Shelf-Life Prediction and Thermodynamic Properties of No Added Sugar Chocolate Spread Fortified with Multiple Micronutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Raw Materials
2.3. Preparation of Magnesium-Calcium Carbonate Nanoparticles
2.4. Preparation of Chocolate Spread Samples
2.5. Moisture and Water Activity
2.6. Storage Condition
2.7. Hydroperoxide Value
2.8. Para-Anisidine Value (p-AnV)
2.9. TOTOX Value
2.10. Study of Oxidative Stability
Thermodynamics of Lipid Oxidation
2.11. Statistical Analysis
3. Results
3.1. Oxidative Stability of Spreadable Chocolates
3.2. Kinetic Study of Spreadable Chocolates Oxidation
3.3. Shelf-Life Estimation of Chocolate Spreads
3.4. Thermodynamic Study of the Chocolate Spreads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tolve, R.; Condelli, N.; Caruso, M.C.; Barletta, D.; Favati, F.; Galgano, F. Fortification of dark chocolate with microencapsulated phytosterols: Chemical and sensory evaluation. Food Funct. 2018, 9, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Paz-Yépez, C.; Peinado, I.; Heredia, A.; Andrés, A. Lipids digestibility and polyphenols release under in vitro digestion of dark, milk and white chocolate. J. Funct. Foods 2019, 52, 196–203. [Google Scholar] [CrossRef]
- Furlán, L.T.R.; Baracco, Y.; Lecot, J.; Zaritzky, N.; Campderrós, M.E. Influence of hydrogenated oil as cocoa butter replacers in the development of sugar-free compound chocolates: Use of inulin as stabilizing agent. Food Chem. 2017, 217, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Talbot, G. Reducing saturated fat in chocolate, compound coatings and filled confectionery products. In Reducing Saturated Fats in Foods; Woodhead Publishing: Sawston, UK, 2011; pp. 318–349. [Google Scholar]
- Tolve, R.; Tchuenbou-Magaia, F.L.; Verderese, D.; Simonato, B.; Puggia, D.; Galgano, F.; Zamboni, A.; Favati, F. Physico-chemical and sensory acceptability of no added sugar chocolate spreads fortified with multiple micronutrients. Food Chem. 2021, 364, 130386. [Google Scholar] [CrossRef]
- Aidoo, R.P.; Depypere, F.; Afoakwa, E.O.; Dewettinck, K. Industrial manufacture of sugar-free chocolates—Applicability of alternative sweeteners and carbohydrate polymers as raw materials in product development. Trends Food Sci. Technol. 2013, 32, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Bootello, M.A.; Chong, P.S.; Máñez, Á.; Garcés, R.; Martínez-Force, E.; Salas, J.J. Characterization of sunflower stearin-based confectionary fats in bulk and in compound coatings. J. Am. Oil Chem. Soc. 2018, 95, 1139–1150. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of magnesium in vitamin D activation and function. J. Am. Osteop. Assoc. 2018, 118, 181. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118. [Google Scholar]
- Souza, S.V.; Borges, N.; Vieira, E.F. Vitamin d-fortified bread: Systematic review of fortification approaches and clinical studies. Food Chem. 2022, 372, 131325. [Google Scholar] [CrossRef]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Semchyshyn, H.M. Reactive carbonyl species in vivo: Generation and dual biological effects. Sci. World J. 2014, 2014, 417842. [Google Scholar] [CrossRef] [Green Version]
- Mc Clements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Santillán-Urquiza, E.; Méndez-Rojas, M.Á.; Vélez-Ruiz, J.F. Fortification of yogurt with nano and micro sized calcium, iron and zinc, effect on the physicochemical and rheological properties. LWT Food Sci. Technol. 2017, 80, 462–469. [Google Scholar] [CrossRef]
- Corradini, M.G.; Peleg, M. Shelf-life estimation from accelerated storage data. Trends Food Sci. Technol. 2007, 18, 37–47. [Google Scholar] [CrossRef]
- Manzocco, L.; Panozzo, A.; Calligaris, S. Accelerated shelf-life testing (ASLT) of oils by light and temperature exploitation. J. Am. Oil Chem. Soc. 2012, 89, 577–583. [Google Scholar] [CrossRef]
- Khan, H.; Ali, M.; Ahuja, A.; Ali, J. Stability Testing of Pharmaceutical Products–Comparison of Stability Testing Guidelines. Curr. Pharm. Anal. 2010, 6, 142–150. [Google Scholar] [CrossRef]
- Winston, P.W.; Bates, D.H. Saturated solutions for the control of humidity in biological research. Ecology 1960, 41, 232–237. [Google Scholar] [CrossRef]
- Katsuda, M.S.; McClements, D.J.; Miglioranza, L.H.S.; Decker, E.A. Physical and oxidative stability of fish oil-in-water emulsions stabilized with β-lactoglobulin and pectin. J. Agric. Food Chem. 2008, 56, 5926–5931. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; Cd 18-P-Anisidine Value; Association of Official Analytical Chemists: Washington, DC, USA, 2011. [Google Scholar]
- Shahidi, F.; Wanasundara, U.N. Methods for measuring oxidative rancidity in fats and oils. Food Lipids Chem. Nutr. Biotechnol. 2002, 17, 387–403. [Google Scholar]
- Shafiei, G.; Ghorbani, M.; Hosseini, H.; Mahoonak, A.S.; Maghsoudlou, Y.; Jafari, S.M. Estimation of oxidative indices in the raw and roasted hazelnuts by accelerated shelf-life testing. J. Food Sci. Technol. 2020, 57, 2433–2442. [Google Scholar] [CrossRef]
- Matissek, R.; Shnepel, F. Analisis de los Alimentos: Fundamentos, Mentodos y Aplicaciones; Editorial Acribia, S.A.: Zaragoza, Spain, 1998. [Google Scholar]
- Vercet, A. Browning of white chocolate during storage. Food Chem. 2003, 81, 371–377. [Google Scholar] [CrossRef]
- Samotyja, U.; Malecka, M. The influence of storage temperature on oxidative stability and shelf-life of peanuts. Pol. J. Nat. Sci. 2015, 30, 439–450. [Google Scholar]
- Lago, C.C.; Noreña, C.P.Z. Thermodynamic and kinetics study of phenolics degradation and color of yacon (Smallanthus sonchifolius) microparticles under accelerated storage conditions. J. Food Sci. Technol. 2017, 54, 4197–4204. [Google Scholar] [CrossRef] [PubMed]
- Martín-Polvillo, M.; Márquez-Ruiz, G.; Dobarganes, M.C. Oxidative stability of sunflower oils differing in un-569 saturation degree during long-term storage at room temperature. J. Am. Oil Chem. Soc. 2004, 81, 577–583. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Peroxide Value of Oils and Fats 965.33. Official METHODS of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2000. [Google Scholar]
- AOCS. Peroxide Value Acetic Acid-Isooctane Method. AOCS Official Method 2009 Cd 8b–AOCS; Association of Official Analytical Chemists: Rockville, MD, USA, 2009. [Google Scholar]
- Zhang, N.; Li, Y.; Wen, S.; Sun, Y.; Chen, J.; Gao, Y.; Sagymbek, A.; Yu, X. Analytical methods for determining the peroxide value of edible oils: A mini-review. Food Chem. 2021, 358, 129834. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.P.; He, L.; McClements, D.J.; Decker, E.A. Effects of water activity, sugars, and proteins on lipid oxidative stability of low moisture model crackers. Food Res. Int. 2020, 130, 108844. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.A.; McClements, D.J.; Decker, E.A. Challenges of utilizing healthy fats in foods. Adv. Nutr. 2015, 6, 309S–317S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Ma, Z.F.; Li, F.; Zhang, H.; Kong, L.; Yang, Z.; Xie, W. Storage quality of walnut oil containing lycopene during accelerated oxidation. J. Food Sci. Technol. 2018, 55, 1387–1395. [Google Scholar] [CrossRef]
- Barden, L.; Vollmer, D.; Johnson, D.; Decker, E. Impact of iron, chelators, and free fatty acids on lipid oxidation in low-moisture crackers. J. Agric. Food Chem. 2015, 63, 1812–1818. [Google Scholar] [CrossRef]
- Yang, X.; Boyle, R.A. Sensory evaluation of oils/fats and oil/fat–based foods. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; AOCS Press: Rockville, MD, USA, 2016; pp. 157–185. [Google Scholar]
- Bravi, E.; Sileoni, V.; Perretti, G.; Marconi, O. Accelerated shelf-life model of gluten-free rusks by using oxidation indices. Food Chem. 2020, 326, 126971. [Google Scholar] [CrossRef]
- Hosseini, H.; Ghorbani, M.; Mahoonak, A.; Maghsoudlou, Y. Monitoring hydroperoxides formation as a measure of predicting walnut oxidative stability. Acta Aliment. 2014, 43, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Sathivel, S.; Huang, J.; Prinyawiwatkul, W. Thermal properties and applications of the Arrhenius equation for evaluating viscosity and oxidation rates of unrefined pollock oil. J. Food Eng. 2008, 84, 187–193. [Google Scholar] [CrossRef]
- Piedrahita, A.M.; Peñaloza, J.; Cogollo, Á.; Rojano, B.A. Kinetic study of the oxidative degradation of choibáoil (Dipteryx oleifera Benth.) with addition of rosemary extract (Rosmarinus officinalis L.) . Food Nutr. Sci. 2015, 6, 466. [Google Scholar]
- Davrieux, F.; Allal, F.; Piombo, G.; Kelly, B.; Okulo, J.B.; Thiam, M.; Diallo, O.B.; Bouvet, J.M. Near infrared spectroscopy for high-throughput characterization of shea tree (Vitellaria paradoxa) nut fat profiles. J. Agric. Food Chem. 2010, 58, 7811–7819. [Google Scholar] [CrossRef] [PubMed]
- Roman, O.; Heyd, B.; Broyart, B.; Castillo, R.; Maillard, M.N. Oxidative reactivity of unsaturated fatty acids from sunflower, high oleic sunflower and rapeseed oils subjected to heat treatment, under controlled conditions. LWT Food Sci. Technol. 2013, 52, 49–59. [Google Scholar] [CrossRef]
- Lawrence, T.E.; Dikeman, M.E.; Hunt, M.C.; Kastner, C.L.; Johnson, D.E. Effects of calcium salts on beef longissimus quality. Meat Sci. 2003, 64, 299–308. [Google Scholar] [CrossRef]
- Elhussein, E.; Bilgin, M.; Şahin, S. Oxidative stability of sesame oil extracted from the seeds with different origins: Kinetic and thermodynamic studies under accelerated conditions. J. Food Process Eng. 2018, 41, e12878. [Google Scholar] [CrossRef]
- Tan, C.P.; Man, Y.B.C.; Selamat, J.; Yusoff, M.S.A. Application of Arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J. Am. Oil Chem. Soc. 2001, 78, 1133. [Google Scholar] [CrossRef]
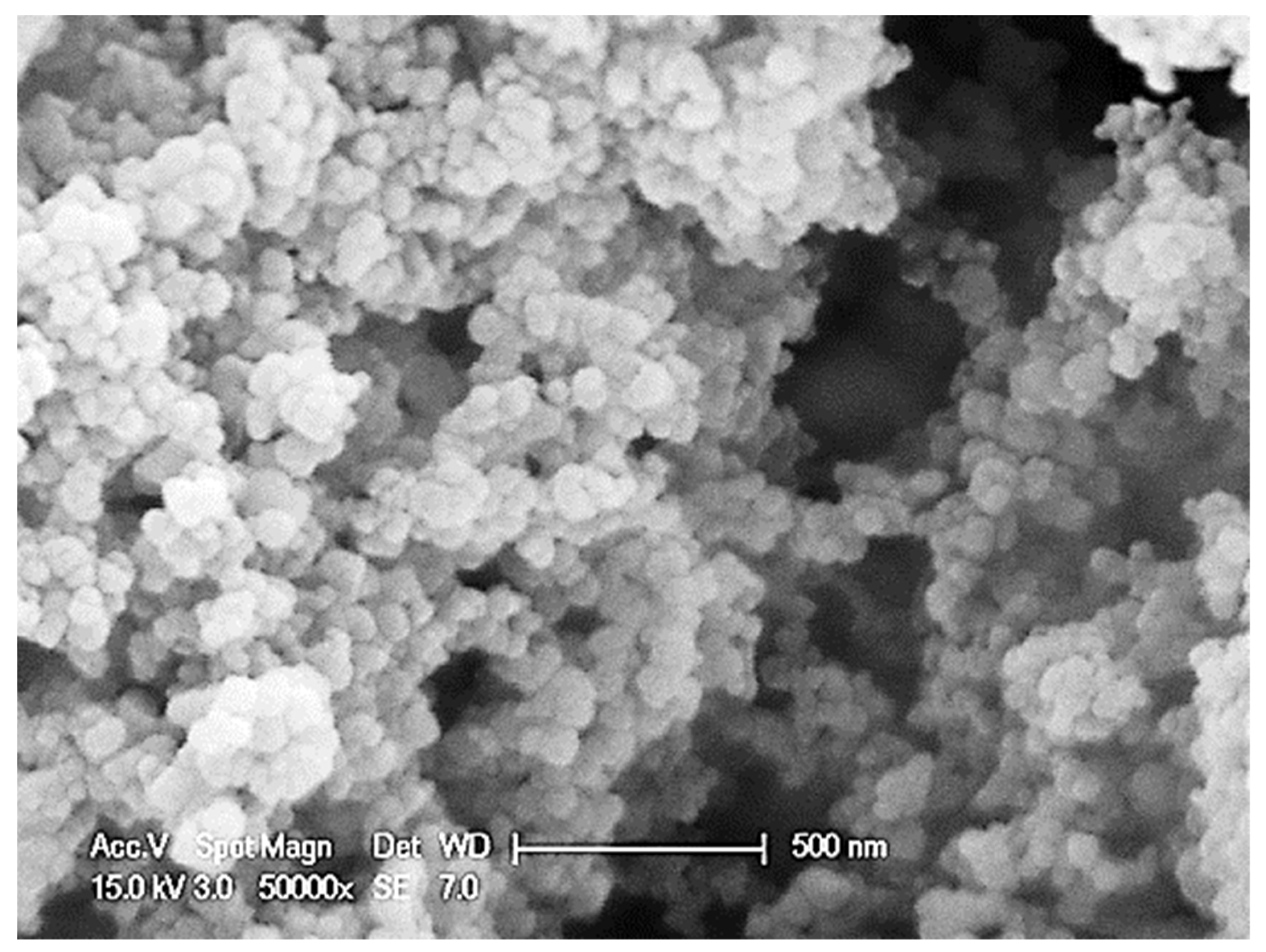
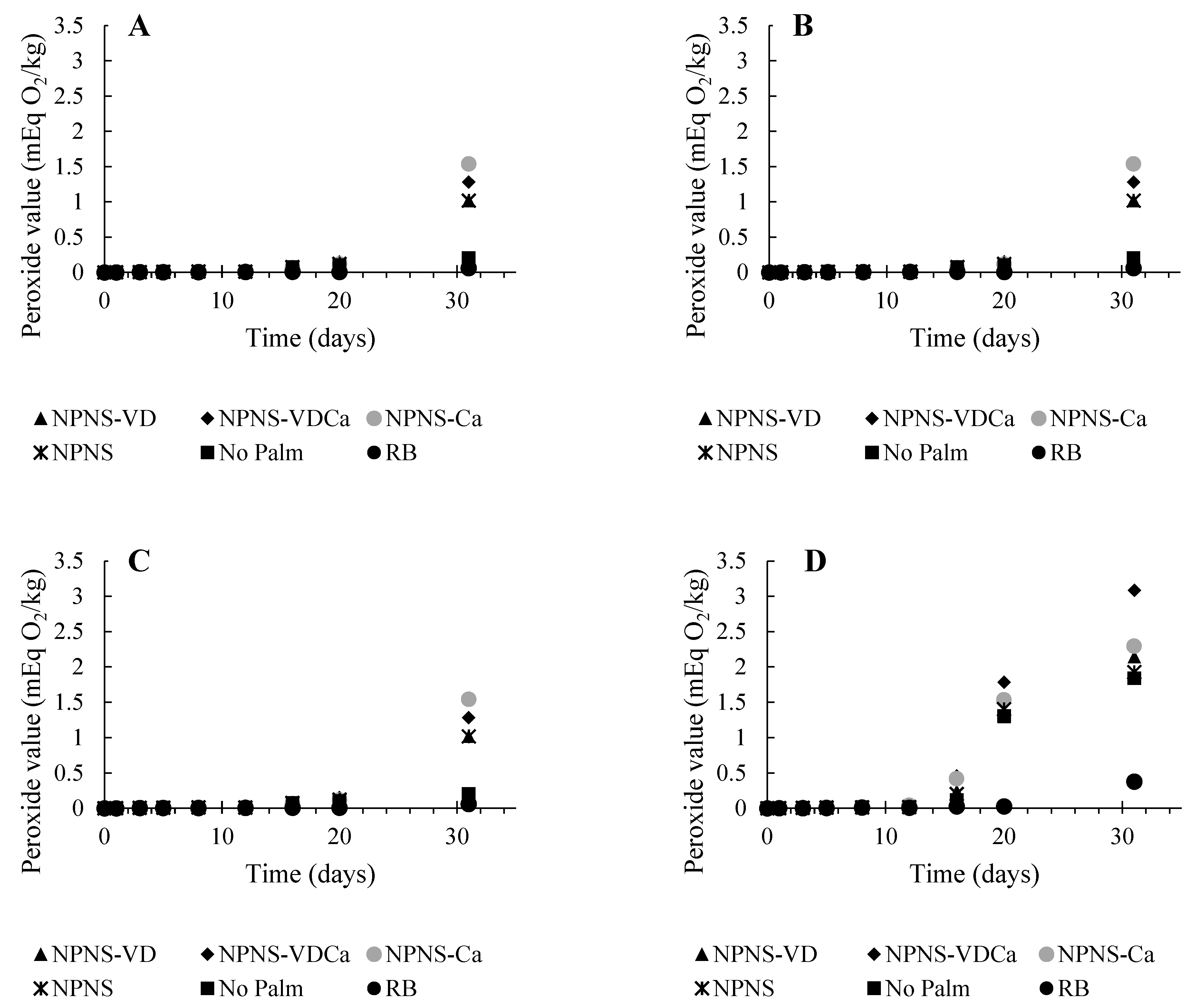
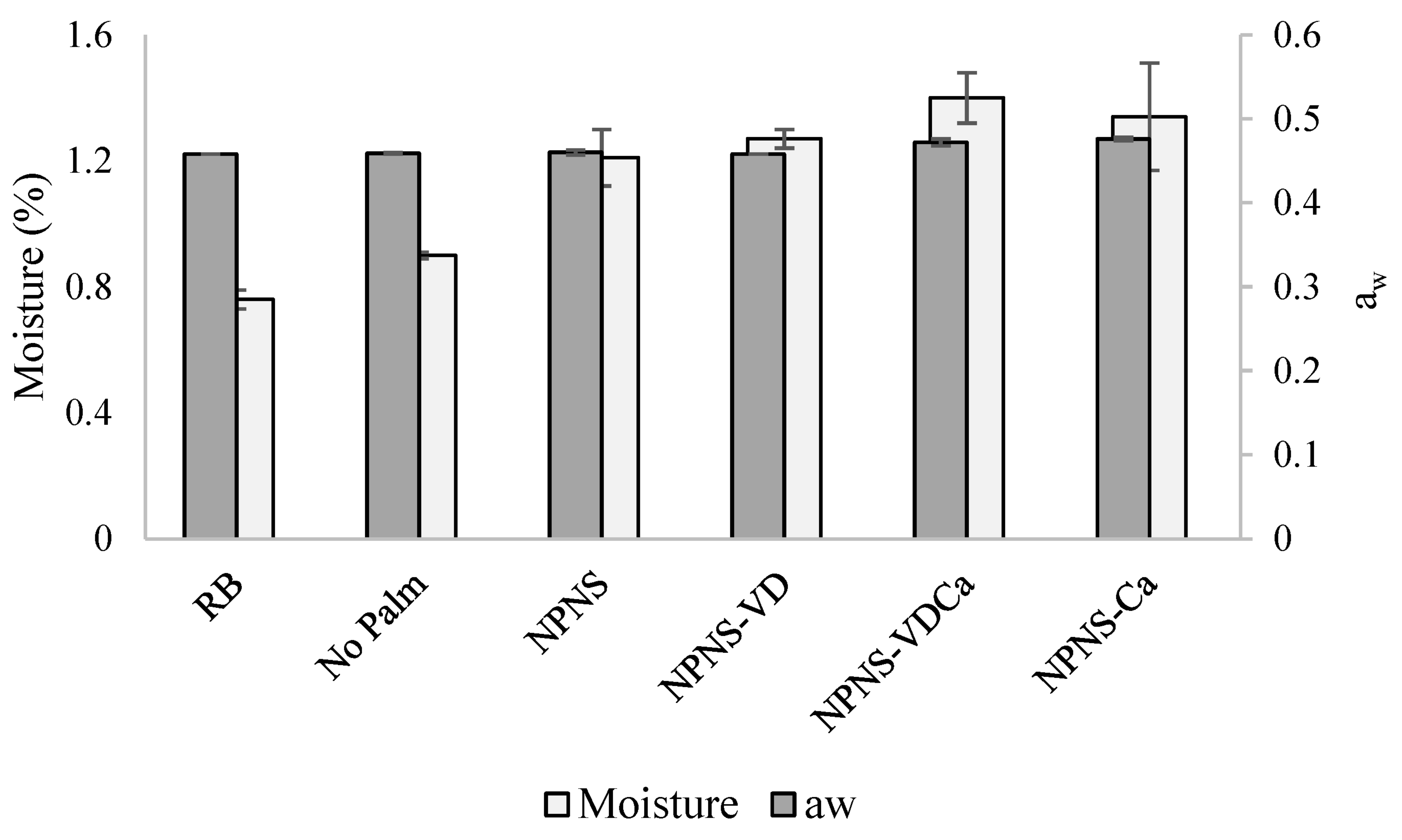
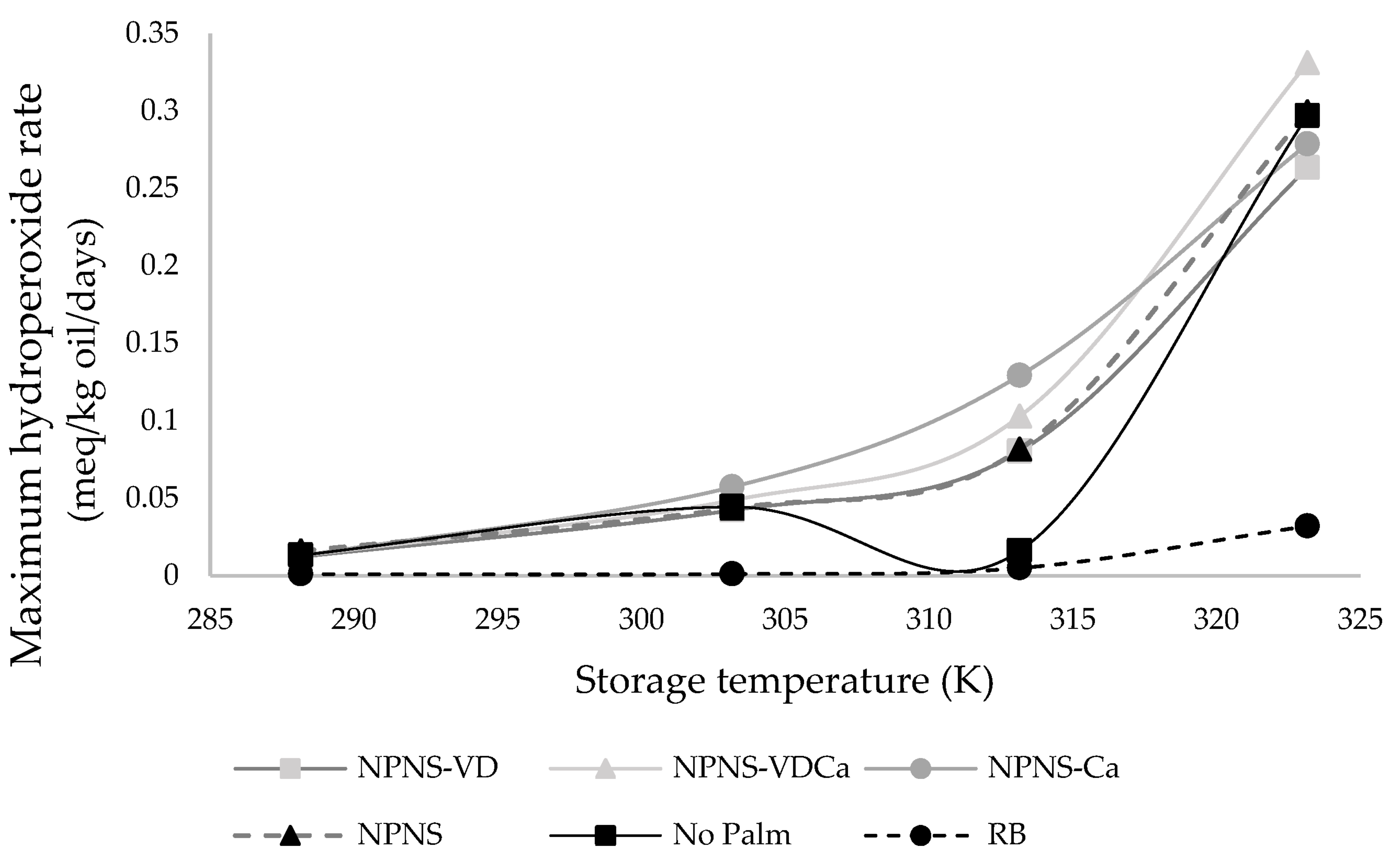
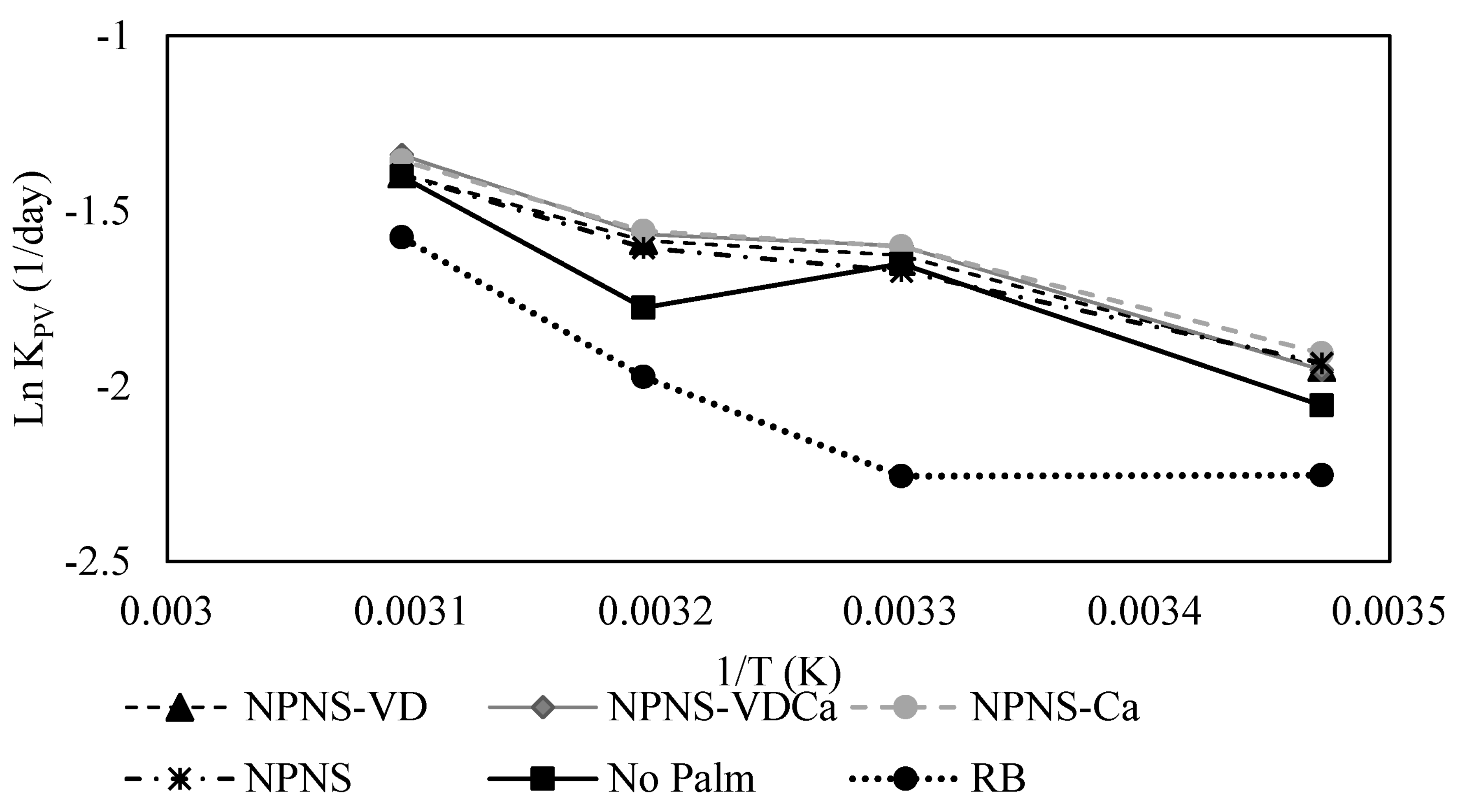
| KERRYPNX | RB | No Palm | NPNS | NPNS-VD | NPNS-VDCa | NPNS-Ca |
|---|---|---|---|---|---|---|
| Energy (KJ/100 g) | 2252 | 2223 | 2074 | 2074 | 2074 | 2074 |
| Total fat (g/100 g) | 30.90 | 30.00 | 36.00 | 36.00 | 36.00 | 36.00 |
| Saturated (g/100 g) | 10.60 | 5.20 | 6.50 | 6.50 | 6.50 | 6.50 |
| Total carbohydrate (g/100 g) | 57.50 | 58.00 | 45.00 | 45.00 | 45.00 | 45.00 |
| Sugars (g) | 56.30 | 56.00 | 13.00 | 13.00 | 13.00 | 13.00 |
| Polyols (g) | - | - | 30.00 | 30.00 | 30.00 | 30.00 |
| Fiber (g) | - | - | 9.80 | 9.80 | 9.80 | 9.80 |
| Protein (g) | 6.30 | 6.10 | 6.40 | 6.40 | 6.40 | 6.40 |
| NaCl salt (g) | 0.11 | 0.08 | 0.14 | 0.14 | 0.14 | 0.14 |
| Vitamin D (μg/100 g) | - | - | <0.50 | 15.20 | 15.10 | <0.50 |
| Calcium | 1.65 | 1.36 | 2.28 | 2.61 | 10.22 | 10.64 |
| Magnesium | 0.84 | 0.65 | 0.79 | 0.84 | 2.06 | 2.12 |
| Chocolate Cream Sample | Storage Temperature (K) | R2 | ||
|---|---|---|---|---|
| Zero Order | First Order | Second Order | ||
| NPNS-VD | 288.15 | 0.61 | 0.94 | 0.63 |
| 303.15 | 0.71 | 0.86 | 0.43 | |
| 313.15 | 0.67 | 0.95 | 0.32 | |
| 323.15 | 0.81 | 0.90 | 0.28 | |
| NPNS-VDCa | 288.15 | 0.61 | 0.93 | 0.58 |
| 303.15 | 0.71 | 0.86 | 0.43 | |
| 313.15 | 0.65 | 0.95 | 0.30 | |
| 323.15 | 0.82 | 0.89 | 0.27 | |
| NPNS-Ca | 288.15 | 0.62 | 0.94 | 0.50 |
| 303.15 | 0.69 | 0.86 | 0.32 | |
| 313.15 | 0.62 | 0.93 | 0.26 | |
| 323.15 | 0.83 | 0.90 | 0.25 | |
| NPNS | 288.15 | 0.60 | 0.92 | 0.64 |
| 303.15 | 0.71 | 0.87 | 0.38 | |
| 313.15 | 0.66 | 0.95 | 0.33 | |
| 323.15 | 0.79 | 0.91 | 0.32 | |
| No Palm | 288.15 | 0.59 | 0.82 | 0.51 |
| 303.15 | 0.71 | 0.89 | 0.37 | |
| 313.15 | 0.90 | 0.89 | 0.37 | |
| 323.15 | 0.77 | 0.91 | 0.33 | |
| RB | 288.15 | 0.75 | 0.72 | 0.43 |
| 303.15 | 0.96 | 0.66 | 0.32 | |
| 313.15 | 0.68 | 0.72 | 0.24 | |
| 323.15 | 0.64 | 0.88 | 0.24 | |
| Storage Temperature | Chocolate Cream | Linear Regression Equation | k PV (1/Day) | Temperature Effect on k PV | Formulation Effect on k PV | R2 |
|---|---|---|---|---|---|---|
| 288.15 K | NPNS-VD | y = 0.1428x − 6.9104 | 0.1428 | 0.1351 | 0.1730 | 0.94 |
| NPNS-VDCa | y = 0.1418x − 6.845 | 0.1418 | 0.2026 | 0.93 | ||
| NPNS-Ca | y = 0.1487x − 6.8993 | 0.1487 | 0.2042 | 0.94 | ||
| NPNS | y = 0.1445x − 6.8506 | 0.1445 | 0.1952 | 0.92 | ||
| No Palm | y = 0.1281x − 6.7932 | 0.1281 | 0.1838 | 0.82 | ||
| RB | y = 0.105x − 7.8284 | 0.105 | 0.1166 | 0.72 | ||
| 303.15 K | NPNS-VD | y = 0.1966x − 6.1561 | 0.1966 | 0.1808 | 0.1730 | 0.86 |
| NPNS-VDCa | y = 0.2019x − 6.1671 | 0.2019 | 0.2026 | 0.86 | ||
| NPNS-Ca | y = 0.2016x − 6.0942 | 0.2016 | 0.2042 | 0.86 | ||
| NPNS | y = 0.1879x − 5.9261 | 0.1879 | 0.1952 | 0.87 | ||
| No Palm | y = 0.1919x − 6.0538 | 0.1919 | 0.1838 | 0.89 | ||
| RB | y = 0.1047x − 7.3513 | 0.1047 | 0.1166 | 0.66 | ||
| 313.15 K | NPNS-VD | y = 0.205x − 6.1991 | 0.2050 | 0.1832 | 0.1730 | 0.95 |
| NPNS-VDCa | y = 0.2087x − 6.1843 | 0.2087 | 0.2026 | 0.95 | ||
| NPNS-Ca | y = 0.2107x − 6.1757 | 0.2107 | 0.2042 | 0.93 | ||
| NPNS | y = 0.2009x − 6.1026 | 0.2009 | 0.1952 | 0.95 | ||
| No Palm | y = 0.1693x − 6.0933 | 0.1693 | 0.1838 | 0.89 | ||
| RB | y = 0.1047x − 7.3513 | 0.1047 | 0.1166 | 0.72 | ||
| 323.15 K | NPNS-VD | y = 0.2479x − 6.0102 | 0.2479 | 0.2446 | 0.1730 | 0.90 |
| NPNS-VDCa | y = 0.2618x − 5.9565 | 0.2618 | 0.2026 | 0.89 | ||
| NPNS-Ca | y = 0.2577x − 5.9401 | 0.2577 | 0.2042 | 0.90 | ||
| NPNS | y = 0.2474x − 6.0628 | 0.2474 | 0.1952 | 0.91 | ||
| No Palm | y = 0.2461x − 6.1799 | 0.2461 | 0.1838 | 0.91 | ||
| RB | y = 0.2070x − 7.176 | 0.2070 | 0.1166 | 0.88 |
| Chocolate Cream | Arrhenius Equation | Ea (KJ/mol K) | k293.15 (1/Day) at 293.15 K | k298.15 (1/Day) at 298.15 K | Q10 | R2 | Estimated Shelf-Life at 293.15 K (Days) | Estimated Shelf-Life at 298.15 K (Days) |
|---|---|---|---|---|---|---|---|---|
| NPNS-VD | y = −1394.8x + 2.9172 | 11.5963 | 0.1583 | 0.1715 | 1.1584 | 0.96 | 59.5 | 55.0 |
| NPNS-VDCa | y = −1537.3x + 3.4057 | 12.7811 | 0.1586 | 0.1732 | 1.1760 | 0.95 | 59.4 | 54.4 |
| NPNS-Ca | y = −1386.0x + 2.9212 | 11.5232 | 0.1638 | 0.1773 | 1.1574 | 0.96 | 59.1 | 54.6 |
| NPNS | y = −1360.6x + 2.7915 | 11.3120 | 0.1569 | 0.1696 | 1.1543 | 0.97 | 59.4 | 54.9 |
| No Palm | y = −1503.4x + 3.189 | 12.4992 | 0.1434 | 0.1563 | 1.1718 | 0.79 | 65.6 | 60.2 |
| RB | y = −1742.1x + 3.6748 | 14.4838 | 0.1032 | 0.1140 | 1.2016 | 0.76 | 107.3 | 97.1 |
| Chocolate Spread | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/K mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 288.15 K | 303.15 K | 313.15 K | 323.15 K | 288.15 K | 303.15 K | 313.15 K | 323.15 K | Mean Value | |
| NPNS-VD | 75.111 | 78.346 | 80.907 | 83.066 | 9.202 | 9.077 | 8.994 | 8.911 | −229.200 + 0.555 |
| NPNS-VDCa | 75.128 | 78.279 | 80.861 | 82.920 | 10.387 | 10.262 | 10.179 | 10.096 | −225.139 + 0.612 |
| NPNS-Ca | 75.014 | 78.283 | 80.836 | 82.962 | 9.129 | 9.004 | 8.921 | 8.838 | −229.165 + 0.544 |
| NPNS | 75.083 | 78.460 | 80.960 | 83.072 | 8.918 | 8.793 | 8.710 | 8.627 | −230.244 + 0.502 |
| No Palm | 75.372 | 78.407 | 81.405 | 83.086 | 10.105 | 9.980 | 9.897 | 9.814 | −226.940 + 1.103 |
| RB | 75.848 | 79.933 | 81.918 | 83.550 | 12.089 | 11.965 | 11.882 | 11.798 | −222.901 + 1.369 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolve, R.; Tchuenbou-Magaia, F.L.; Sportiello, L.; Bianchi, F.; Radecka, I.; Favati, F. Shelf-Life Prediction and Thermodynamic Properties of No Added Sugar Chocolate Spread Fortified with Multiple Micronutrients. Foods 2022, 11, 2358. https://doi.org/10.3390/foods11152358
Tolve R, Tchuenbou-Magaia FL, Sportiello L, Bianchi F, Radecka I, Favati F. Shelf-Life Prediction and Thermodynamic Properties of No Added Sugar Chocolate Spread Fortified with Multiple Micronutrients. Foods. 2022; 11(15):2358. https://doi.org/10.3390/foods11152358
Chicago/Turabian StyleTolve, Roberta, Fideline Laure Tchuenbou-Magaia, Lucia Sportiello, Federico Bianchi, Iza Radecka, and Fabio Favati. 2022. "Shelf-Life Prediction and Thermodynamic Properties of No Added Sugar Chocolate Spread Fortified with Multiple Micronutrients" Foods 11, no. 15: 2358. https://doi.org/10.3390/foods11152358
APA StyleTolve, R., Tchuenbou-Magaia, F. L., Sportiello, L., Bianchi, F., Radecka, I., & Favati, F. (2022). Shelf-Life Prediction and Thermodynamic Properties of No Added Sugar Chocolate Spread Fortified with Multiple Micronutrients. Foods, 11(15), 2358. https://doi.org/10.3390/foods11152358










