Composition and Antioxidant Properties of Pigments of Mediterranean Herbs and Spices as Affected by Different Extraction Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction Conditions
2.3.1. Agitation-Assisted Extraction (AAE)
2.3.2. Pressurized Liquid Extraction (PLE)
2.3.3. Ultrasound-Assisted Extraction (UAE)
2.4. HPLC-DAD Analysis
2.5. Spectrophotometric Determination of Chlorophylls and Carotenoids
2.6. The Ferric Reducing Antioxidant Power (FRAP)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Determination of Carotenoids and Chlorophylls
3.2. Effect of Extraction Method and Solvent
3.3. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Jug-Dujaković, M.; Dolina, K.; Jeričević, M.; Vitasović-Kosić, I. Insular Pharmacopoeias: Ethnobotanical Characteristics of Medicinal Plants Used on the Adriatic Islands. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Ramos, C.; Teixeira, B.; Batista, I.; Matos, O.; Serrano, M.D.C.; Neng, N.; Nogueira, J.; Nunes, M.L.; Marques, A. Antioxidant and antibacterial activity of essential oil and extracts of bay laurel Laurus nobilis Linnaeus (Lauraceae) from Portugal. Nat. Prod. Res. 2012, 26, 518–529. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2016, 220, 153–161. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Congiu, R.; Falconieri, D.; Marongiu, B.; Piras, A.; Porcedda, S. Extraction and isolation of Pistacia lentiscus L. essential oil by supercritical CO2. Flavour Fragr. J. 2002, 17, 239–244. [Google Scholar] [CrossRef]
- Zimmermann, B.F.; Walch, S.G.; Tinzoh, L.N.; Stühlinger, W.; Lachenmeier, D.W. Rapid UHPLC determination of polyphenols in aqueous infusions of Salvia officinalis L. (sage tea). J. Chromatogr. B 2011, 879, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Hennia, A.; Nemmiche, S.; Guerreiro, A.; Faleiro, M.L.; Antunes, M.D.; Aazza, S.; Miguel, M.G. Antioxidant and Antiproliferative Activities of Myrtus communis L. Essential Oils from Different Algerian Regions. J. Essent. Oil Bear. Plants 2019, 22, 1488–1499. [Google Scholar] [CrossRef]
- Ozcan, B.; Esen, M.; Sangun, M.K.; Coleri, A.; Caliskan, M. Effective antibacterial and antioxidant properties of methanolic extract of Laurus nobilis seed oil. J. Environ. Biol. 2010, 31. [Google Scholar]
- Kaliora, A.C.; Kogiannou, D.A.; Kefalas, P.; Papassideri, I.S.; Kalogeropoulos, N. Phenolic profiles and antioxidant and anticarcinogenic activities of Greek herbal infusions; balancing delight and chemoprevention? Food Chem. 2014, 142, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.; Brutnon, N.P.; Fitzgerald, R.J.; Wilkinson, M.G. The Use of Herbs, Spices, and Whey Proteins as Natural Flavor Enhancers and Their Effect on the Sensory Acceptability of Reduced-Salt Chilled Ready-Meals. J. Culin. Sci. Technol. 2013, 11, 222–240. [Google Scholar] [CrossRef]
- Feketea, G.; Tsabouri, S. Common food colorants and allergic reactions in children: Myth or reality? Food Chem. 2017, 230, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Di Venere, D.; Gatto, M.A.; Ippolito, A.; Bianco, V.V. Antimicrobial potential of wild edible herbaceous species. In Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; de Cortes Sánchez-Mata, M., Tardio, J., Eds.; Springer: New York, NY, USA, 2016; pp. 233–252. ISBN 9781493933297. [Google Scholar]
- Lee, J.H.; Schwartz, S.J. Analysis of Carotenoids and Chlorophylls in Foods. In Methods of Analysis of Food Components and Additives; Chemical & Functional Properties of Food Components Series; Otles, S., Ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 231–252. ISBN 9781439815533. [Google Scholar]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Vági, E.; Simándi, B.; Daood, H.G.; Deák, A.; Sawinsky, J. Recovery of Pigments from Origanum majorana L. by Extraction with Supercritical Carbon Dioxide. J. Agric. Food Chem. 2002, 50, 2297–2301. [Google Scholar] [CrossRef]
- Halliop, W.; McAlary, G.M.; George, R.A. Fuel Cell System with Degradation Protected Anode. WO Patent 2004012288A2, 5 February 2004. [Google Scholar]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Tattini, M.; Remorini, D.; Pinelli, P.; Agati, G.; Saracini, E.; Traversi, M.L.; Massai, R. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs: Myrtus communis and Pistacia lentiscus. New Phytol. 2006, 170, 779–794. [Google Scholar] [CrossRef]
- Daly, T.; Jiwan, M.A.; O’Brien, N.M.; Aherne, S.A. Carotenoid Content of Commonly Consumed Herbs and Assessment of Their Bioaccessibility Using an In Vitro Digestion Model. Plant Foods Hum. Nutr. 2010, 65, 164–169. [Google Scholar] [CrossRef]
- Ain-Lhout, F.; Barradas, M.D.; Zunzunegui, M.; Rodríguez, H.; Novo, F.G.; Vargas, M. Seasonal Differences in Photochemical Efficiency and Chlorophyll and Carotenoid Contents in Six Mediterranean Shrub Species under Field Conditions. Photosynthetica 2004, 42, 399–407. [Google Scholar] [CrossRef]
- Hamdan, S.; Daood, H.G. Changes in the chlorophyll and carotenoid content and composition of ground thyme leaves as a function of supercritical carbon dioxide and subcritical propane extraction. Acta Aliment 2011, 40, 8–18. [Google Scholar] [CrossRef]
- Murkovic, M.; Gams, K.; Draxl, S.; Pfannhauser, W. Development of an Austrian Carotenoid Database. J. Food Compos. Anal. 2000, 13, 435–440. [Google Scholar] [CrossRef]
- Di Caprio, F.; Altimari, P.; Pagnanelli, F. Sequential extraction of lutein and β-carotene from wet microalgal biomass. J. Chem. Technol. Biotechnol. 2020, 95, 3024–3033. [Google Scholar] [CrossRef]
- Kumar, M.; Mondal, P.; Borah, S.; Mahato, K. Physico-chemical evaluation, preliminary phytochemical investigation, fluo-rescence and TLC analysis of leaves of the plant Lasia spinosa (Lour) Thwaites. Int. J. Pharm. Pharm. Sci. 2013, 5, 306–310. [Google Scholar]
- Öquist, G.; Samuelsson, G. Sequential extraction of chlorophyll from chlorophyll-protein complexes in lyophilized pea thylakoids with solvents of different polarity. Physiol. Plant. 1980, 50, 57–62. [Google Scholar] [CrossRef]
- Laguerre, M.; Giraldo, L.J.L.; Piombo, G.; Figueroa-Espinoza, M.C.; Pina, M.; Benaissa, M.; Combe, A.; Castera, A.R.; Lecomte, J.; Villeneuve, P. Characterization of Olive-Leaf Phenolics by ESI-MS and Evaluation of their Antioxidant Capacities by the CAT Assay. J. Am. Oil Chem. Soc. 2009, 86, 1215–1225. [Google Scholar] [CrossRef]
- Barapatre, A.; Meena, A.; Mekala, S.; Das, A.; Jha, H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int. J. Biol. Macromol. 2016, 86, 443–453. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Žlabur, J.; Voća, S.; Dobričević, N.; Pliestić, S.; Galić, A.; Boričević, A.; Borić, N. Ultrasound-assisted extraction of bioactive compounds from lemon balm and peppermint leaves. Int. Agrophysics 2016, 30, 95–104. [Google Scholar] [CrossRef]
- Zaghdoudi, K.; Pontvianne, S.; Framboisier, X.; Achard, M.; Kudaibergenova, R.; Ayadi-Trabelsi, M.; Kalthoum-Cherif, J.; Vanderesse, R.; Frochot, C.; Guiavarc’H, Y. Accelerated solvent extraction of carotenoids from: Tunisian Kaki (Diospyros kaki L.), peach (Prunus persica L.) and apricot (Prunus armeniaca L.). Food Chem. 2015, 184, 131–139. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.; Suarez-Álvarez, S.; Ferragut, J.; Micol, V.; Cifuentes, A.; Ibáñez, E.; Garcia-Cañas, V. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafeuille, J.-L.; Lefèvre, S.; Lebuhotel, J. Quantitation of Chlorophylls and 22 of Their Colored Degradation Products in Culinary Aromatic Herbs by HPLC-DAD-MS and Correlation with Color Changes During the Dehydration Process. J. Agric. Food Chem. 2014, 62, 1926–1935. [Google Scholar] [CrossRef]
- Almela, L.; Fernández-López, J.A.; Roca, M.J. High-performance liquid chromatographic screening of chlorophyll derivatives produced during fruit storage. J. Chromatogr. A 2000, 870, 483–489. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Munekata, P.E.S.; Alcántara, C.; Žugčić, T.; Abdelkebir, R.; Collado, M.C.; García-Pérez, J.V.; Jambrak, A.R.; Gavahian, M.; Barba, F.J.; Lorenzo, J.M. Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary. Food Res. Int. 2020, 134, 109242. [Google Scholar] [CrossRef] [PubMed]
- Miri, S.M.; Ahmadi, S.; Moradi, P. Influence of Salicylic Acid and Citric Acid on the Growth, Biochemical Characteristics and Essential Oil Content of Thyme (Thymus vulgaris L.). J. Med. Plants Prod. 2015, 4, 141–146. [Google Scholar] [CrossRef]
- Karalija, E.; Parić, A. The effect of BA and IBA on the secondary metabolite production by shoot culture of Thymus vulgaris L. Biol. Nyssana 2011, 2, 29–35. [Google Scholar]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.-L.; Neilan, B.A.; Scheer, H. A Red-Shifted Chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Šircelj, H.; Mikulic-Petkovsek, M.; Veberic, R.; Hudina, M.; Slatnar, A. Lipophilic antioxidants in edible weeds from agricultural areas. Turk. J. Agric. For. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Hadif, W.M.; Rahim, S.A.; Sahid, I.; Rahman, A.; Ibrahima, I. Influence of chromium metal on chlorophyll content in leaves of paddy Oryza sativa L. Int. J. Chem. Sci. 2015, 13, 1238–1252. [Google Scholar]
- Pavlovic, D.; Nikolic, B.; Djurovic, S.; Waisi, H.; Anđelković, A.; Marisavljevic, D. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Fitomed. 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Pumilia, G.; Cichon, M.J.; Cooperstone, J.L.; Giuffrida, D.; Dugo, G.; Schwartz, S.J. Changes in chlorophylls, chlorophyll degradation products and lutein in pistachio kernels (Pistacia vera L.) during roasting. Food Res. Int. 2014, 65, 193–198. [Google Scholar] [CrossRef]
- Pareek, S.; Sagar, N.A.; Sharma, S.; Kumar, V.; Agarwal, T.; González-Aguilar, G.A.; Yahia, E.M. Chlorophylls: Chemistry and Biological Functions. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 269–284. [Google Scholar] [CrossRef]
- Takamiya, K.-I.; Tsuchiya, T.; Ohta, H. Degradation pathway(s) of chlorophyll: What has gene cloning revealed? Trends Plant Sci. 2000, 5, 426–431. [Google Scholar] [CrossRef]
- Teng, S. Formation of pyrochlorophylls and their derivatives in spinach leaves during heating. Food Chem. 1999, 65, 367–373. [Google Scholar] [CrossRef]
- Yilmaz, C.; Gökmen, V. Chlorophyll. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2015; pp. 37–41. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chao, P.-Y.; Hu, S.-P.; Yang, C.-M. The Antioxidant and Free Radical Scavenging Activities of Chlorophylls and Pheophytins. Food Nutr. Sci. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Oshima, H.; Ueno, E.; Saito, I.; Matsumoto, H. Development of a Solid-Phase Extraction Method for Determination of Pheophorbide a and Pyropheophorbide a in Health Foods by Liquid Chromatography. J. AOAC Int. 2004, 87, 937–942. [Google Scholar] [CrossRef] [Green Version]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.-G.; Lee, D.-U.; Pan, C.-H. Optimization of Pressurized Liquid Extraction of Carotenoids and Chlorophylls fromChlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kundu, A.; Sharma, K.; Paul, R. Optimal extraction and fingerprinting of carotenoids by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem. 2015, 177, 369–375. [Google Scholar] [CrossRef]
- Lazzerini, C.; Cifelli, M.; Domenici, V. Pigments in extra virgin olive oils produced in different mediterranean countries in 2014: Near UV-vis spectroscopy versus HPLC-DAD. Food Sci. Technol. 2017, 84, 586–594. [Google Scholar] [CrossRef]
- Pniewski, F. HPLC separation of cyanobacterial and algal photosynthetic pigments. Biologia 2020, 75, 223–233. [Google Scholar] [CrossRef]
- Maatallah, S.; Nasri, N.; Hajlaoui, H.; Albouchi, A.; Elaissi, A. Evaluation changing of essential oil of laurel (Laurus nobilis L.) under water deficit stress conditions. Ind. Crop. Prod. 2016, 91, 170–178. [Google Scholar] [CrossRef]
- Jacobsen, T.R.; Rai, H. Comparison of Spectrophotometric, Fluorometric and High Performance Liquid Chromatography Methods for Determination of Chlorophylla in Aquatic Samples: Effects of Solvent and Extraction Procedures. Int. Rev. Hydrobiol. 1990, 75, 207–217. [Google Scholar] [CrossRef]
- Pinckney, J.; Papa, R.; Zingmark, R. Comparison of high-performance liquid chromatographic, spectrophotometric, and fluorometric methods for determining chlorophyll a concentrations in estaurine sediments. J. Microbiol. Methods 1994, 19, 59–66. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Kruk, V.; Pedisić, S.; Çınar, F.; Kovačević, D.B.; Žutić, I.; Dragović-Uzelac, V. Accelerated Solvent Extraction as a Green Tool for the Recovery of Polyphenols and Pigments from Wild Nettle Leaves. Processes 2020, 8, 803. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Avalo, B.; Cifuentes, A.; Reglero, G.; Reina, G.G.-B.; Señoráns, F.J.; Ibanez, E. Comprehensive characterization of the functional activities of pressurized liquid and ultrasound-assisted extracts from Chlorella vulgaris. Food Sci. Technol. 2012, 46, 245–253. [Google Scholar] [CrossRef]
- Samarin, A.M.; Poorazarang, H.; Hematyar, N.; Elhamirad, A. Phenolics in potato peels: Extraction and utilization as natural antioxidants. World Appl. Sci. J. 2012, 18, 191–195. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Gerola, A.P.; Tsubone, T.M.; Santana, A.; de Oliveira, H.P.M.; Hioka, N.; Caetano, W. Properties of Chlorophyll and Derivatives in Homogeneous and Microheterogeneous Systems. J. Phys. Chem. B 2011, 115, 7364–7373. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; De Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2015, 53, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Gião, M.S.; González-SanJosé, M.L.; Rivero-Pérez, M.D.; I Pereira, C.; Pintado, M.M.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Amensour, M.; Sendra, E.; Abrini, J.; Bouhdid, S.; Pérez-Alvarez, J.A.; Fernandez-Lopez, J. Total Phenolic Content and Antioxidant Activity of Myrtle (Myrtus communis) Extracts. Nat. Prod. Commun. 2009, 4, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Gardeli, C.; Vassiliki, P.; Athanasios, M.; Kibouris, T.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Dorman, H.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M.F. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2015, 197, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ou, X.; Zhang, X.; Zhou, Z.; Ma, L. Effect of Different Solvents on the Measurement of Phenolics and the Antioxidant Activity of Mulberry (Morus atropurpurea Roxb.) with Accelerated Solvent Extraction. J. Food Sci. 2017, 82, 605–612. [Google Scholar] [CrossRef]
- Rajha, H.N.; Ziegler, W.; Louka, N.; Hobaika, Z.; Vorobiev, E.; Boechzelt, H.G.; Maroun, R.G. Effect of the Drying Process on the Intensification of Phenolic Compounds Recovery from Grape Pomace Using Accelerated Solvent Extraction. Int. J. Mol. Sci. 2014, 15, 18640–18658. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Rabalski, I.; Bryan, M. Accelerated, Microwave-Assisted, and Conventional Solvent Extraction Methods Affect Anthocyanin Composition from Colored Grains. J. Food Sci. 2014, 79, C138–C146. [Google Scholar] [CrossRef]
- Oszmiański, J.; Nowicka, P.; Teleszko, M.; Wojdyło, A.; Cebulak, T.; Oklejewicz, K. Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruits. Int. J. Mol. Sci. 2015, 16, 14540–14553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gololo, S.S.; Mogale, M.A.; Agyei, N.M.; Shai, L.J. Phytochemical, Antioxidant and Antibacterial Screening of the Leaves of Barleria dinteri (Oberm), Grewia flava (DC) and Jatropha lagarinthoides (Sond). J. Chem. Pharm. Res. 2016, 8, 56–60. [Google Scholar]
- Thavamoney, N.; Sivanadian, L.; Tee, L.H.; Khoo, H.E.; Prasad, K.N.; Kong, K.W. Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents. J. Food Sci. Technol. 2018, 55, 2523–2532. [Google Scholar] [CrossRef]
- Nikousaleh, A.; Prakash, J. Antioxidant components and properties of dry heat treated clove in different extraction solvents. J. Food Sci. Technol. 2015, 53, 1993–2000. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, S.F.; Ooi, K.L. Supriatno Antioxidant and α-Glucosidase Inhibitory Activities of Cucurbit Fruit Vegetables and Identification of Active and Major Constituents from Phenolic-rich Extracts of Lagenaria siceraria and Sechium edule. J. Agric. Food Chem. 2013, 61, 10080–10090. [Google Scholar] [CrossRef] [PubMed]
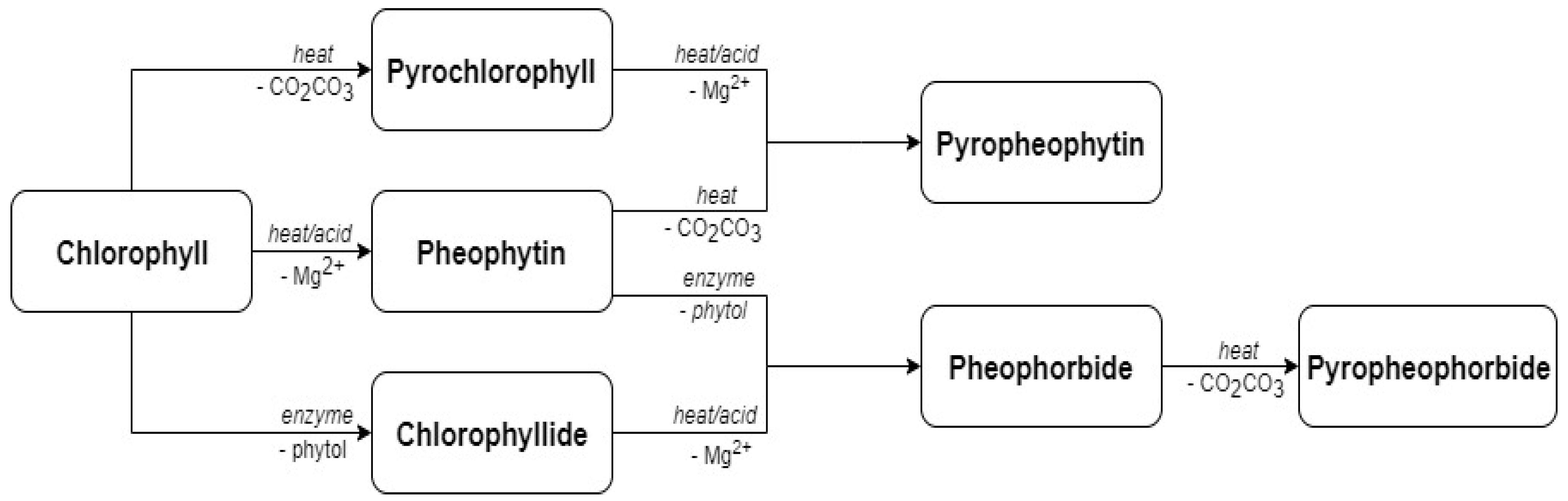
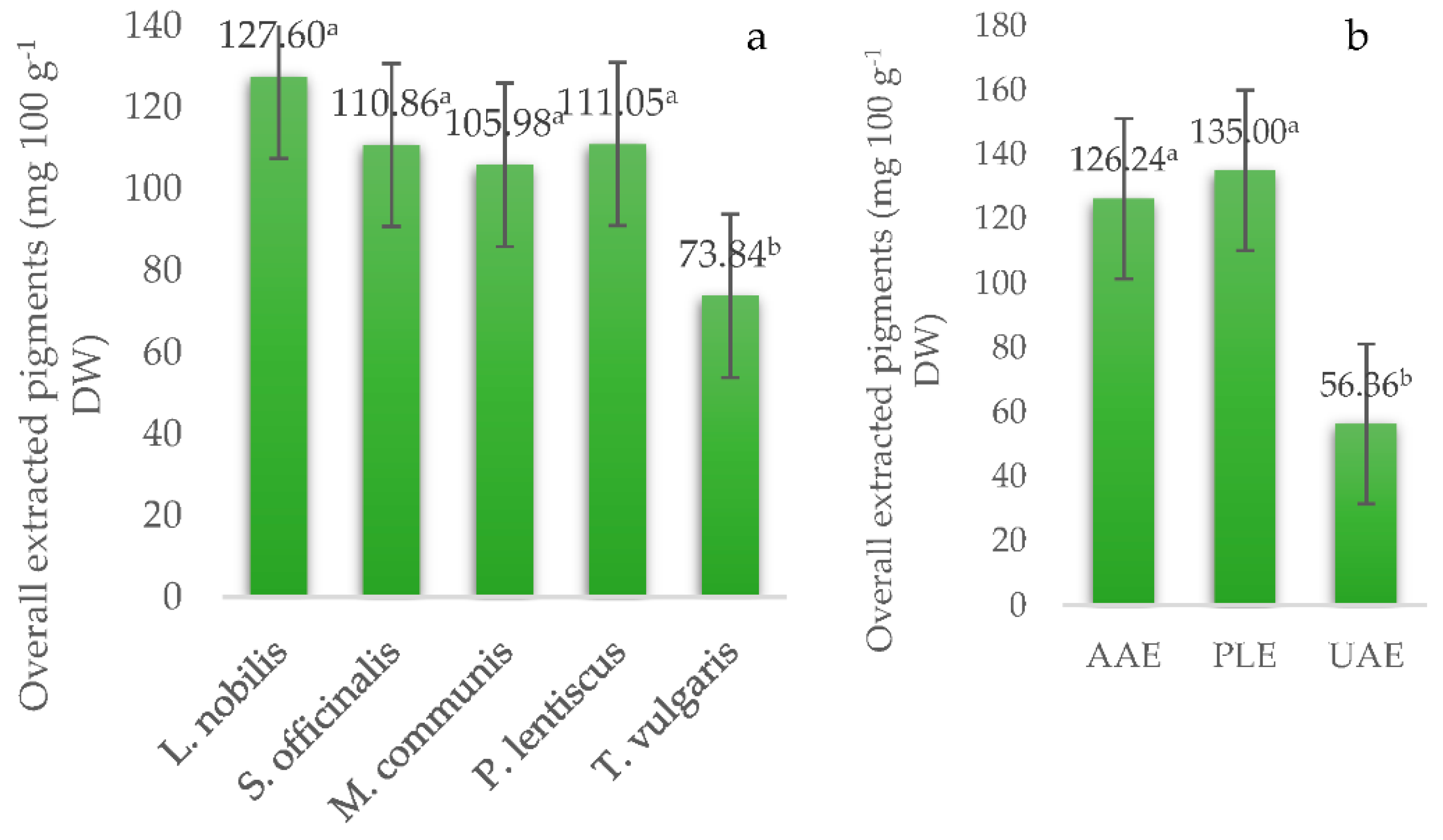
| Source of Variation | Lutein | Zeaxanthin | 9-cis Lutein | β-Carotene | Total Carotenoids |
|---|---|---|---|---|---|
| Herb species | p < 0.01 | p = 0.03 | p = 0.13 | p < 0.01 | p < 0.01 |
| L. nobilis | 6.84 ± 1.11 ab | 0.43 ± 0.13 ab | 0.58 ± 0.18 a | 1.83 ± 0.41 bc | 9.68 ± 1.61 ab |
| S. officinalis | 5.85 ± 1.11 ab | 0.36 ± 0.13 ab | 0.24 ± 0.18 a | 2.99 ± 0.41 ab | 9.43 ± 1.61 ab |
| M. communis | 9.32 ± 1.11 a | 0.65 ± 0.13 a | 0.69 ± 0.18 a | 3.58 ± 0.41 a | 14.24 ± 1.61 a |
| P. lentiscus | 6.63 ± 1.11 ab | 0.47 ± 0.13 ab | 0.40 ± 0.18 a | 1.76 ± 0.41 bc | 9.26 ± 1.61 ab |
| T. vulgaris | 4.25 ± 1.11 b | 0.20 ± 0.13 b | 0.33 ± 0.18 a | 1.29 ± 0.41 c | 6.02 ± 1.61 b |
| Method | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| AAE | 7.32 ± 0.86 a | 0.47 ± 0.10 a | 0.61 ± 0.14 a | 2.93 ± 0.31 a | 11.33 ± 1.25 a |
| PLE | 8.97 ± 0.86 a | 0.59 ± 0.10 a | 0.58 ± 0.14 a | 3.13 ± 0.31 a | 13.27 ± 1.25 a |
| UAE | 3.45 ± 0.86 b | 0.21 ± 0.10 b | 0.15 ± 0.14 b | 0.78 ± 0.31 b | 4.58 ± 1.25 b |
| Solvent | p < 0.01 | p = 0.04 | p < 0.01 | p < 0.01 | p < 0.01 |
| Hexane | 6.81 ± 0.86 a | 0.42 ± 0.10 ab | 0.27 ± 0.14 b | 5.94 ± 0.31 a | 13.45 ± 1.25 a |
| Acetone-80% | 8.81 ± 0.86 a | 0.56 ± 0.10 a | 1.01 ± 0.14 a | 0.59 ± 0.31 b | 10.97 ± 1.25 a |
| Ethanol-96% | 4.11 ± 0.86 b | 0.29 ± 0.10 b | 0.06 ± 0.14 b | 0.31 ± 0.31 b | 4.76 ± 1.25 b |
| Grand mean | 6.58 | 0.42 | 0.45 | 2.28 | 9.73 |
| Source of Variation | Chl b | Chl b’ | Piro b | Chl a | Chl a’ | Piro a | Phe b | Phe b’ | Phe a | Phe a’ | Total Chlorophyll |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Herb species | p = 0.12 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p = 0.02 | p = 0.04 |
| L. nobilis | 4.72 ± 1.52 a | 0.36 ± 0.22 ab | 0.11 ± 0.19 bc | 3.11 ± 1.34 ab | 0.04 ± 0.11 b | 0.22 ± 0.04 a | 10.47 ± 1.36 a | 8.63 ± 1.18 a | 4.22 ± 1.25 c | 0.97 ± 0.31 ab | 32.86 ± 4.45 a |
| S. officinalis | 4.99 ± 1.52 a | 0.95 ± 0.22 a | 0.50 ± 0.19 ab | 4.77 ± 1.34 a | 0.44 ± 0.11 a | 0.00 ± 0.04 b | 4.70 ± 1.36 bc | 4.45 ± 1.18 bc | 5.77 ± 1.25 bc | 0.96 ± 0.31 ab | 27.52 ± 4.45 ab |
| M. communis | 3.22 ± 1.52 a | 0.00 ± 0.22 b | 0.00 ± 0.19 c | 0.22 ± 1.34 b | 0.00 ± 0.11 b | 0.00 ± 0.04 b | 1.15 ± 1.36 c | 2.66 ± 1.18 c | 12.04 ± 1.25 a | 1.80 ± 0.31 a | 21.09 ± 4.45 ab |
| P. lentiscus | 2.31 ± 1.52 a | 0.34 ± 0.22 ab | 0.00 ± 0.19 c | 0.51 ± 1.34 b | 0.04 ± 0.11 b | 0.09 ± 0.04 ab | 6.97 ± 1.36 ab | 7.48 ± 1.18 ab | 8.87 ± 1.25 ab | 1.15 ± 0.31 ab | 27.75 ± 4.45 ab |
| T. vulgaris | 4.51 ± 1.52 a | 0.44 ± 0.22 ab | 0.66 ± 0.19 a | 4.78 ± 1.34 a | 0.11 ± 0.11 b | 0.00 ± 0.04 b | 1.48 ± 1.36 c | 2.36 ± 1.18 c | 3.68 ± 1.25 c | 0.58 ± 0.31 b | 18.60 ± 4.45 b |
| Method | p = 0.01 | p = 0.44 | p = 0.20 | p = 0.03 | p = 0.06 | p = 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p = 0.00 |
| AAE | 5.33 ± 1.18 a | 0.52 ± 0.17 a | 0.45 ± 0.15 a | 4.21 ± 1.04 a | 0.25 ± 0.09 a | 0.11 ± 0.03 a | 5.40 ± 1.05 ab | 4.69 ± 0.91 b | 8.61 ± 0.97 a | 1.17 ± 0.24 ab | 30.75 ± 3.44 a |
| PLE | 4.01 ± 1.18 ab | 0.44 ± 0.17 a | 0.26 ± 0.15 ab | 1.57 ± 1.04 b | 0.04 ± 0.09 a | 0.07 ± 0.03 ab | 6.53 ± 1.05 a | 7.84 ± 0.91 a | 9.46 ± 0.97 a | 1.52 ± 0.24 a | 31.73 ± 3.44 a |
| UAE | 2.51 ± 1.18 b | 0.29 ± 0.17 a | 0.06 ± 0.15 b | 2.26 ± 1.04 ab | 0.10 ± 0.09 a | 0.00 ± 0.03 b | 2.93 ± 1.05 b | 2.82 ± 0.91 b | 2.67 ± 0.97 b | 0.58 ± 0.24 b | 14.21 ± 3.44 b |
| Solvent | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p = 0.12 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| Hexane | 0.05 ± 1.18 c | 0.00 ± 0.17 b | 0.00 ± 0.15 c | 0.15 ± 1.04 b | 0.05 ± 0.09 a | 0.15 ± 0.03 a | 9.31 ± 1.05 a | 8.20 ± 0.91 a | 8.80 ± 0.97 a | 1.63 ± 0.24 a | 28.34 ± 3.44 a |
| Acetone-80% | 7.45 ± 1.18 a | 0.60 ± 0.17 ab | 0.52 ± 0.15 a | 4.24 ± 1.04 a | 0.10 ± 0.09 a | 0.04 ± 0.03 b | 3.07 ± 1.05 b | 4.48 ± 0.91 b | 8.39 ± 0.97 a | 1.15 ± 0.24 a | 30.03 ± 3.44 a |
| Ethanol-96% | 4.35 ± 1.18 b | 0.65 ± 0.17 a | 0.25 ± 0.15 ab | 3.64 ± 1.04 a | 0.23 ± 0.09 a | 0.00 ± 0.03 b | 2.49 ± 1.05 b | 2.66 ± 0.91 b | 3.56 ± 0.97 b | 0.49 ± 0.24 b | 18.31 ± 3.44 b |
| Grand mean | 3.95 | 0.42 | 0.26 | 2.68 | 0.13 | 0.06 | 4.95 | 5.12 | 6.92 | 1.09 | 25.56 |
| Source of Variation | Car | Chl a | Chl b | Total Chl | FRAP |
|---|---|---|---|---|---|
| Herb species | p = 0.01 | p = 0.04 | p = 0.20 | p = 0.07 | p < 0.01 |
| L. nobilis | 8.56 ± 0.65 a | 24.28 ± 2.34 a | 10.07 ± 1.23 a | 34.35 ± 3.34 a | 8.29 ± 3.98 c |
| S. officinalis | 7.40 ± 0.65 ab | 16.76 ± 2.34 ab | 9.29 ± 1.23 a | 26.05 ± 3.34 a | 12.28 ± 3.98 b |
| M. communis | 7.40 ± 0.65 ab | 14.38 ± 2.34 ab | 6.31 ± 1.23 a | 20.70 ± 3.34 a | 31.37 ± 3.98 a |
| P. lentiscus | 5.64 ± 0.65 b | 16.27 ± 2.34 ab | 6.72 ± 1.23 a | 22.98 ± 3.34 a | 29.06 ± 3.98 ab |
| T. vulgaris | 5.32 ± 0.65 b | 13.89 ± 2.34 b | 8.10 ± 1.23 a | 21.99 ± 3.34 a | 4.38 ± 3.98 c |
| Method | p < 0.01 | p = 0.01 | p = 0.15 | p = 0.02 | p = 0.23 |
| AAE | 7.39 ± 0.50 a | 19.22 ± 1.81 a | 7.91 ± 0.95 a | 27.13 ± 2.59 ab | 17.06 ± 3.08 a |
| PLE | 8.65 ± 0.50 a | 20.26 ± 1.81 a | 9.57 ± 0.95 a | 29.83 ± 2.59 a | 21.02 ± 3.08 a |
| UAE | 4.55 ± 0.50 b | 11.87 ± 1.81 b | 6.81 ± 0.95 a | 18.68 ± 2.59 b | 13.16 ± 3.08 a |
| Solvent | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| Hexane | 6.49 ± 0.50 b | 14.97 ± 1.81 b | 1.47 ± 0.95 c | 16.44 ± 2.59 b | 0.77 ± 3.08 c |
| Acetone-80% | 10.20 ± 0.50 a | 24.27 ± 1.81 a | 14.88 ± 0.95 a | 39.14 ± 2.59 a | 37.00 ± 3.08 a |
| Ethanol-96% | 3.89 ± 0.50 c | 12.11 ± 1.81 b | 7.95 ± 0.95 b | 20.07 ± 2.59 b | 13.46 ± 3.08 b |
| Grand mean | 6.86 | 17.12 | 8.10 | 25.21 | 17.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvitković, D.; Lisica, P.; Zorić, Z.; Repajić, M.; Pedisić, S.; Dragović-Uzelac, V.; Balbino, S. Composition and Antioxidant Properties of Pigments of Mediterranean Herbs and Spices as Affected by Different Extraction Methods. Foods 2021, 10, 2477. https://doi.org/10.3390/foods10102477
Cvitković D, Lisica P, Zorić Z, Repajić M, Pedisić S, Dragović-Uzelac V, Balbino S. Composition and Antioxidant Properties of Pigments of Mediterranean Herbs and Spices as Affected by Different Extraction Methods. Foods. 2021; 10(10):2477. https://doi.org/10.3390/foods10102477
Chicago/Turabian StyleCvitković, Daniela, Patricija Lisica, Zoran Zorić, Maja Repajić, Sandra Pedisić, Verica Dragović-Uzelac, and Sandra Balbino. 2021. "Composition and Antioxidant Properties of Pigments of Mediterranean Herbs and Spices as Affected by Different Extraction Methods" Foods 10, no. 10: 2477. https://doi.org/10.3390/foods10102477







