after 24 h;
- At = average of the common logarithm of the number of viable bacteria (cells/cm2), recovered from the treated test specimens after 24 h.
2.8. Statistical Analysis
3. Results and Discussion
3.1. Spray-Drying Encapsulation Yields
3.2. Characterization of Commercial Extracts and Spray-Dried Encapsulated Extracts
3.2.1. Moisture Content, Water Activity, and Color
3.2.2. Differential Scanning Calorimetry
3.2.3. Particle Morphology
3.2.4. Total Phenolic Content and In Vitro Antioxidant Capacity (FRAP and ABTS Assays)
3.2.5. Antimicrobial Activity
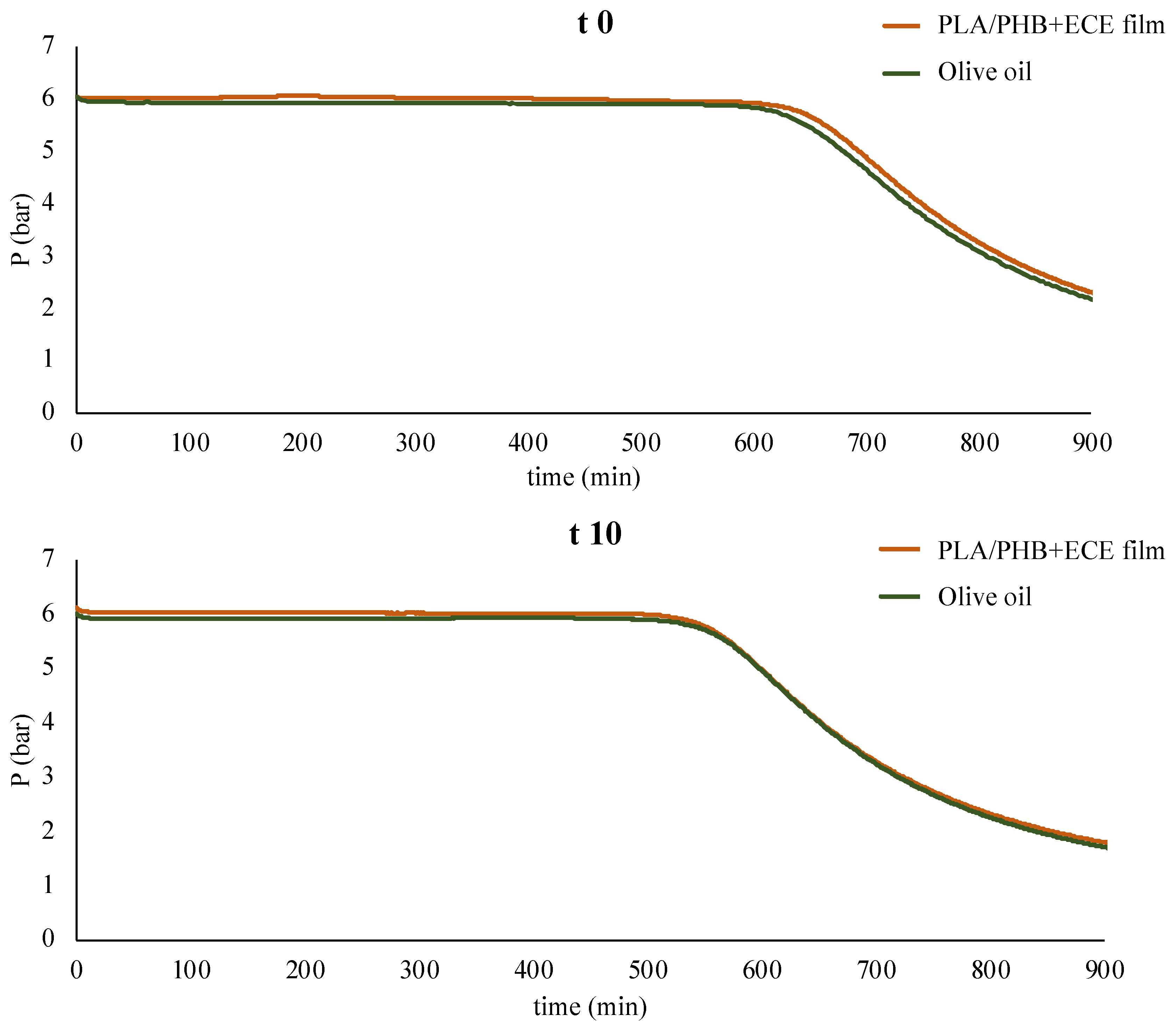
3.3. Overall Migration Test
3.4. Release of Bioactive Compounds
3.5. Antimicrobial Activity of the Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuai, L.; Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An overview of natural extracts with antioxidant activity for the improvement of the oxidative stability and shelf life of edible oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Wong, D.E.; Roman, M.J.; Lin, Z.; Goddard, J.M. Active packaging coatings. Coatings 2015, 5, 771–791. [Google Scholar] [CrossRef]
- Abdin, M.; El-Beltagy, A.E.; El-sayed, M.E.; Naeem, M.A. Production and Characterization of Sodium Alginate/Gum Arabic Based Films Enriched with Syzygium cumini Seeds Extracts for Food Application. J. Polym. Environ. 2021, 29, 4027–4039. [Google Scholar] [CrossRef]
- Damian-Reyna, A.A.; González-Hernández, J.C.; Ayala-Zavala, J.F.; Penagos, C.d.J.C.; Maya-Yescas, R.; Chávez-Parga, M.d.C. Antioxidant Capacity and Food Pathogenic Bacteria Inhibition of Citrus limetta and Citrus reticulata. In Citrus-Health Benefits and Production Technology; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Eltabakh, M.; Kassab, H.; Badawy, W.; Abdin, M.; Abdelhady, S. Active Bio-composite Sodium Alginate/Maltodextrin Packaging Films for Food Containing Azolla pinnata Leaves Extract as Natural Antioxidant. J. Polym. Environ. 2021, 1–11. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Fu, Y.; Dudley, E.G. Antimicrobial-coated films as food packaging: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3404–3437. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Castro-lo, M.; Rayo, E.; Barral-losada, L.F.; Manuel, J. Plasticized Poly(lactic acid) − Poly(hydroxybutyrate) (PLA − PHB) Blends Incorporated with Catechin Intended for Active Food-Packaging Applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef]
- Ha, J.U.; Kim, Y.M.; Lee, D.S. Multilayered antimicrobial polyethylene films applied to the packaging of ground beef. Packag. Technol. Sci. 2001, 14, 55–62. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-Drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M. Encapsulation of pomegranate peel extract with a new carrier material from orange juice by-products. J. Food Eng. 2019, 253, 1–13. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; A Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Donsì, F.; Barba, F.J.; Režek Jambrak, A. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; The Association of Official Analytical Chemist: Rockville, MD, USA, 2007. [Google Scholar]
- Shrestha, M.; Ho, T.M.; Bhandari, B.R. Encapsulation of tea tree oil by amorphous beta-cyclodextrin powder. Food Chem. 2017, 221, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Bassani, A.; Fiorentini, C.; Vadivel, V.; Moncalvo, A.; Spigno, G. Implementation of auto-hydrolysis process for the recovery of antioxidants and cellulose from wheat straw. Appl. Sci. 2020, 10, 6112. [Google Scholar] [CrossRef]
- Rocchetti, G.; Rebecchi, A.; Dallolio, M.; Braceschi, G.; Domínguez, R.; Dallolio, G.; Trevisan, M.; Lorenzo, J.M.; Lucini, L. Changes in the chemical and sensory profile of ripened Italian salami following the addition of different microbial starters. Meat Sci. 2021, 180, 108584. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- EC Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89.
- EN 1186–7: 2003. ; Materials and Articles in Contact with Foodstuffs–Plastics–Part 7: Test Methods for Overall Migration into Aqueous Food Simulants Using a Pouch. European Commission: Brussels, Belgium, 2003. Available online: http://store.uni.com/catalogo/uni-en-1186-7-2003 (accessed on 15 December 2021).
- EN 1186–6: 2003. ; Materials and Articles in Contact with Foodstuffs–Plastics–Part 6: Test Methods for Overall Migration into Olive Oil Using a Pouch. European Commission: Brussels, Belgium, 2003. Available online: http://store.uni.com/catalogo/uni-en-1186-6-2003 (accessed on 15 December 2021).
- Bassani, A.; Montes, S.; Jubete, E.; Palenzuela, J.; Sanjuán, A.P.; Spigno, G. Incorporation of waste orange peels extracts into PLA films. Chem. Eng. Trans. 2019, 74, 1063–1068. [Google Scholar] [CrossRef]
- Marcos, B.; Sárraga, C.; Castellari, M.; Kappen, F.; Schennink, G.; Arnau, J. Development of biodegradable films with antioxidant properties based on polyesters containing α-tocopherol and olive leaf extract for food packaging applications. Food Packag. Shelf Life. 2014, 1, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, N.; Pola, C.C.; Teixeira, B.N.; Hill, L.E.; Bayrak, A.; Gomes, C.L. Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: A comparative study. LWT-Food Sci. Technol. 2018, 91, 439–445. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; MacÊdo, R.O.; Medeiros, A.C.D. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Ma, S.-X.; Cheng, H.-L.; Yang, L.-J.; Chen, W.; Yin, Y.-Q.; Shi, Y.-M.; Yang, X.-D. Host-guest interaction between pinocembrin and cyclodextrins: Characterization, solubilization and stability. J. Mol. Struct. 2014, 1058, 181–188. [Google Scholar] [CrossRef]
- do Nascimento, T.G.; Redondo, G.D.P.; de Araújo Abreu, C.T.; Silva, V.C.; Lira, G.M.; Meireles Grillo, L.A.; da Conceição, M.M.; Freitas, J.D.; Souza, J.S.; Araújo Júnior, J.X.; et al. Modified release microcapsules loaded with red propolis extract obtained by spray-dryer technique: Phytochemical, thermal and physicochemical characterizations. J. Therm. Anal. Calorim. 2019, 138, 3559–3569. [Google Scholar] [CrossRef]
- Ficarra, R.; Tommasini, S.; Raneri, D.; Calabrò, M.L.; Di Bella, M.R.; Rustichelli, C.; Gamberini, M.C.; Ficarra, P. Study of flavonoids/β-cyclodextrins inclusion complexes by NMR, FT-IR, DSC, X-ray investigation. J. Pharm. Biomed. Anal. 2002, 29, 1005–1014. [Google Scholar] [CrossRef]
- Ibrahim, S.; Bowra, S. Improving Oxidative Stability of Polyphenolic Fraction of Apple Pomace by Encapsulation Using Naturally Occurring Polymers. J. Encapsul. Adsorpt. Sci. 2019, 9, 83–108. [Google Scholar] [CrossRef] [Green Version]
- Escobar-Avello, D.; Avendaño-Godoy, J.; Santos, J.; Lozano-Castellón, J.; Mardones, C.; von Baer, D.; Luengo, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Gómez-Gaete, C. Encapsulation of phenolic compounds from a grape cane pilot-plant extract in hydroxypropyl beta-cyclodextrin and maltodextrin by spray drying. Antioxidants 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Shofinita, D.; Feng, S.; Langrish, T.A.G. Comparing yields from the extraction of different citrus peels and spray drying of the extracts. Adv. Powder Technol. 2015, 26, 1633–1638. [Google Scholar] [CrossRef]
- Ramírez, M.J.; Giraldo, G.I.; Orrego, C.E. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technol. 2015, 277, 89–96. [Google Scholar] [CrossRef]
- Chanthaphon, S.; Chanthachum, S.; Hongpattarakere, T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. Against food-related microorganisms. Songklanakarin J. Sci. Technol. 2008, 30, 125–131. [Google Scholar]
- Egbuonu, A.; Osuji, C. Proximate Compositions and Antibacterial Activity of Citrus sinensis (Sweet Orange) Peel and Seed Extracts. Eur. J. Med. Plants 2016, 12, 1–7. [Google Scholar] [CrossRef]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT-Food Sci. Technol. 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Santos, E.H.; Kamimura, J.A.; Hill, L.E.; Gomes, C.L. Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT-Food Sci. Technol. 2015, 60, 583–592. [Google Scholar] [CrossRef]
- Torres-Alvarez, C.; Castillo, S.; Sánchez-García, E.; Aguilera González, C.; Galindo-Rodríguez, S.A.; Gabaldón-Hernández, J.A.; Báez-González, J.G. Inclusion Complexes of Concentrated Orange Oils and β-Cyclodextrin: Physicochemical and Biological Characterizations. Molecules 2020, 25, 5109. [Google Scholar] [CrossRef] [PubMed]
- Mlalila, N.; Hilonga, A.; Swai, H.; Devlieghere, F.; Ragaert, P. Antimicrobial packaging based on starch, poly(3-hydroxybutyrate) and poly(lactic-co-glycolide) materials and application challenges. Trends Food Sci. Technol. 2018, 74, 1–11. [Google Scholar] [CrossRef]
- Majumdar, S.; Srirangam, R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: A natural bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerin, C.; Silva, F.; Manso, S.; Becerril, R. The Downside of Antimicrobial Packaging: Migration of Packaging Elements into Food; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
| Encapsulated Extracts | Recovery Mass Yields (% dm) |
|---|---|
| N3-70 | 119.25 ± 1.36 a |
| N10-60 | 104.17 ± 1.19 c |
| N40-60 | 120.95 ± 1.27 a |
| N28-20 | 109.11 ± 1.70 b |
| Citrus Extracts before and after Encapsulation | Moisture (%) | Water Activity (aw) | Trichromatic Coordinates | H* (°) | C* | Color | ||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| N3-70 | 2.67 ± 0.02 b | 0.26 ± 0.01 c | 74.80 ± 0.89 a | 2.18 ± 0.29 c | 20.19 ± 1.40 a | 83.84 | 20.31 |  |
| N3-70 E. | 4.84 ± 0.30 CB | 0.29 ± 0.01 A | 66.94 ± 0.01 A | 15.71 ± 0.01 D | 74.02 ± 0.02 B | 78.02 | 75.67 |  |
| N10-60 | 1.18 ± 0.02 d | 0.27 ± 0.01 b | 72.80 ± 0.50 b | 3.93 ± 0.33 b | 16.64 ± 1.96 b | 76.71 | 17.10 |  |
| N10-60 E. | 4.60 ± 0.02 C | 0.21 ± 0.01 B | 63.66 ± 0.01 B | 16.95 ± 0.01 C | 71.70 ± 0.10 C | 76.70 | 73.68 |  |
| N40-60 | 4.19 ± 0.02 a | 0.40 ± 0.01 a | 70.86 ± 0.19 c | 4.08 ± 0.22 b | 17.97 ± 0.59 ab | 77.21 | 18.43 |  |
| N40-60 E. | 5.38 ± 0.02 A | 0.21 ± 0.01 B | 61.95 ± 0.01 C | 19.89 ± 0.01 A | 75.99 ± 0.03 A | 75.33 | 78.55 |  |
| N28-20 | 2.02 ± 0.03 c | 0.40 ± 0.01 a | 71.27 ± 0.12 c | 5.07 ± 0.10 a | 18.59 ± 0.19 ab | 74.74 | 19.27 |  |
| N28-20 E. | 5.22 ± 0.05 AB | 0.21 ± 0.01 B | 60.74 ± 0.01 D | 17.55 ± 0.01 B | 70.00 ± 0.01 D | 75.93 | 72.17 |  |
| CITRUS EXTRACT | BEFORE Encapsulation | AFTER Encapsulation | ||||
|---|---|---|---|---|---|---|
| mgGAE/g dm | mgHE/g dm | mgGAE/g dm | mgGAE/gEXT dm | mgHE/g dm | mgHE/gEXT dm | |
| N3-70 | 315.09 ± 19.44 a | 963.13 ± 82.89 a | 142.19 ± 8.35 A | 235.68 ± 13.84 A | 422.28 ± 37.29 A | 699.94 ± 61.80 A |
| N10-60 | 286.16 ± 17.54 b | 858.63 ± 72.10 b | 130.38 ± 7.78 A | 217.74 ± 12.99 A | 379.23 ± 34.99 AB | 633.31 ± 58.44 A |
| N40-60 | 274.36 ± 16.11 b | 809.71 ± 64.08 b | 116.19 ± 11.27 B | 194.03 ± 18.82 B | 329.60 ± 53.26 B | 550.38 ± 88.93 B |
| N28-20 | 282.19 ± 17.87 b | 844.29 ± 60.93 b | 130.30 ± 10.37 A | 217.18 ± 17.29 A | 378.66 ± 24.70 AB | 631.11 ± 41.16 AB |
| Citrus Extracts before and after Encapsulation | Listeria monocytogenes DSM 15675 | Escherichia coli ATCC 25922 | Salmonella enterica DSM 17058 | Staphylococcus aureus ATCC 33591 |
|---|---|---|---|---|
| MIC mg/mL | ||||
| N3-70 | >5 | >5 | >5 | 1.25 |
| N3-70 E. | 10 | >10 | >10 | 2.5 |
| N10-60 | >5 | >5 | >5 | 1.25 |
| N10-60 E. | 10 | 10 | 10 | 2.5 |
| N40-60 | 5 | 5 | 5 | 0.625 |
| N40-60 E. | 10 | 10 | 10 | 2.5 |
| N28-20 | >5 | 5 | 5 | 0.625 |
| N28-20 E. | 10 | 10 | 10 | 2.5 |
| Film | M (mg/dm2) | ||
|---|---|---|---|
| Simulant A | Simulant B | Simulant D2 | |
| PLA/PHB | 1.07 ± 0.20 a | 1.14 ± 0.32 a | 6.00 ± 1.11 b |
| PLA/PHB+ECE | 1.00 ± 0.13 a | 0.56 ± 0.21 a | 7.45 ± 1.12 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorentini, C.; Duserm Garrido, G.; Bassani, A.; Cortimiglia, C.; Zaccone, M.; Montalbano, L.; Martinez-Nogues, V.; Cocconcelli, P.S.; Spigno, G. Citrus Peel Extracts for Industrial-Scale Production of Bio-Based Active Food Packaging. Foods 2022, 11, 30. https://doi.org/10.3390/foods11010030
Fiorentini C, Duserm Garrido G, Bassani A, Cortimiglia C, Zaccone M, Montalbano L, Martinez-Nogues V, Cocconcelli PS, Spigno G. Citrus Peel Extracts for Industrial-Scale Production of Bio-Based Active Food Packaging. Foods. 2022; 11(1):30. https://doi.org/10.3390/foods11010030
Chicago/Turabian StyleFiorentini, Cecilia, Guillermo Duserm Garrido, Andrea Bassani, Claudia Cortimiglia, Marta Zaccone, Luana Montalbano, Vanesa Martinez-Nogues, Pier Sandro Cocconcelli, and Giorgia Spigno. 2022. "Citrus Peel Extracts for Industrial-Scale Production of Bio-Based Active Food Packaging" Foods 11, no. 1: 30. https://doi.org/10.3390/foods11010030








