Applications of Cannabis Sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement
Abstract
:1. Introduction
2. Potential Health Benefits of Cannabis
2.1. Cardiovascular Health
2.2. Cancer
2.3. Disorders of the Central Nervous System
2.4. Rheumatoid Arthritis
2.5. Dermatitis and Skin Disorders
2.6. Sleep Disorders and Mental Health
3. Negative Health Impacts of Cannabis
3.1. Driving and Psychomotor Consequences
3.2. Respiratory System
3.3. Reproductive Effects
4. Consequences of Increased THC Content
5. Cannabis Uses
5.1. Medicinal Uses
5.2. Cannabis Addition in Food and Its Biological Effects
6. Cannabis-Infused Food Products
6.1. Cannabis Preparation for Incorporation in Edibles
6.2. Chocolate Products
6.3. Beverages
6.4. Gluten-Free Crackers
6.5. Pasta
6.6. Cookies
6.7. Brownies
6.8. Bread
6.9. Extruded Rice
6.10. Gluten-Free Biscuits
7. Safety Remains a Concern
8. Future Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional quality and potential functionality for human health and nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- Sirikantaramas, S.; Taura, F.; Tanaka, Y.; Ishikawa, Y.; Morimoto, S.; Shoyama, Y. Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol. 2005, 46, 1578–1582. [Google Scholar] [CrossRef]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L.(hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Singh, A.; Saluja, S.; Kumar, A.; Agrawal, S.; Thind, M.; Nanda, S.; Shirani, J. Cardiovascular complications of marijuana and related substances: A review. Cardiol. Ther. 2018, 7, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Naderi, S. Cannabis and cardiovascular disease. Curr. Atheroscler. Rep. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- Callaway, J. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Baldini, M.; Ferfuia, C.; Piani, B.; Sepulcri, A.; Dorigo, G.; Zuliani, F.; Danuso, F.; Cattivello, C. The performance and potentiality of monoecious hemp (Cannabis sativa L.) cultivars as a multipurpose crop. Agronomy 2018, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef] [Green Version]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajaram, S. Health benefits of plant-derived α-linolenic acid. Am. J. Clin. Nutr. 2014, 100, 443S–448S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batalla, A.; Janssen, H.; Gangadin, S.S.; Bossong, M.G. The Potential of Cannabidiol as a Treatment for Psychosis and Addiction: Who Benefits Most? A Systematic Review. J. Clin. Med. 2019, 8, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.K.; Lee, S.J.; Kang, Y.Y.; Lee, Y.; Mok, H.; Ahn, J.-H. Biological synthesis and anti-inflammatory activity of arylalkylamine. Appl. Biol. Chem. 2017, 60, 597–602. [Google Scholar] [CrossRef]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Okwuosa, I.S.; Lewsey, S.C.; Adesiyun, T.; Blumenthal, R.S.; Yancy, C.W. Worldwide disparities in cardiovascular disease: Challenges and solutions. Int. J. Cardiol. 2016, 202, 433–440. [Google Scholar] [CrossRef]
- Howlett, A.; Barth, F.; Bonner, T.; Cabral, G.; Casellas, P.; Devane, W.; Felder, C.; Herkenham, M.; Mackie, K.; Martin, B. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Di Marzo, V. At the heart of the matter: The endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol. Sci. 2012, 33, 331–340. [Google Scholar] [CrossRef]
- Steffens, S.; Pacher, P. Targeting cannabinoid receptor CB2 in cardiovascular disorders: Promises and controversies. Br. J. Pharmacol. 2012, 167, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Waldman, M.; Hochhauser, E.; Fishbein, M.; Aravot, D.; Shainberg, A.; Sarne, Y. An ultra-low dose of tetrahydrocannabinol provides cardioprotection. Biochem. Pharmacol. 2013, 85, 1626–1633. [Google Scholar] [CrossRef]
- Karch, S.B. Cannabis and cardiotoxicity. Forensic Sci. Med. Pathol. 2006, 2, 13–18. [Google Scholar] [CrossRef]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M.T. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom Manag. 2010, 39, 167–179. [Google Scholar] [CrossRef]
- Lowin, T.; Tingting, R.; Zurmahr, J.; Classen, T.; Schneider, M.; Pongratz, G. Cannabidiol (CBD): A killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, M.; Uliel-Siboni, S.; Linder, I.; Kramer, U.; Epstein, O.; Menascu, S.; Nissenkorn, A.; Yosef, O.B.; Hyman, E.; Granot, D. CBD-enriched medical cannabis for intractable pediatric epilepsy: The current Israeli experience. Seizure 2016, 35, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Baron, E.P.; Lucas, P.; Eades, J.; Hogue, O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J. Headache Pain 2018, 19, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J. Dermatol. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef]
- Flygare, J.; Sander, B. The endocannabinoid system in cancer—Potential therapeutic target? Semin. Cancer Biol. 2008, 3, 176–189. [Google Scholar] [CrossRef]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H. Cannabidiol enhances the inhibitory effects of Δ9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, Y.; Huang, S.; Liu, G.; Xie, C.; Zhou, J.; Fan, W.; Li, Q.; Wang, Q.; Zhong, D. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2006, 171, 31–38. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Grimaldi, C.; Santoro, A.; Gazzerro, P.; Laezza, C.; Bifulco, M. Use of cannabinoid receptor agonists in cancer therapy as palliative and curative agents. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 117–131. [Google Scholar] [CrossRef]
- Bowles, D.W.; O’Bryant, C.L.; Camidge, D.R.; Jimeno, A. The intersection between cannabis and cancer in the United States. Crit. Rev. Oncol. /Hematol. 2012, 83, 1–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Neurological Disorders Affect Millions of People Worldwide, New WHO Report Shows; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Nutbrown, D.L. A Review of Research into the Health Benefits of Cannabidiol (CBD); The Neighborhood Academy: Pittsburgh, PA, USA, 2021. [Google Scholar]
- Iffland, K.; Grotenhermen, F. An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamaschi, M.; Helena Costa Queiroz, R.; Waldo Zuardi, A.; Crippa, A.S. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Lorenzetti, V.; Chye, Y.; Silva, P.; Solowij, N.; Roberts, C.A. Does regular cannabis use affect neuroanatomy? An updated systematic review and meta-analysis of structural neuroimaging studies. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 59–71. [Google Scholar] [CrossRef]
- Hammell, D.; Zhang, L.; Ma, F.; Abshire, S.; McIlwrath, S.; Stinchcomb, A.; Westlund, K. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef]
- Wei, J.; Jaleel, T.; MacLeod, A.S.; Ji, J.S. Inverted U-shaped relationship between vitamin D and ever-reported eczema in US adults. Allergy 2019, 74, 964–975. [Google Scholar] [CrossRef]
- Buxton, O.M.; Broussard, J.L.; Zahl, A.K.; Hall, M. Effects of sleep deficiency on hormones, cytokines, and metabolism. In Impact of Sleep and Sleep Disturbances on Obesity and Cancer; Springer: Berlin/Heidelberg, Germany, 2014; pp. 25–50. [Google Scholar]
- Murawski, B.; Wade, L.; Plotnikoff, R.C.; Lubans, D.R.; Duncan, M.J. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Med. Rev. 2018, 40, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of healthy sleep duration among adults—United States, 2014. Morb. Mortal. Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Hoch, E.; Niemann, D.; von Keller, R.; Schneider, M.; Friemel, C.M.; Preuss, U.W.; Hasan, A.; Pogarell, O. How effective and safe is medical cannabis as a treatment of mental disorders? A systematic review. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 87–105. [Google Scholar] [CrossRef] [Green Version]
- D'Souza, D.C.; Perry, E.; MacDougall, L.; Ammerman, Y.; Cooper, T.; Braley, G.; Gueorguieva, R.; Krystal, J.H. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 2004, 29, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.; McGlade, E.; Yurgelun-Todd, D. Safety and toxicology of cannabinoids. Neurotherapeutics 2015, 12, 735–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, A.; Livny, A.; Weizman, A. Brain imaging studies on the cognitive, pharmacological and neurobiological effects of cannabis in humans: Evidence from studies of adult users. Curr. Pharm. Des. 2016, 22, 6366–6379. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Road Safety 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Sevigny, E.L. Cannabis and driving ability. Curr. Opin. Psychol. 2021, 38, 75–79. [Google Scholar] [CrossRef]
- Schulze, H.; Schumacher, M.; Urmeew, R.; Alvarez, J.; Bernhoft, I.M.; de Gier, H.d.G.; Hagenzieker, M.; Houwing, S.; Knoche, A.; Pilgerstorfer, M. Driving under the Influence of Drugs, Alcohol and Medicines in Europe—Findings from the DRUID Project; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2012; 58p, Available online: https://orbit.dtu.dk/en/publications/driving-under-the-influence-of-drugs-alcohol-and-medicines-in-eur (accessed on 15 December 2021).
- Senna, M.-C.; Augsburger, M.; Aebi, B.; Briellmann, T.A.; Donzé, N.; Dubugnon, J.-L.; Iten, P.X.; Staub, C.; Sturm, W.; Sutter, K. First nationwide study on driving under the influence of drugs in Switzerland. Forensic Sci. Int. 2010, 198, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Asbridge, M.; Hayden, J.A.; Cartwright, J.L. Acute cannabis consumption and motor vehicle collision risk: Systematic review of observational studies and meta-analysis. BMJ 2012, 344, e536. [Google Scholar] [CrossRef] [Green Version]
- Ramaekers, J.G.; Kauert, G.; Theunissen, E.; Toennes, S.W.; Moeller, M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J. Psychopharmacol. 2009, 23, 266–277. [Google Scholar] [CrossRef]
- Chuchalin, A.G.; Khaltaev, N.; Antonov, N.S.; Galkin, D.V.; Manakov, L.G.; Antonini, P.; Murphy, M.; Solodovnikov, A.G.; Bousquet, J.; Pereira, M.H. Chronic respiratory diseases and risk factors in 12 regions of the Russian Federation. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 963. [Google Scholar] [CrossRef] [Green Version]
- Tashkin, D.P.; Baldwin, G.C.; Sarafian, T.; Dubinett, S.; Roth, M.D. Respiratory and immunologic consequences of marijuana smoking. J. Clin. Pharmacol. 2002, 42, 71S–81S. [Google Scholar] [CrossRef]
- Hancox, R.J.; Poulton, R.; Ely, M.; Welch, D.; Taylor, D.R.; McLachlan, C.R.; Greene, J.M.; Moffitt, T.E.; Caspi, A.; Sears, M.R. Effects of cannabis on lung function: A population-based cohort study. Eur. Respir. J. 2010, 35, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Tashkin, D.P.; Simmons, M.S.; Tseng, C.-H. Impact of changes in regular use of marijuana and/or tobacco on chronic bronchitis. COPD: J. Chronic Obstr. Pulm. Dis. 2012, 9, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.A.; Augustson, E.M.; Moser, R.P.; Budney, A.J. Respiratory effects of marijuana and tobacco use in a US sample. J. Gen. Intern. Med. 2005, 20, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M. Cannabis use in pregnancy and early life and its consequences: Animal models. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Leemaqz, S.Y.; Dekker, G.A.; McCowan, L.M.; Kenny, L.C.; Myers, J.E.; Simpson, N.A.; Poston, L.; Roberts, C.T.; Consortium, S. Maternal marijuana use has independent effects on risk for spontaneous preterm birth but not other common late pregnancy complications. Reprod. Toxicol. 2016, 62, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Blackard, C.; Tennes, K. Human placental transfer of cannabinoids. New Engl. J. Med. 1984, 311, 797. [Google Scholar]
- Bertrand, K.A.; Hanan, N.J.; Honerkamp-Smith, G.; Best, B.M.; Chambers, C.D. Marijuana use by breastfeeding mothers and cannabinoid concentrations in breast milk. Pediatrics 2018, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luke, S.; Hutcheon, J.; Kendall, T. Cannabis use in pregnancy in British Columbia and selected birth outcomes. J. Obstet. Gynaecol. Can. 2019, 41, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Filion, K.; Abenhaim, H.; Eisenberg, M. Prevalence and outcomes of prenatal recreational cannabis use in high-income countries: A scoping review. BJOG: Int. J. Obstet. Gynaecol. 2020, 127, 8–16. [Google Scholar] [CrossRef]
- Gunn, J.; Rosales, C.; Center, K.; Nuñez, A.; Gibson, S.; Christ, C.; Ehiri, J. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open 2016, 6, e009986. [Google Scholar] [CrossRef] [Green Version]
- Colizzi, M.; Bhattacharyya, S. Does cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Curr. Addict. Rep. 2017, 4, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Charlebois, S.; Somogyi, S.; Sterling, B. Cannabis-infused food and Canadian consumers’ willingness to consider “recreational” cannabis as a food ingredient. Trends Food Sci. Technol. 2018, 74, 112–118. [Google Scholar] [CrossRef]
- Balant, M.; Gras, A.; Gálvez, F.; Garnatje, T.; Vallès, J.; Vitales, D. CANNUSE, a database of traditional Cannabis uses—an opportunity for new research. Database 2021, 2021. [Google Scholar] [CrossRef]
- Balant, M.; Gras, A.; Ruz, M.; Vallès, J.; Vitales, D.; Garnatje, T. Traditional uses of Cannabis: An analysis of the CANNUSE database. J. Ethnopharmacol. 2021, 279, 114362. [Google Scholar] [CrossRef] [PubMed]
- Chouvy, P.-A.; Afsahi, K. Hashish revival in Morocco. Int. J. Drug Policy 2015, 25, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.X.; Rogers, M.A. Opportunities and challenges in developing orally administered cannabis edibles. Curr. Opin. Food Sci. 2019, 28, 7–13. [Google Scholar] [CrossRef]
- King, J.W. The relationship between cannabis/hemp use in foods and processing methodology. Curr. Opin. Food Sci. 2019, 28, 32–40. [Google Scholar] [CrossRef]
- Rasera, G.B.; Ohara, A.; de Castro, R.J.S. Innovative and emerging applications of cannabis in food and beverage products: From an illicit drug to a potential ingredient for health promotion. Trends Food Sci. Technol. 2021, 115, 31–41. [Google Scholar] [CrossRef]
- Marzorati, S.; Friscione, D.; Picchi, E.; Verotta, L. Cannabidiol from inflorescences of Cannabis sativa L.: Green extraction and purification processes. Ind. Crops Prod. 2020, 155, 112816. [Google Scholar] [CrossRef]
- Knutson, K. Food Safety Lessons for Cannabis-Infused Edibles; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Beal, K. Considerations in the addition of cannabis to chocolate. Curr. Opin. Food Sci. 2019, 28, 14–17. [Google Scholar] [CrossRef]
- Ramírez, A.; Viveros, J.M. Brewing with Cannabis sativa vs. Humulus lupulus: A review. J. Inst. Brew. 2021, 4, 55–67. [Google Scholar] [CrossRef]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. (CCLM) 2017, 55, 1555–1563. [Google Scholar] [CrossRef]
- Pongtanavong, A. Encapsulated cannabis oil Oolong tea formulation. journal of Biotechnology 2018, 2, 1–35. [Google Scholar]
- Dabija, A.; Codină, G.G.; Gâtlan, A.-M.; Sănduleac, E.T.; Rusu, L. Effects of some vegetable proteins addition on yogurt quality. Sci. Study Research. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 181–192. [Google Scholar]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis sativa subsp. sativa) flour and protein preparation as natural nutrients and structure forming agents in starch based gluten-free bread. LWT 2017, 84, 143–150. [Google Scholar] [CrossRef]
- Schlienz, N.J.; Spindle, T.R.; Cone, E.J.; Herrmann, E.S.; Bigelow, G.E.; Mitchell, J.M.; Flegel, R.; LoDico, C.; Vandrey, R. Pharmacodynamic dose effects of oral cannabis ingestion in healthy adults who infrequently use cannabis. Drug Alcohol Depend. 2020, 211, 107969. [Google Scholar] [CrossRef] [PubMed]
- Radočaj, O.; Dimić, E.; Tsao, R. Effects of hemp (Cannabis sativa L.) seed oil press-cake and decaffeinated green tea leaves (Camellia sinensis) on functional characteristics of gluten-free crackers. J. Food Sci. 2014, 79, 318–325. [Google Scholar] [CrossRef]
- Jančíková, S.; Dordevic, D. The use of a pectin–cannabis flour coating on freshly cut apple pieces. J. Food Sci. 2020, 1, 73–78. [Google Scholar]
- Pasquali, F.; Schinzari, M.; Lucchi, A.; Mandrioli, M.; Toschi, T.G.; De Cesare, A.; Manfreda, G. Preliminary data on the antimicrobial effect of Cannabis sativa L. variety Futura 75 against food-borne pathogens in vitro as well as against naturally occurring microbial populations on minced meat during storage. Ital. J. Food Saf. 2020, 9, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Teterycz, D.; Sobota, A.; Przygodzka, D.; Łysakowska, P. Hemp seed (Cannabis sativa L.) enriched pasta: Physicochemical properties and quality evaluation. PLoS ONE 2021, 16, e0248790. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, N.; Aslan, M. Antioxidant and physicochemical properties of cookies containing raw and roasted hemp flour. Acta Sci. Pol. Technol. Aliment. 2020, 19, 177–184. [Google Scholar]
- Wolf, C.E.; Poklis, J.L.; Poklis, A. Stability of tetrahydrocannabinol and cannabidiol in prepared quality control medible brownies. J. Anal. Toxicol. 2017, 41, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulec, A.; Kowalski, S.; Sabat, R.; Skoczylas, Ł.; Tabaszewska, M.; Wywrocka-Gurgul, A. Hemp flour as a valuable component for enriching physicochemical and antioxidant properties of wheat bread. LWT 2019, 102, 164–172. [Google Scholar] [CrossRef]
- Norajit, K.; Gu, B.-J.; Ryu, G.-H. Effects of the addition of hemp powder on the physicochemical properties and energy bar qualities of extruded rice. Food Chem. 2011, 129, 1919–1925. [Google Scholar] [CrossRef]
- Korus, A.; Gumul, D.; Krystyjan, M.; Juszczak, L.; Korus, J. Evaluation of the quality, nutritional value and antioxidant activity of gluten-free biscuits made from corn-acorn flour or corn-hemp flour composites. Eur. Food Res. Technol. 2017, 243, 1429–1438. [Google Scholar] [CrossRef]
- Ritter, S.; Zadik-Weiss, L.; Almogi-Hazan, O.; Or, R. Cannabis, One Health, and Veterinary Medicine: Cannabinoids’ Role in Public Health, Food Safety, and Translational Medicine. Rambam Maimonides Med. J. 2020, 11, e0006. [Google Scholar] [CrossRef] [Green Version]
- Tallon, M.J. Cannabis sativa L. and its extracts: Regulation of cannabidiol in the European Union and United Kingdom. J. Diet. Suppl. 2020, 17, 503–516. [Google Scholar] [CrossRef]
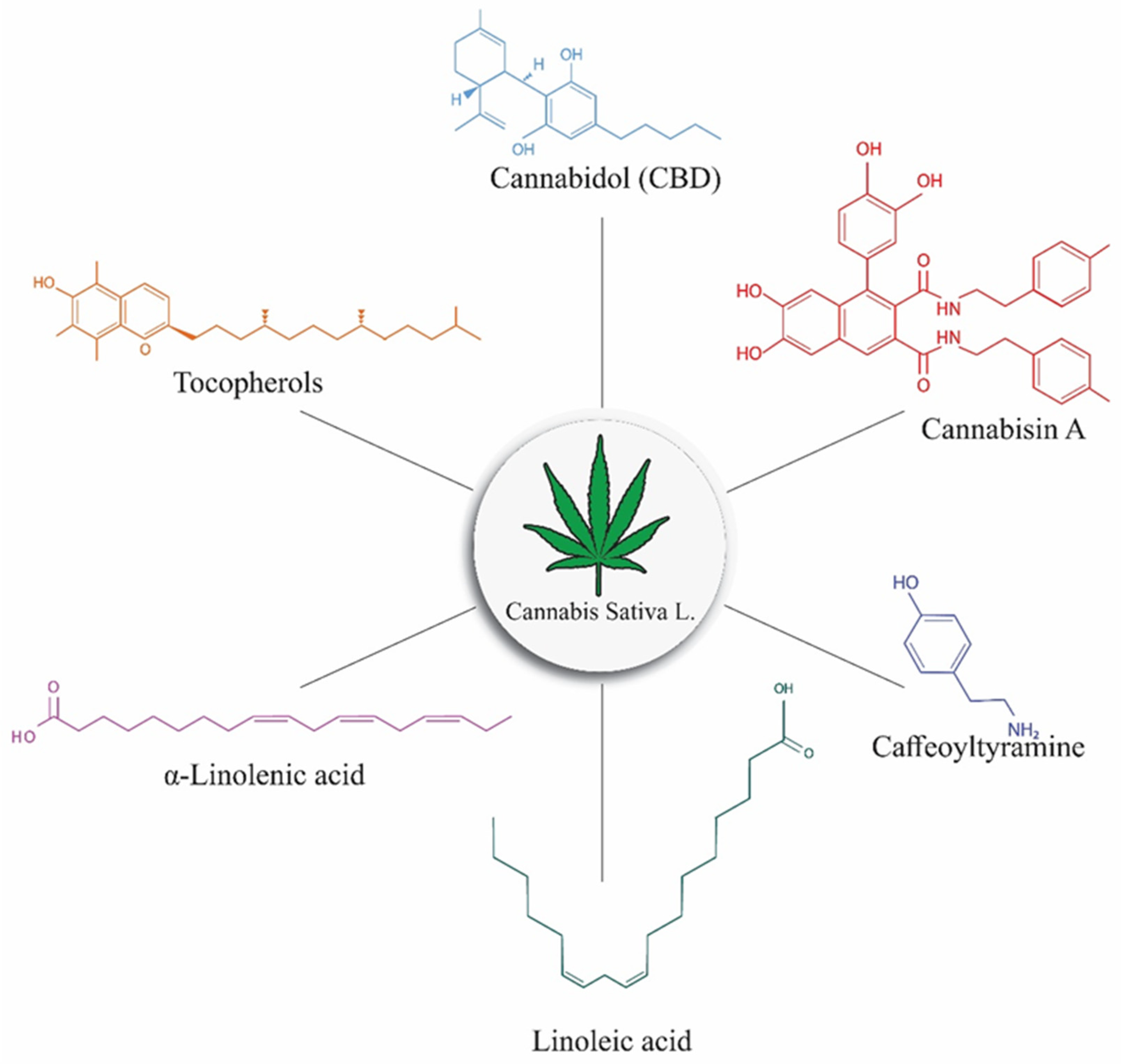
| Formulation and Route of Administration | Medical Condition | Cannabinoids Composition | Number of Subjects | Therapeutic Effects | Country | Reference |
|---|---|---|---|---|---|---|
| THC:CBD and THC extract were used | Cancer | 1:1:1 of THC:CBD, THC, and placebo | 177 patients | THC:CBD extract showed significant change as compared to the placebo. THC extract showed non-significant change. Induced nausea and vomiting. | Europe | [22] |
| CBD was used along with TRPA1 receptor, supplemented through diet | Inflammatory rheumatoid arthritis | Cannabinoids CBD and TRPA1 were used | 40 patients with long-standing rheumatoid arthritis | CBD showed anti-arthritis effects, it increased the intracellular calcium levels, reduces cell viability, and IL-6/IL-8/MMP-3 production of rheumatoid arthritis synovial fibroblasts (RASF) by activating TRPA1 and mitochondrial targets. | USA | [23] |
| Extracts of CBD and THC was inhaled or supplemented through diet | Epilepsy | 20% CBD and 1% THC were used | 74 patients, aged 1–18 years | Intractable epilepsy could be effectively treated with CBD-rich medicinal cannabis. There were just a few modest and occasional adverse effects mentioned. | IL | [24] |
| Five different cannabis strains were used. Hybrid, Indica, Sativa, 3:1 CBD: THC, and 1:1 CBD: THC They were inhaled or supplemented through diet | Headache, migraine, arthritis, and chronic pain | 3:1 ratio of CBD: THC and 1:1 ratio of CBD: THC were used | 2032 patients, 62.6% male (n = 1271), 37.3% females (n = 758) | Out of a total of 2032 people, cannabis was used to treat 21 ailments. Overall, 42.4% (n = 861) of people had pain syndromes, with chronic pain accounting for 29.4% (n = 598), arthritis accounting for 9.3% (n = 188), and headache accounting for 3.7% (n = 75). | UK | [25] |
| Hemp seed oil was consumed orally | Dermatitis and diseases of the skin | 30 mL (2tbsp) hemp seed oil and olive oil were used | 20 patients with atopic dermatitis | Hempseed oil consumption resulted in significant alterations in plasma lipid content and reduced clinical signs of atopic dermatitis as compared to olive oil. | USA | [26] |
| Product | Country | Cannabis to Product Ratio | Results | Citations |
|---|---|---|---|---|
| Chocolate | Canada | Cannabis was added up to 20% of the chocolate (in non-concentrated form). | The cannabis’s plant extract was effectively added to chocolate, which enhanced the physical and nutritional characteristics of chocolate without altering its qualities. | [75] |
| Brewing | United States | Cannabis-infused beers usually contain 10 mg of CBD and 3.5 to 6% alcohol by volume. | When people drink CBD beers, they report feeling “elevated” and “naturally relaxing.” | [76] |
| Tea | Italy | For 500 mL of water, 500 mg medicinal cannabis was used. | The maximum concentration of cannabinoids in the cannabis tea was obtained after 15 min of boiling. | [77] |
| Oolong tea | Thailand | 2.5 g of Oolong tea contains 5% of encapsulated cannabis oil | Phenolics, antioxidants, flavor, aroma, and therapeutic potential were improved making the product healthier. | [78] |
| Yogurt | Romania | The protein content of the yogurt was increased by adding 4% cannabis protein. | Yogurt with improved nutritional, physicochemical, rheological, and sensory characteristics was produced. | [79] |
| Gluten-free bread | Poland | 60–120 g of cannabis was utilized for substitution of 10–20% of the starch. | Cannabis enhanced the nutritional value and sensory acceptability of the bread. | [80] |
| Brownies | US | About 0 to 50 mg concentration of cannabis was added in brownies. | The cannabis-infused brownies were successfully produced, revealing that even the smallest cannabis dose causes discernible drug effects. | [81] |
| Gluten-free Crackers | Canada | 20% cannabis oil press cake was utilized for the formation of gluten-free crackers. | The enrichment resulted in a substantial modification in the cracker’s physicochemical, as well as sensory characteristics | [82] |
| Apples-coating | Germany | Apple slices were coated with 1% pectin and 5% cannabis flour. | There was an increase in polyphenol and antioxidant capacity of apple slices and less weight loss. | [83] |
| Meat | Italy | 50 mL of extract, containing 322.70 g/mL of CBD, was applied to 2.5 kg of meat | Cannabis extract showed antimicrobial activity against foodborne pathogens in meat. | [84] |
| Pasta | Italy | 30–40% concentration of cannabis flour was added to pasta. | Cannabis ingredients improved the nutritional content of pasta, while maintaining its safety. | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iftikhar, A.; Zafar, U.; Ahmed, W.; Shabbir, M.A.; Sameen, A.; Sahar, A.; Bhat, Z.F.; Kowalczewski, P.Ł.; Jarzębski, M.; Aadil, R.M. Applications of Cannabis Sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement. Molecules 2021, 26, 7699. https://doi.org/10.3390/molecules26247699
Iftikhar A, Zafar U, Ahmed W, Shabbir MA, Sameen A, Sahar A, Bhat ZF, Kowalczewski PŁ, Jarzębski M, Aadil RM. Applications of Cannabis Sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement. Molecules. 2021; 26(24):7699. https://doi.org/10.3390/molecules26247699
Chicago/Turabian StyleIftikhar, Amna, Umaima Zafar, Waqar Ahmed, Muhammad Asim Shabbir, Aysha Sameen, Amna Sahar, Zuhaib F. Bhat, Przemysław Łukasz Kowalczewski, Maciej Jarzębski, and Rana Muhammad Aadil. 2021. "Applications of Cannabis Sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement" Molecules 26, no. 24: 7699. https://doi.org/10.3390/molecules26247699
APA StyleIftikhar, A., Zafar, U., Ahmed, W., Shabbir, M. A., Sameen, A., Sahar, A., Bhat, Z. F., Kowalczewski, P. Ł., Jarzębski, M., & Aadil, R. M. (2021). Applications of Cannabis Sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement. Molecules, 26(24), 7699. https://doi.org/10.3390/molecules26247699









