The Land Snail, Eobania vermiculata, as a Bioindicator of the Heavy Metal Pollution in the Urban Areas of Sulaimani, Iraq
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Soil and Snail Samples Processing
2.4. Soil pH and Organic Matter
2.5. Soil and Snail Digestion
2.6. Quality Control
2.7. Data Analysis
3. Results and Discussions
3.1. Concentration of Heavy Metals in Soil Samples
3.2. Concentration of Heavy Metals in Snail Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murtaza, G.; Ditta, A.; Ullah, N.; Usman, M.; Ahmed, Z. Biochar for the management of nutrient impoverished and metal contaminated soils: Preparation, applications, and prospects. J. Soil Sci. Plant Nutr. 2021, 21, 2191–2213. [Google Scholar] [CrossRef]
- Mehmood, S.; Wang, X.; Ahmed, W.; Imtiaz, M.; Ditta, A.; Rizwan, M.; Irshad, S.; Bashir, S.; Saeed, Q.; Mustafa, A.; et al. Removal mechanisms of slag against potentially toxic elements in soil and plants for sustainable agriculture development: A critical review. Sustainability 2021, 13, 5255. [Google Scholar] [CrossRef]
- Mehmood, S.; Ahmed, W.; Rizwan, M.; Imtiaz, M.; Elnahal, A.S.M.A.; Ditta, A.; Irshad, S.; Ikram, M.; Li, W. Comparative efficacy of raw and HNO3-modified biochar derived from rice straw on vanadium transformation and its uptake by rice (Oryza sativa L.): Insights from photosynthesis, antioxidative response, and gene-expression profile. Environ. Pollut. 2021, 289, 117916. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Xuebin, Q.; Riaz, L.; Yasin, G.; Shah, A.N.; Shahzad, U.; Jahan, M.S.; Ditta, A.; Bashir, M.A.; Rehim, A.; et al. The interactive effect of pH variation and cadmium stress on wheat (Triticum aestivum L.) growth, physiological and biochemical parameters. PLoS ONE 2021, 16, e0253798. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.; El-Sayed, M.M.; Li, X.; Liu, Z.; Mustafa, S.K.; Ditta, A.; Hessini, K. Diminishing Heavy Metal Hazards of Contaminated Soil via Biochar Supplementation. Sustainability 2021, 13, 12742. [Google Scholar] [CrossRef]
- Viard, B.; Pihan, F.; Promeyrat, S.; Pihan, J.C. Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: Bioaccumulation in soil, Graminaceae and land snails. Chemosphere 2004, 55, 1349–1359. [Google Scholar] [CrossRef]
- Divrikli, U.; Mendil, D.; Tuzen, M.; Soylak, M.; Elci, L. Trace metal pollution from traffic in Denizli-Turkey during dry season. Biomed. Environ. Sci. 2006, 19, 254–261. [Google Scholar]
- Keith, L.S.; Moffett, D.B.; Rosemond, Z.A.; Wohlers, D.W. ATSDR evaluation of health effects of tungsten and relevance to public health. Toxicol. Ind. Health 2007, 23, 347–387. [Google Scholar] [CrossRef]
- Nica, D.V.; Bura, M.; Gergen, I.; Harmanescu, M.; Bordean, D.M. Bioaccumulative and conchological assessment of heavy metal transfer in a soil-plant-snail food chain. Chem. Cent. J. 2012, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordaens, K.; De Wolf, H.; Van Houtte, N.; Vandecasteele, B.; Backeljau, T. Genetic variation in two land snails, Cepaea nemoralis and Succinea putris (Gastropoda, Pulmonata), from sites differing in heavy metal content. Genetica 2006, 128, 227–239. [Google Scholar] [CrossRef]
- Louzon, M.; Gimbert, F.; Belly, T.; Amiot, C.; Pauget, B.; de Vaufleury, A.; Capelli, N. From Environmental Bioavailability of Metal(Loid)s to Their Ecogenotoxicological Effects in Land Snails. Environ. Sci. Pollut. Res. 2021, 28, 43629–43642. [Google Scholar] [CrossRef]
- De Vaufleury, A.; Cœurdassier, M.; Pandard, P.; Scheifler, R.; Lovy, C.; Crini, N.; Badot, P.M. How terrestrial snails can be used in risk assessment of soils. Environ. Toxicol. Chem. Int. J. 2006, 25, 797–806. [Google Scholar] [CrossRef]
- Regoli, F.; Gorbi, S.; Fattorini, D.; Tedesco, S.; Notti, A.; Machella, N.; Bocchetti, R.; Benedetti, M.; Piva, F. Use of the land snail Helix aspersa as sentinel organism for monitoring ecotoxicologic effects of urban pollution: An integrated approach. Environ. Health Perspect. 2006, 114, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Baroudi, F.; Al Alam, J.; Fajloun, Z.; Millet, M. Snail as sentinel organism for monitoring the environmental pollution: A review. Ecol. Indic. 2020, 113, 106240. [Google Scholar] [CrossRef]
- Berandah, F.E.; Kong, Y.C.; Ismail, A. Bioaccumulation and distribution of heavy metals (Cd, Cu, Fe, Ni, Pb and Zn) in the different tissues of Chicoreus capucinus Lamarck (Mollusca: Muricidae)’, Sungai Janggut, Kuala Langat, Malaysia. Environ. Asia 2010, 3, 65–71. [Google Scholar]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef]
- Yap, C.K.; Cheng, W.H. Distributions of heavy metal concentrations in different tissues of the mangrove snail Nerita lineata. Sains Malays. 2013, 42, 597–603. [Google Scholar]
- Samsi, A.N.; Asaf, R.; Santi, A.; Wamnebo, M.I. Gastropods as a bioindicator and biomonitoring metal pollution. Aquac. Indones. 2017, 18, 54. [Google Scholar] [CrossRef] [Green Version]
- Dallinger, R.; Lagg, B.; Egg, M.; Schipflinger, R.; Chabicovsky, M. Cd accumulation and Cd–metallothionein as a biomarker in Cepaea hortensis (Helicidae, Pulmonata) from laboratory exposure and metal-polluted habitats. Ecotoxicology 2004, 13, 757–772. [Google Scholar] [CrossRef]
- Habib, M.R.; Mohamed, A.H.; Osman, G.Y.; Mossalem, H.S.; El-Din, A.T.S.; Croll, R.P. Biomphalaria alexandrina as a bioindicator of metal toxicity. Chemosphere 2016, 157, 97–106. [Google Scholar] [CrossRef]
- Scheifler, R.; Gomot-De Vaufleury, A.; Toussaint, M.L.; Badot, P.M. Transfer and effects of cadmium in an experimental food chain involving the snail Helix aspersa and the predatory carabid beetle Chrysocarabus splendens. Chemosphere 2002, 48, 571–579. [Google Scholar] [CrossRef]
- Arrébola, J.R.; Cárcaba, A.; Álvarez, R.M.; Ruiz, A. Characterization of Andalusian helicola sector: Terrestrial snails consumption in Western Andalusia. Iberus 2004, 22, 31–41. [Google Scholar]
- Rashid, K.A. Environmental Implications of Tanjaro Waste Disposal Site in the City of Sulaimani. Ph.D. Thesis, University of Sulaimani, Sulaymaniyah, Egypt, 2010. [Google Scholar]
- Simonson, R.W. Historical Highlights of Soil Survey and Soil Classification with Emphasis on the United States, 1899–1970; International Soil Reference and Information Centre: Wageningen, The Netherlands, 1989. [Google Scholar]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic pollution sources. Rev. Environ. Contam. 2009, 197, 17–60. [Google Scholar]
- Irshad, S.; Xie, Z.; Mehmood, S.; Nawaz, A.; Ditta, A.; Mahmood, Q. Insights into conventional and recent technologies for arsenic bioremediation: A systematic review. Environ. Sci. Pollut. Res. 2021, 28, 18870–18892. [Google Scholar] [CrossRef]
- Rastegari Mehr, M.; Keshavarzi, B.; Moore, F.; Hooda, P.S.; Busquets, R.; Ghorbani, Z. Arsenic in the rock–soil-plant system and related health risk in a magmatic–metamorphic belt, West of Iran. Environ. Geochem. Health 2020, 42, 3659–3673. [Google Scholar] [CrossRef]
- Meharg, A.A.; Rahman, M.M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 2003, 37, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Using multivariate analyses and GIS to identify pollutants and their spatial patterns in urban soils in Galway, Ireland. Environ. Pollut. 2006, 142, 501–511. [Google Scholar] [CrossRef]
- Fayad, N.; Al-Noor, T.H.; Al-Noor, N.H. Analysis and assessment of essential toxic heavy metals, pH and EC in Ishaqi River and adjacent soil. Rev. Adv. Phys. Theor. Appl. 2013, 16, 25–37. [Google Scholar]
- Salah, E.; Turki, A.; Earth, S.N. Heavy metals concentration in urban soils of Fallujah City, Iraq. Environ. Earth Sci. 2013, 3, 100–112. [Google Scholar]
- Sulaivany, R.O.H.; Al-Mezori, H.A.M. Heavy Metals Concentration in Selected Vegetables Grown in Dohuk City, Kurdistan Region, Iraq. WIT Trans. Built Environ. 2007, 94, 255–265. [Google Scholar]
- Ali, H.A. Heavy metals concentrations in surface soils of the Haweja area southwestern of Kirkuk, Iraq. Kirkuk Univ.-Sci. Stud. 2007, 2, 35–48. [Google Scholar] [CrossRef]
- Zeb, H.; Hussain, A.; Naveed, M.; Ditta, A.; Ahmad, S.; Jamshaid, M.U.; Ahmad, H.T.; Hussain, B.; Aziz, R.; Haider, M.S. Compost enriched with ZnO and Zn-solubilizing bacteria improves yield and Zn-fortification in flooded rice. Ital. J. Agron. 2018, 13, 310–316. [Google Scholar] [CrossRef]
- Manta, D.S.; Angelone, M.; Bellanca, A.; Neri, R.; Sprovieri, M. Heavy metals in urban soils: A case study from the city of Palermo (Sicily), Italy. Sci. Total Environ. 2002, 300, 229–243. [Google Scholar] [CrossRef]
- Rizo, O.D.; Hernández, I.C.; López, J.A.; Arado, O.D.; Pino, N.L. Chromium, cobalt and nickel contents in urban soils of Moa, northeastern Cuba. Bull. Environ. Contam. Toxicol. 2011, 86, 189–193. [Google Scholar] [CrossRef]
- Pfleiderer, S.; Englisch, M.; Reiter, R. Current state of heavy metal contents in Vienna soils. Environ. Geochem. Health 2012, 34, 665–675. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Mo, C.H.; Li, H.Q.; Lü, H.; Zeng, Q.Y.; Li, Y.W.; Wu, X.L. Heavy metal contamination of urban soils and dusts in Guangzhou, South China. Environ. Monit. Assess. 2013, 185, 1095–1106. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, D.; Xie, Q.; Chen, X. An approach to studying heavy metal pollution caused by modern city development in Nanjing, China. Environ. Geol. 1999, 38, 223–228. [Google Scholar] [CrossRef]
- Ward, N.I. Multielement contamination of British motorway environments. Sci. Total Environ. 1990, 93, 393–401. [Google Scholar] [CrossRef]
- Khodadoust, A.P.; Reddy, K.R.; Maturi, K. Removal of nickel and phenanthrene from kaolin soil using different extractants. Environ. Eng. Sci. 2004, 21, 691–704. [Google Scholar] [CrossRef]
- Naveed, M.; Ditta, A.; Ahmad, M.; Mustafa, A.; Ahmad, Z.; Conde-Cid, M.; Tahir, S.; Shah, S.A.A.; Abrar, M.M.; Fahad, S. Processed animal manure improves morpho-physiological and biochemical characteristics of Brassica napus L. under nickel and salinity stress. Environ. Sci. Pollut. Res. 2021, 28, 45629–45645. [Google Scholar] [CrossRef]
- Naveed, M.; Bukhari, S.S.; Mustafa, A.; Ditta, A.; Alamri, S.; El-Esawi, M.A.; Rafique, M.; Ashraf, S.; Siddiqui, M.H. Mitigation of nickel toxicity and growth promotion in sesame through the application of a bacterial endophyte and zeolite in nickel contaminated soil. Int. J. Environ. Res. Public Health 2020, 17, 8859. [Google Scholar] [CrossRef]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson correlation coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- World Health Organization. List of Maximum Levels Recommended for Contaminants by the Joint FAO/WHO Codex Alimentarius Commission-Third Series; CAC/FAL: Rome, Italy, 1978; Available online: https://agris.fao.org/agris-search/search.do?recordID=XF7703780 (accessed on 14 May 2020).
- Eneji, I.S. Trace Metals Levels in African Giant Land Snails (Achatina achatina) from Selected Local Government Areas in Akwa Ibom State, Nigeria. Libr. J. 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Thanh-Nho, N.; Marchand, C.; Strady, E.; Huu-Phat, N.; Nhu-Trang, T.T. Bioaccumulation of some trace elements in tropical mangrove plants and snails (Can Gio, Vietnam). Environ. Pollut. 2019, 248, 635–645. [Google Scholar] [CrossRef]
- Sabarina, M.Y.; Zaini, H.; Nik, A.N.A.; Mohd, B.M. Cadmium, Chromium, Copper, Lead, Ferum and Zinc Levels in the Cockles (Anadara granosa) From Kuala Selangor, Malaysia. Malays. J. Anal. Sci. 2014, 18, 514–521. [Google Scholar]
- Nwoko, C.I.A.; Ukiwe, L.N.; Oshoakpeme, G.S. Heavy Metals Concentrations in the Shell and Tissue of Periwinkle (Tympanotonus fuscatus) and Giant Land Snail (Achatina fulica) in Soku Community of Niger. J. Adv. Chem. 2014, 10, 2426–2429. [Google Scholar]
- Swaileh, K.M.; Rabay’a, N.; Salim, R.; Ezzughayyar, A.; Rabbo, A.A. Concentrations of heavy metals in roadside soils, plants, and land snails from the West Bank, Palestine. J. Environ. Sci. Health A Toxic Hazard. Subst. Eng. 2001, 36, 765–778. [Google Scholar] [CrossRef]
- Aziz, N.M.; Marina, B.A.; Qzar, E.A. The Potential of some snails as bioindicator of Trace metals level in East Hammar marsh, south of Iraq. Basrah J. Sci. 2010, 28, 228–242. [Google Scholar]
- Radwan, M.A.; El-Gendy, K.S.; Gad, A.F. Biomarkers of oxidative stress in the land snail, Theba pisana for assessing ecotoxicological effects of urban metal pollution. Chemosphere 2010, 79, 40–46. [Google Scholar] [CrossRef]
- Mohammadein, A.; El-Shenawy, N.S.; Al-Fahmie, Z.H.H. Italian Journal of Zoology Bioaccumulation and histopathological changes of the digestive gland of the land snail Eobania vermiculata (Mollusca: Gastropoda), as biomarkers of terrestrial heavy metal pollution in Taif city Bioaccumulation and histopathology. Ital. J. Zool. 2013, 80, 345–357. [Google Scholar] [CrossRef]
- Altuğ, G.; Güler, N. Determination of the levels of indicator bacteria, Salmonella spp. and heavy metals in sea snails (Rapana venosa) from the Northern Marmara Sea, Turkey. Turk. J. Fish. Aquat. Sci. 2002, 2, 141–144. [Google Scholar]
- Aboho, S.Y.; Anhwange, B.A.; Ber, G.A. Screening of Achatina achatina and Pila ovata for trace metals in Makurdi metropolis. Pak. J. Nutr. 2009, 8, 1170–1171. [Google Scholar] [CrossRef] [Green Version]
- Mariam, I.; Iqbal, S.; Nagra, S.A. Distribution of some trace and macrominerals in beef, mutton and poultry. Int. J. Agric. Biol. 2004, 6, 816–820. [Google Scholar]
- Harmsen, J. Measuring Bioavailability: From a Scientific Approach to Standard Methods. J. Environ. Qual. 2007, 36, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, S.; Ikegami, M.; Sakanakura, H.; Kanjo, Y. Test methods for the evaluation of heavy metals in contaminated soil. In Environmental Remediation Technologies for Metal-Contaminated Soils; Springer: Tokyo, Japan, 2016; pp. 67–97. [Google Scholar]
- Vranković, J.; Janković-Tomanić, M.; Vukov, T. Comparative assessment of biomarker response to tissue metal concentrations in urban populations of the land snail Helix pomatia (Pulmonata: Helicidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 245, 110448. [Google Scholar] [CrossRef]
- Mourier, B.; Fritsch, C.; Dhivert, E.; Gimbert, F.; Cœurdassier, M.; Pauget, B.; De Vaufleury, A.; Scheifler, R. Chemical extractions and predicted free ion activities fail to estimate metal transfer from soil to field land snails. Chemosphere 2011, 85, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, P.C.J.; Van Der Zee, S.E.A.T.M.; Ma, W.C. Heavy metal concentrations in soil and earthworms in a floodplain grassland. Environ. Pollut. 2005, 138, 505–516. [Google Scholar] [CrossRef] [PubMed]
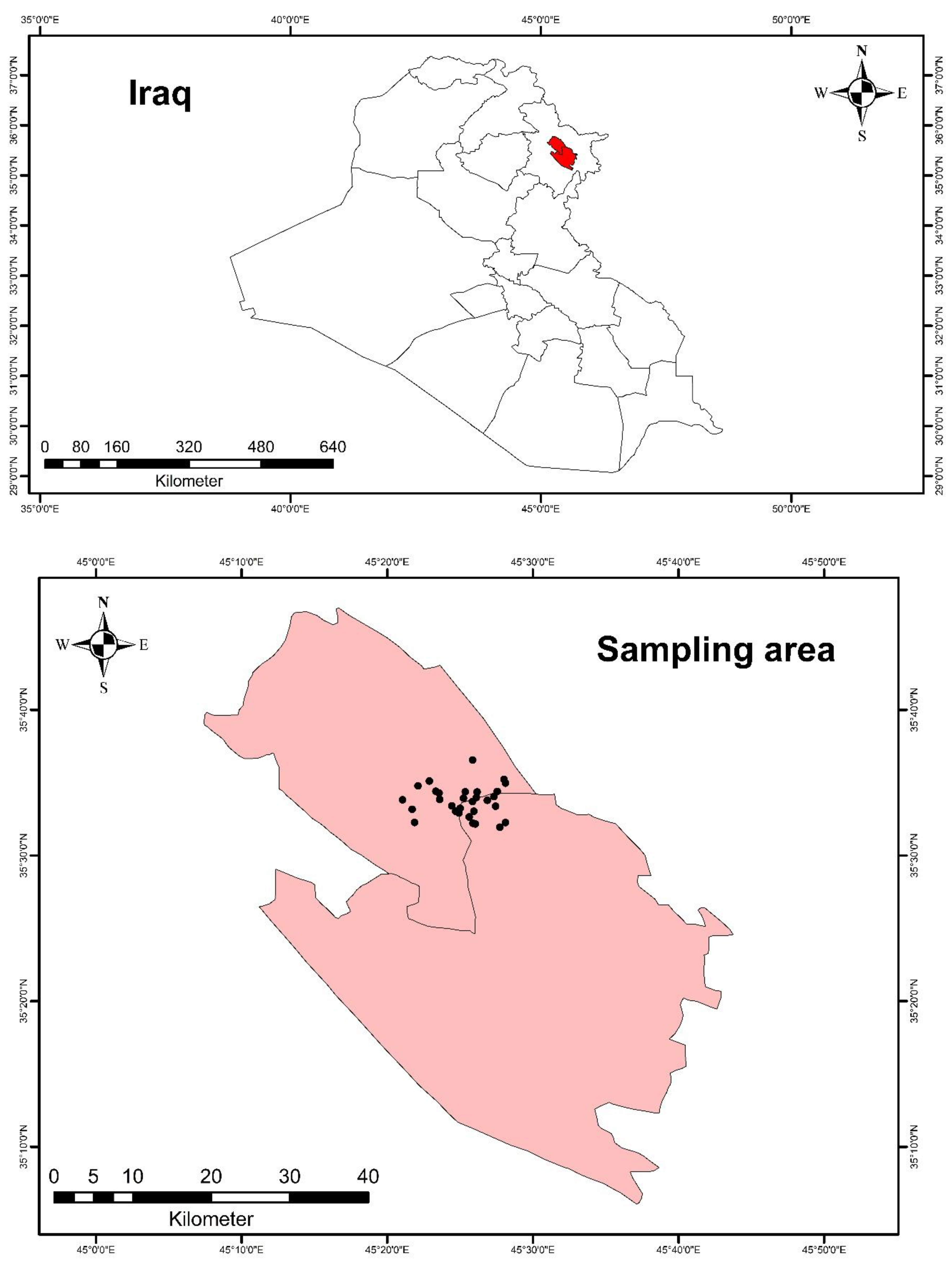
| Site No. | Site Name | Latitude | Longitude | Location Description |
|---|---|---|---|---|
| 1 | Rizgary taza | 35.5567927 | 45.4073041 | Residential area |
| 2 | Sharawani | 35.5441263 | 45.4270755 | Main road |
| 3 | Chwarbakh | 35.5506672 | 45.4325128 | Residential area, street |
| 4 | Shekhmuheddin | 35.5539817 | 45.416746 | Residential area, street |
| 5 | Chaviland 1 | 35.58285 | 45.46867 | Amusement park |
| 6 | Chaviland 2 | 35.58707 | 45.46696 | Amusement park |
| 7 | Rizgary | 35.5640917 | 45.3933773 | Beside bus park |
| 8 | Grdi sarchnar | 35.5734994 | 45.3890346 | Residential area |
| 9 | Baxtiary | 35.571448 | 45.3927084 | Main road |
| 10 | Alikamal | 35.567429 | 45.4553902 | Main road |
| 11 | Azadi | 35.556414 | 45.4573128 | Residential area, street |
| 12 | Hawarabarza | 35.57325 | 45.45918 | Residential area |
| 13 | Azadi Park | 35.5616684 | 45.4307625 | Amusement park |
| 14 | Daik Park | 35.5632342 | 45.447859 | Very crowded street |
| 15 | Wuluba | 35.536912 | 45.4313598 | Main road |
| 16 | Xabat | 35.53613 | 45.43422 | Main road |
| 17 | Qirga | 35.53772 | 45.46860 | Near petrol station |
| 18 | Kaniba | 35.53239 | 45.46221 | Main road, near petrol station |
| 19 | Bakrajoi Taza | 35.563746 | 45.350795 | American University |
| 20 | Bakrajo | 35.55288 | 45.36179 | Main road |
| 21 | Qalawa | 35.54876 | 45.41539 | Residential area, school |
| 22 | Ablax | 35.55051 | 45.41162 | Residential area, school |
| 23 | Sarchnar | 35.58520 | 45.38159 | Office park |
| 24 | Mamostayan | 35.56637 | 45.43520 | Main road |
| 25 | Hawkari | 35.57267 | 45.43614 | Very crowded road |
| 26 | Baxan | 35.57300 | 45.42260 | Main road |
| 27 | Baranan | 35.56561 | 45.42056 | Main road, near school |
| 28 | Alban | 35.53787 | 45.36453 | Agriculture college park |
| 29 | Rapareen | 35.57972 | 45.36850 | UOS * park |
| 30 | Hawary-shar | 35.60929 | 45.43117 | Amusement park |
| Sampling Sites | pH | OM | Pb | As | Cr | Ni | Zn |
|---|---|---|---|---|---|---|---|
| Rizgary taza | 8.07 ± 0.12 * | 11.47 ± 1.25 | 4.17 ± 0.12 | 2.11 ± 0.44 | 28.94 ± 2.28 | 54.66± 0.17 | 46.95 ± 0.36 |
| Sharawany | 8.09 ± 0.13 | 8.51 ± 1.23 | 5.38 ± 0.30 | 1.93 ± 0.25 | 26.47 ± 7.89 | 58.55± 0.08 | 51.25 ± 0.12 |
| Chwarbakh | 7.94 ± 0.54 | 10.73 ± 2.03 | 7.50 ± 0.16 | 2.74 ± 0.09 | 27.30 ± 6.39 | 59.70 ± 0.07 | 98.58 ± 0.27 |
| Shexmuhedin | 8.11 ± 0.56 | 10.51 ± 2.05 | 3.84 ± 0.30 | 2.58 ± 0.24 | 27.39 ± 0.72 | 48.53 ± 0.16 | 52.37 ± 0.14 |
| Chaviland (1) | 8.14 ± 0.65 | 8.60 ± 1.03 | 1.58 ± 0.23 | 2.68 ± 0.27 | 29.21 ± 3.23 | 59.32 ± 0.16 | 36.57 ± 0.10 |
| Chaviland (2) | 8.08 ± 0.57 | 8.56 ± 1.05 | 0.75 ± 0.35 | 1.33 ± 0.14 | 44.73 ± 1.00 | 95.45 ± 0.22 | 30.58 ± 0.10 |
| Rizgary | 8.26 ± 0.59 | 9.84 ± 1.25 | 2.14 ± 0.26 | 3.01 ± 0.14 | 32.67 ± 0.38 | 58.86 ± 0.14 | 43.50 ± 0.10 |
| Grdi sarchnar | 8.57 ± 0.56 | 11.71 ± 1.22 | 2.48 ± 0.31 | 2.03 ± 0.25 | 31.20 ± 0.62 | 52.27 ± 0.18 | 37.71 ± 0.09 |
| Baxtiary | 8.13 ± 0.41 | 12.68 ± 2.01 | 1.65 ± 0.11 | 2.38 ± 0.23 | 26.17 ± 2.38 | 48.38 ± 0.31 | 69.07 ± 0.42 |
| Alikamal | 8.18 ± 0.31 | 10.86 ± 1.32 | 5.23 ± 0.03 | 2.22 ± 0.41 | 30.57 ± 3.13 | 55.73 ± 0.17 | 47.54 ± 0.06 |
| Azadi | 8.22 ± 0.22 | 9.09 ± 1.65 | 1.93 ± 0.32 | 2.39 ± 0.11 | 31.36 ± 3.05 | 58.40 ± 0.21 | 36.52 ± 0.19 |
| Hawarabarza | 8.26 ± 0.55 | 11.55 ± 1.23 | 6.00 ± 0.19 | 2.68 ± 0.52 | 78.45 ± 6.23 | 72.24 ± 0.19 | 93.76 ± 0.19 |
| Azadi Park | 8.20 ± 0.85 | 9.85 ± 1.47 | 4.38 ± 0.30 | 3.22 ± 0.36 | 68.25 ± 4.36 | 73.28 ± 0.38 | 47.56 ± 0.25 |
| Daik Park | 8.51 ± 0.58 | 9.19 ± 1.22 | 6.85 ± 0.11 | 2.55 ± 0.34 | 81.16 ± 6.78 | 95.21 ± 0.41 | 65.33 ± 0.40 |
| Wuluba | 8.26 ± 0.66 | 13.79 ± 1.33 | 5.42 ± 0.28 | 3.23 ± 0.52 | 76.59 ± 5.23 | 83.90 ± 0.21 | 73.41 ± 0.19 |
| Xabat | 8.15 ± 0.69 | 11.48 ± 1.58 | 2.80 ± 0.52 | 2.84 ± 0.82 | 68.40 ± 3.15 | 77.18 ± 0.55 | 48.14 ± 0.25 |
| Qirga-SPU | 8.08 ± 0.47 | 8.41 ± 1.06 | 2.99 ± 0.21 | 3.30 ± 0.20 | 72.36 ± 1.31 | 80.72 ± 0.24 | 46.58 ± 0.12 |
| Kaniba | 8.28 ± 0.55 | 8.69 ± 1.05 | 11.56 ± 0.19 | 2.44 ± 0.43 | 64.55 ± 0.98 | 72.82 ± 0.15 | 75.84 ± 0.33 |
| Bakrajoi Taza | 8.06 ± 0.54 | 11.31 ± 1.13 | 6.15 ± 0.51 | 2.72 ± 0.12 | 58.82 ± 4.97 | 78.50 ± 0.25 | 46.71 ± 0.26 |
| Bakrajo | 8.24 ± 0.66 | 10.07 ± 1.15 | 3.65 ± 0.34 | 2.31 ± 0.08 | 48.49 ± 7.06 | 63.42 ± 0.15 | 50.79 ± 0.02 |
| Qalawa | 8.48 ± 0.63 | 10.37 ± 1.26 | 5.26 ± 0.16 | 3.26 ± 0.24 | 62.67 ± 4.09 | 72.48 ± 0.37 | 53.53 ± 0.24 |
| Ablax | 8.79 ± 0.36 | 9.11 ± 1.08 | 2.85 ± 0.65 | 3.11 ± 0.34 | 64.96 ± 0.29 | 78.14 ± 0.20 | 46.95 ± 0.18 |
| Sarchnar | 8.19 ± 0.44 | 8.91 ± 1.09 | 4.26 ± 0.35 | 3.50 ± 0.20 | 84.65 ± 11.95 | 73.18 ± 0.14 | 48.32 ± 0.14 |
| Mamostayan | 8.32 ± 0.65 | 7.18 ± 0.95 | 3.43 ± 0.29 | 3.82 ± 0.19 | 78.16 ± 23.10 | 76.98 ± 0.25 | 45.63 ± 0.12 |
| Hawkari | 8.26 ± 0.45 | 9.24 ± 0.89 | 18.37 ± 0.18 | 3.14 ± 0.13 | 71.75 ± 24.30 | 66.10 ± 0.09 | 55.95 ± 0.04 |
| Baxan | 8.25 ± 0.55 | 9.42 ± 1.23 | 6.94 ± 0.59 | 2.88 ± 0.39 | 72.15 ± 27.48 | 67.58 ± 0.40 | 57.76 ± 0.41 |
| Baranan | 8.22 ± 0.52 | 10.02 ± 1.47 | 8.21 ± 0.28 | 3.11 ± 0.31 | 83.86 ± 7.31 | 69.88 ± 0.18 | 60.03 ± 0.19 |
| College of Agriculture | 8.47 ± 0.12 | 9.23 ± 0.86 | 4.01 ± 0.05 | 3.65 ± 0.32 | 83.95 ± 6.88 | 75.09 ± 0.06 | 59.76 ± 0.09 |
| UOS-New Campus | 8.45 ± 0.12 | 9.60 ± 1.23 | 2.15 ± 0.26 | 3.53 ± 0.53 | 91.01 ± 18.73 | 87.16 ± 0.37 | 44.68 ± 0.23 |
| Hawarishar Park | 8.38 ± 0.32 | 10.48 ± 1.32 | 3.70 ± 0.20 | 4.46 ± 0.14 | 95.45 ± 19.75 | 85.84 ± 0.24 | 53.71 ± 0.14 |
| Soil quality guidelines by Canadian standards | NL ** | NL ** | 12 | 70 | 64 | 45 | 200 |
| OM * | pH | Pb | As | Cr | Ni | |
|---|---|---|---|---|---|---|
| pH | 0.583 | |||||
| Pb | 0.778 | 0.823 | ||||
| As | 0.519 | 0.072 | 0.502 | |||
| Cr | 0.385 | 0.022 | 0.031 | 0.000 | ||
| Ni | 0.204 | 0.107 | 0.955 | 0.002 | 0.000 | |
| Zn | 0.048 | 0.544 | 0.000 | 0.366 | 0.143 | 0.892 |
| Sampling Sites | Pb | As | Cr | Ni | Zn |
|---|---|---|---|---|---|
| Rizgary taza | 1.295 | 0.971 | 1.795 | * BDL | 87.305 |
| Sharawany | 0.986 | 0.552 | 1.318 | 0.308 | 90.791 |
| Chwarbakh | 1.056 | 0.398 | 1.227 | 0.364 | 84.173 |
| Shexmuhedin | 1.293 | 0.694 | 1.577 | BDL | 87.408 |
| Chaviland (1) | 1.691 | 0.273 | 3.372 | 1.657 | 79.591 |
| Chaviland (2) | 1.007 | 0.26 | 7.522 | 4.157 | 78.941 |
| Rizgary | 0.961 | 0.576 | 0.996 | 0.677 | 128.265 |
| Grdi sarchnar | 0.888 | 0.569 | 5.939 | 3.479 | 71.263 |
| Baxtiary | 0.864 | 0.129 | 1.102 | 0.282 | 95.552 |
| Alikamal | 1.388 | 1.004 | 15.877 | 10.924 | 99.307 |
| Azadi | 0.876 | 0.531 | 4.209 | BDL | 96.033 |
| Hawarabarza | 0.979 | 0.593 | 4.449 | 1.931 | 82.505 |
| Azadi Park | 1.506 | 0.829 | 9.332 | 5.216 | 128.348 |
| Daik Park | 0.677 | 0.46 | 1.548 | BDL | 83.404 |
| Wuluba | 1.829 | 0.251 | 4.764 | 2.404 | 107.322 |
| Xabat | 0.845 | 0.708 | 6.628 | 4.165 | 71.805 |
| Qirga-SPU | 0.993 | 0.449 | 1.914 | BDL | 167.31 |
| Kaniba | 1.684 | 0.428 | 5.264 | 1.953 | 101.607 |
| Bakrajoi Taza | 0.708 | 0.223 | 2.407 | 0.594 | 147.847 |
| Bakrajo | 1.414 | 0.08 | 1.246 | 0.597 | 97.933 |
| Qalawa | 1.626 | 0.213 | 2.124 | BDL | 135.818 |
| Ablax | 0.642 | 0.463 | 1.764 | BDL | 83.627 |
| Sarchnar | 0.707 | 0.327 | 1.321 | BDL | 70.746 |
| Mamostayan | 2.25 | 0.829 | 7.783 | 3.945 | 203.635 |
| Hawkari | 1.435 | 0.307 | 2.197 | 0.121 | 106.395 |
| Baxan | 0.915 | 0.116 | 2.565 | 0.044 | 71.593 |
| Baranan | 1.263 | 0.405 | 2.24 | 1.624 | 107.501 |
| College of Agriculture | 0.325 | 0.473 | 1.211 | BDL | 105.028 |
| UOS-New Campus | 2.092 | 0.328 | 2.062 | 0.647 | 125.951 |
| Hawarishar Park | 1.186 | 0.332 | 3.156 | 2.777 | 93.178 |
| WHO Permissible Limit Value | 1.5 | 1.0 | NL ** | 0.1 | 100 |
| Pb in Soil | As in Soil | Cr in Soil | Ni in Soil | Zn in Soil | |
|---|---|---|---|---|---|
| Pb in Snail | 0.0158 | 0.300 | 0.627 | 0.836 | 0.858 |
| As in Snail | 0.585 | 0.0072 | 0.281 | 0.202 | 0.469 |
| Cr in Snail | 0.703 | 0.345 | 0.0054 | 0.835 | 0.293 |
| Ni in Snail | 0.675 | 0.549 | 0.822 | 0.0017 | 0.498 |
| Zn in Snail | 0.991 | 0.017 | 0.185 | 0.254 | 0.0213 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, A.H.S.H.; Hama, A.A.; Hawrami, K.A.M.; Ditta, A. The Land Snail, Eobania vermiculata, as a Bioindicator of the Heavy Metal Pollution in the Urban Areas of Sulaimani, Iraq. Sustainability 2021, 13, 13719. https://doi.org/10.3390/su132413719
Salih AHSH, Hama AA, Hawrami KAM, Ditta A. The Land Snail, Eobania vermiculata, as a Bioindicator of the Heavy Metal Pollution in the Urban Areas of Sulaimani, Iraq. Sustainability. 2021; 13(24):13719. https://doi.org/10.3390/su132413719
Chicago/Turabian StyleSalih, Aso H. Saeed H., Abdullah A. Hama, Karzan A. M. Hawrami, and Allah Ditta. 2021. "The Land Snail, Eobania vermiculata, as a Bioindicator of the Heavy Metal Pollution in the Urban Areas of Sulaimani, Iraq" Sustainability 13, no. 24: 13719. https://doi.org/10.3390/su132413719
APA StyleSalih, A. H. S. H., Hama, A. A., Hawrami, K. A. M., & Ditta, A. (2021). The Land Snail, Eobania vermiculata, as a Bioindicator of the Heavy Metal Pollution in the Urban Areas of Sulaimani, Iraq. Sustainability, 13(24), 13719. https://doi.org/10.3390/su132413719









