Polysaccharide from Patinopecten yessoensis Skirt Boosts Immune Response via Modulation of Gut Microbiota and Short-Chain Fatty Acids Metabolism in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PS
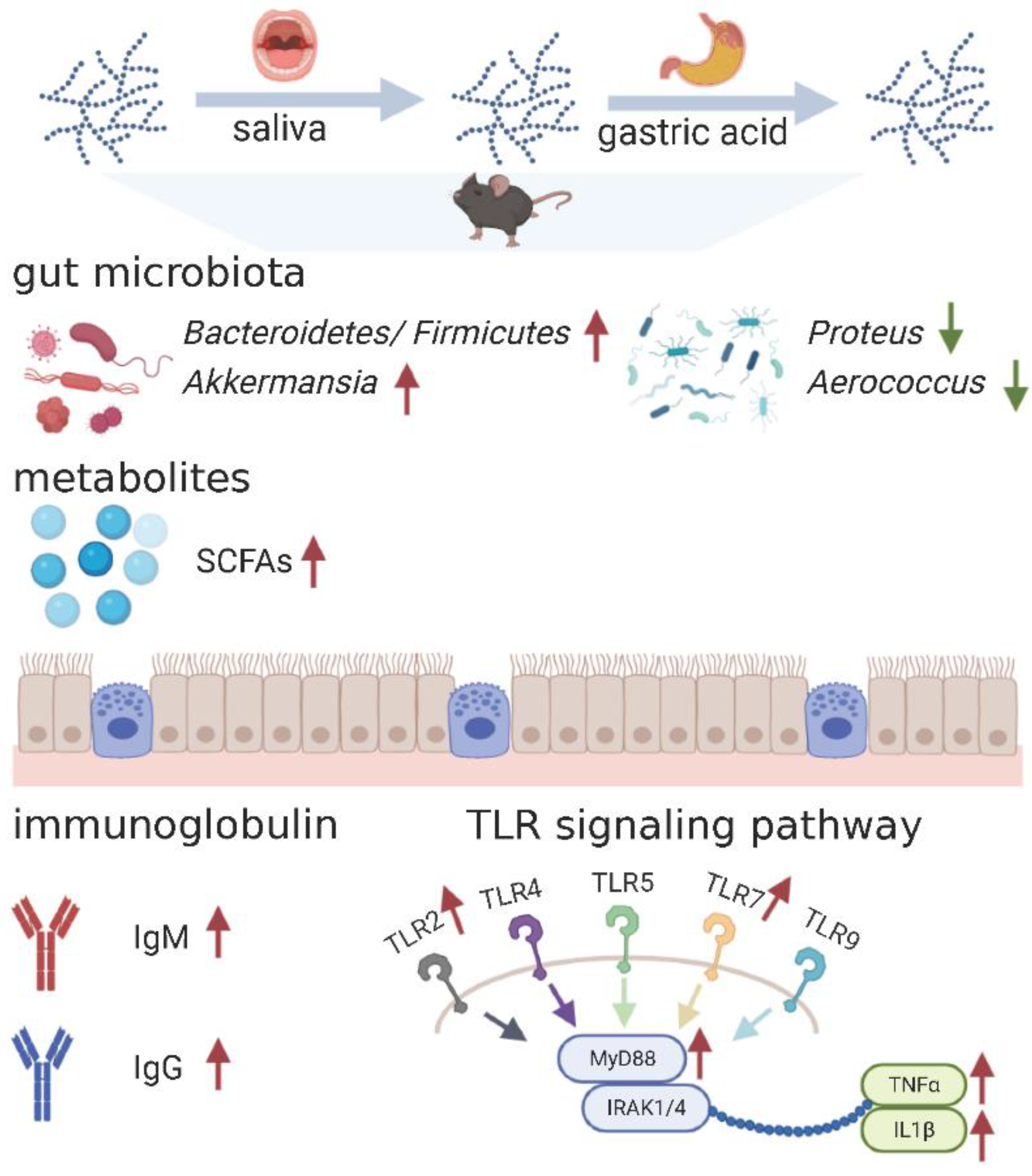
2.2. Digestibility of PS by Artificial Human Saliva and Gastric Acid
2.3. Animal Experiment
2.4. Sample Collection
2.5. Immune Index
2.6. Gut Microbiota Analysis
2.7. Determination of SCFA Contents
2.8. Intestinal Immune Related Gene Expression
2.9. Statistical Analyses
3. Results
3.1. Digestibility of PS
3.2. Effect of PS on the Immune Indexes
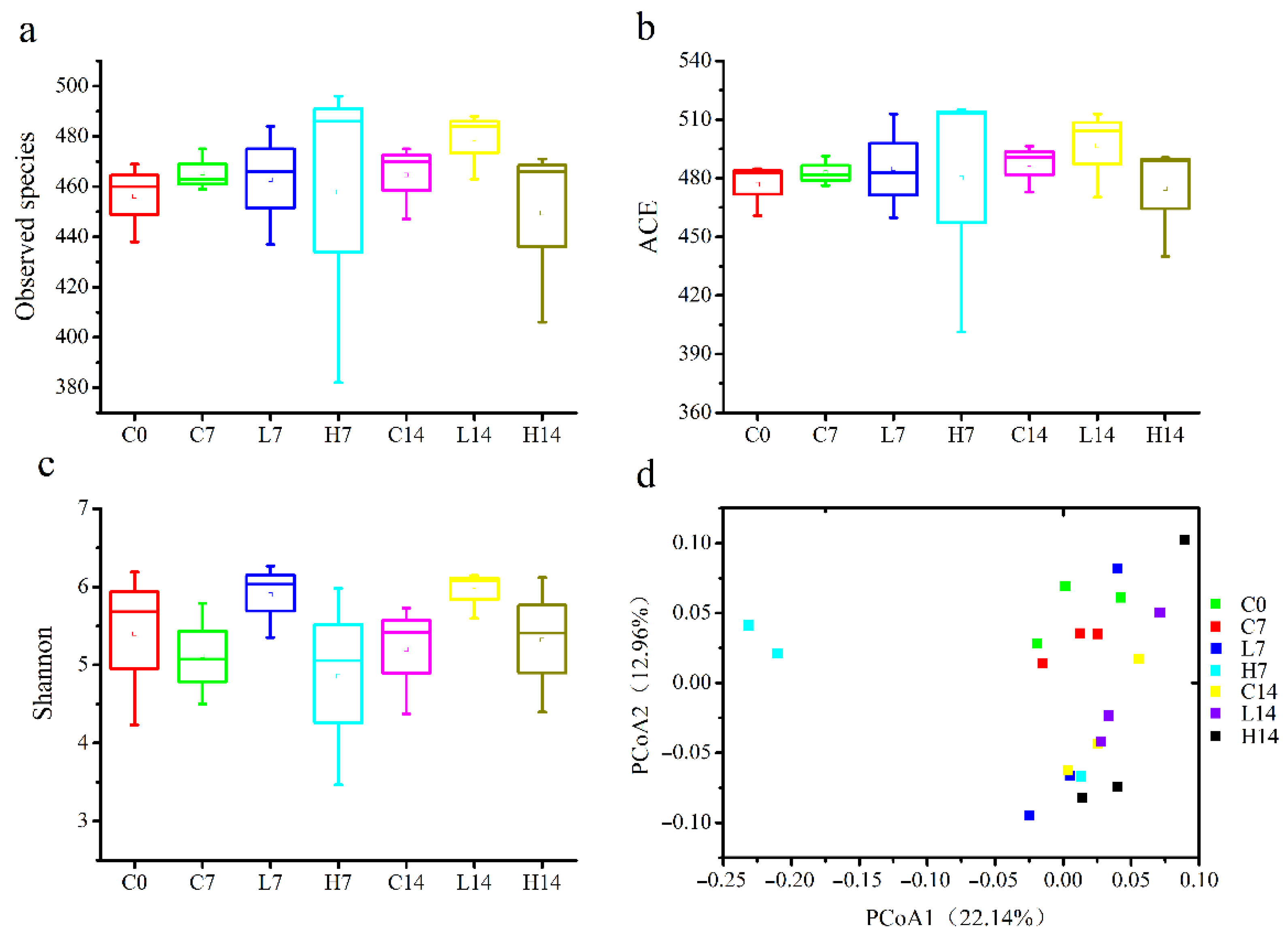
3.3. Microbial Community Diversity in the Mice Gut
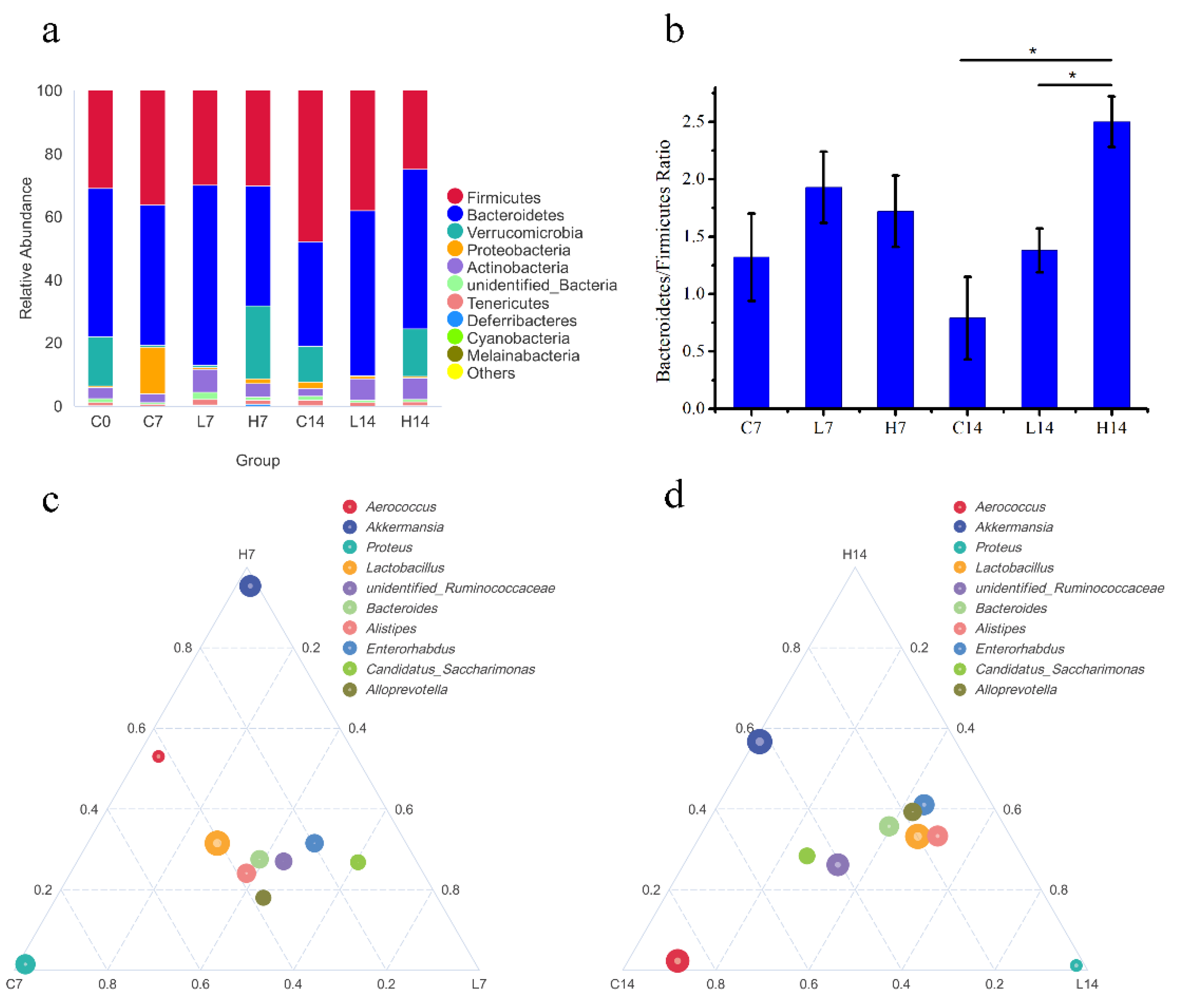
3.4. Changes in Gut Microbiota
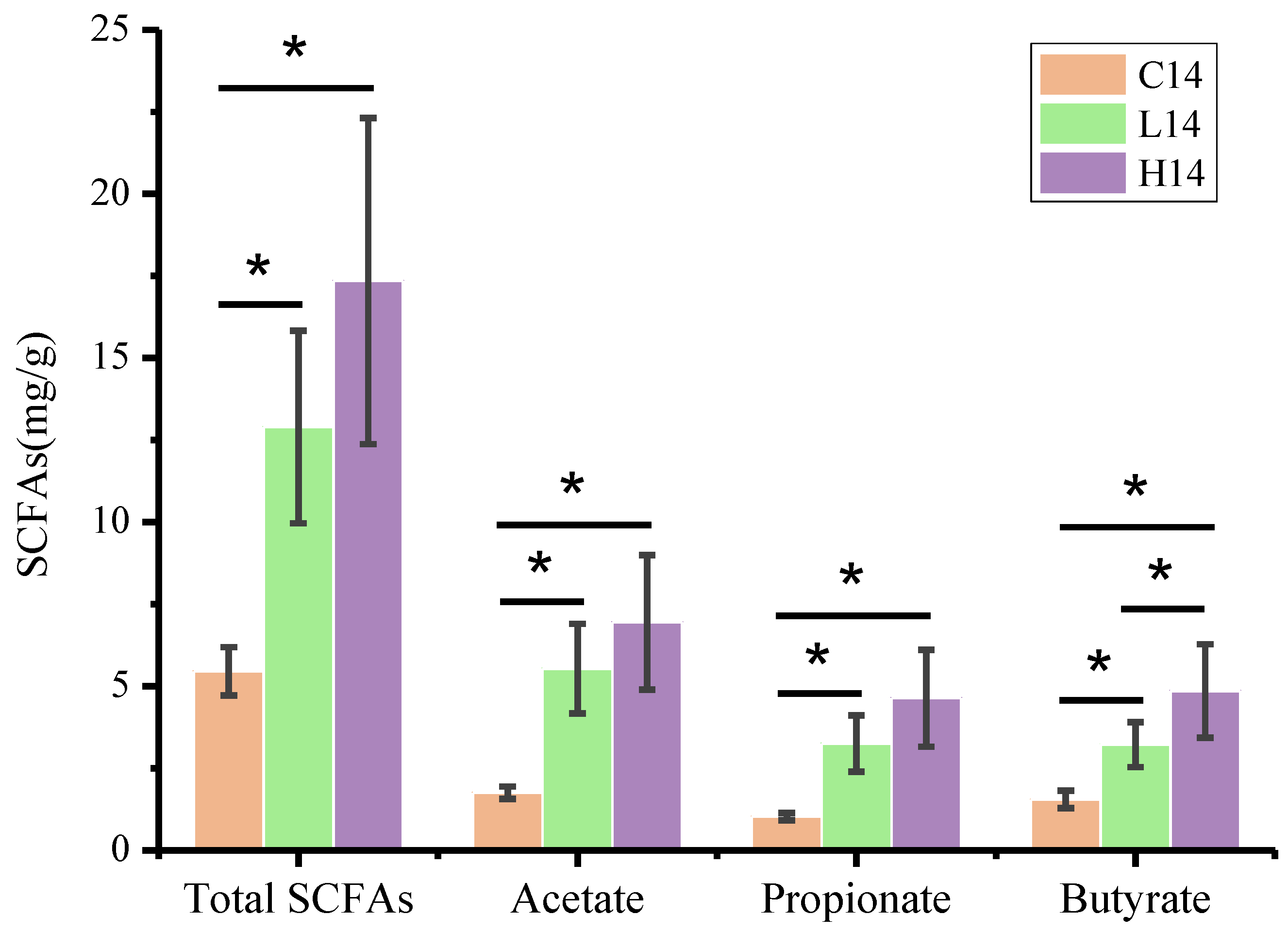
3.5. SCFA Content
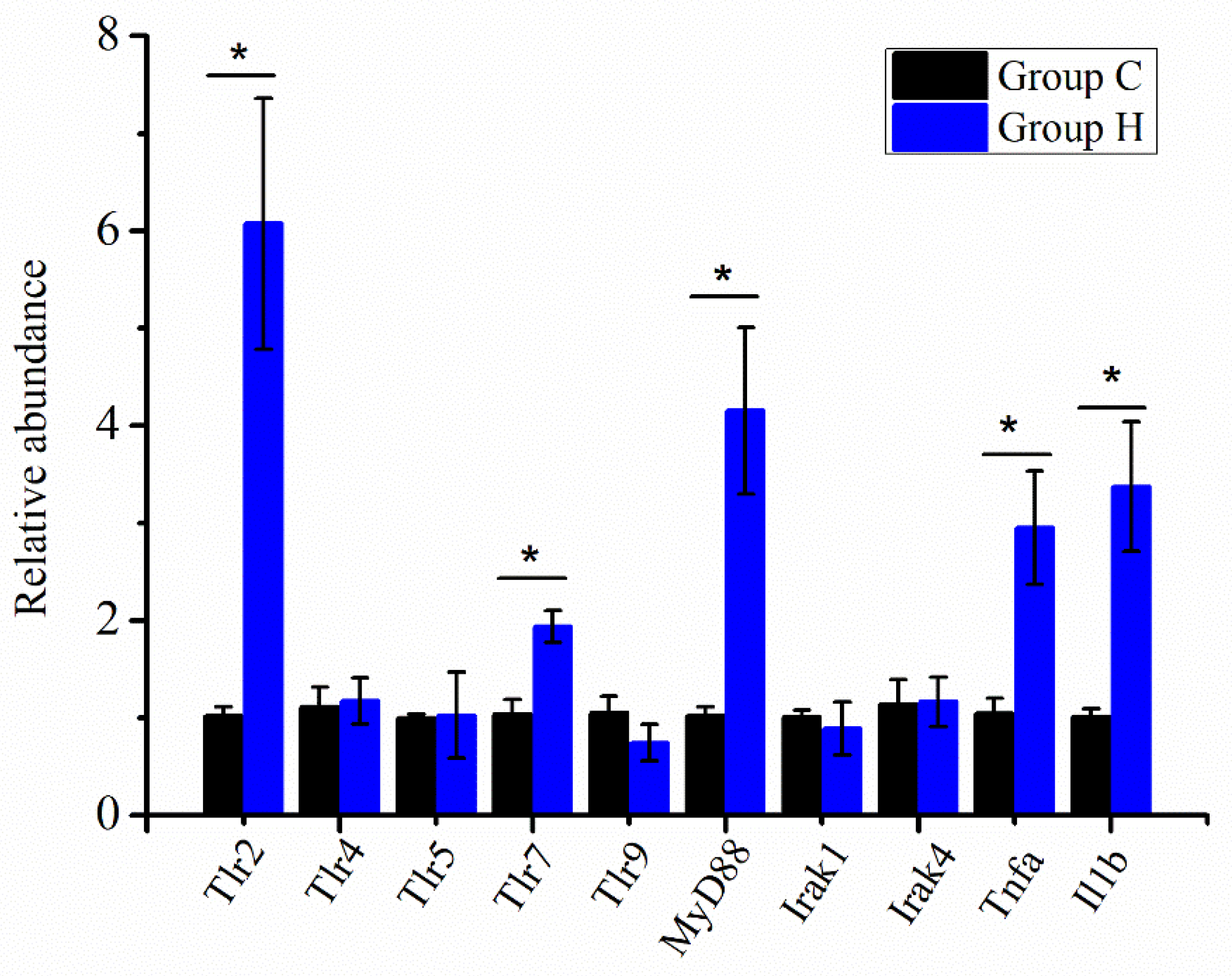
3.6. Immune-Related Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Steinach, M.; Gunga, H.-C. Circadian Rhythm and Stress. In Stress Challenges and Immunity in Space: From Mechanisms to Monitoring and Preventive Strategies; Chouker, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 87–106. [Google Scholar]
- Pansai, N.; Chakree, K.; Takahashi Yupanqui, C.; Raungrut, P.; Yanyiam, N.; Wichienchot, S. Gut microbiota modulation and immune boosting properties of prebiotic dragon fruit oligosaccharides. Int. J. Food Sci. Technol. 2020, 55, 55–64. [Google Scholar] [CrossRef]
- Yu, Z.; Song, G.; Liu, J.; Wang, J.; Zhang, P.; Chen, K. Beneficial effects of extracellular polysaccharide from Rhizopus nigricans on the intestinal immunity of colorectal cancer mice. Int. J. Biol. Macromol. 2018, 115, 718–726. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Mao, G.; Yan, L.; Hu, Y.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. Effect of the sulfation pattern of sea cucumber-derived fucoidan oligosaccharides on modulating metabolic syndromes and gut microbiota dysbiosis caused by HFD in mice. J. Funct. Foods 2019, 55, 193–210. [Google Scholar] [CrossRef]
- Ying, N.; Lin, Q.; Luo, F. Effects of Non-Starch Polysaccharides on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 1372. [Google Scholar]
- Huo, W.; Feng, Z.; Hu, S.; Cui, L.; Qiao, T.; Dai, L.; Qi, P.; Zhang, L.; Liu, Y.; Li, J. Effects of polysaccharides from wild morels on immune response and gut microbiota composition in non-treated and cyclophosphamide-treated mice. Food Funct. 2020, 11, 4291–4303. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Zhi, Z.; Hu, Y.; Ge, J.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. 4-O-Sulfation in sea cucumber fucodians contribute to reversing dyslipidiaemia caused by HFD. Int. J. Biol. Macromol. 2017, 99, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, B.K.; Chen, Y.F.; Su, L.; Wang, J.; Li, S.H.; Wang, Q.B.; Li, S.P.; Chen, M.S.; Lin, X.J. Antitumour activity of Lycium chinensis polysaccharides in liver cancer rats. Int. J. Biol. Macromol. 2012, 51, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Han, Y.; Ding, Y.; Zhu, B.; Song, S.; Xiao, H. Health effects of dietary sulfated polysaccharides from seafoods and their interaction with gut microbiota. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2882–2913. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Ai, C.; Wen, C.; Dong, X.; Sun, X.; Cao, C.; Zhang, X.; Zhu, B.; Song, S. Gut microbiota response to sulfated sea cucumber polysaccharides in a differential manner using an in vitro fermentation model. Food Res. Int. 2021, 148, 110562. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, G.; Cao, H.; Yu, D.; Fang, X.; de Vos, W.M.; Wu, H. Gut dysbacteriosis and intestinal disease: Mechanism and treatment. J. Appl. Microbiol. 2020, 129, 787–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Ma, Y.; Yang, Q.; Deng, S. Modulation of inflammatory response and gut microbiota in ankylosing spondylitis mouse model by bioactive peptide IQW. J. Appl. Microbiol. 2020, 128, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Y.; Jiang, P.; Song, S.; Ai, C. Interaction of sulfated polysaccharides with intestinal Bacteroidales plays an important role in its biological activities. Int. J. Biol. Macromol. 2021, 168, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Modulation of inflammatory and immune responses by short-chain fatty acids. In Diet, Immunity and Inflammation; Calder, P.C., Yaqoob, P., Eds.; Woodhead Publishing: London, UK, 2013; pp. 435–458. [Google Scholar]
- Barton, G.M.; Medzhitov, R. Toll-like receptor signaling pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Seo, Y.; Park, H.-Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, R.; Abdollahi, M. Marine bioactives. In Food Bioactives and Health; Galanakis, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 195–235. [Google Scholar]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Zhao, L.; Cha, Q.; Sun, Y.; Ye, H.; Zeng, X. Structural characterization and immunostimulatory activity of a homogeneous polysaccharide from Sinonovacula constricta. J. Agric. Food Chem. 2015, 63, 7986–7994. [Google Scholar] [CrossRef]
- Du, Z.; Jia, X.; Chen, J.; Zhou, S.; Chen, J.; Liu, X.; Cao, X.; Zhong, S.; Hong, P. Isolation and characterization of a heparin-like compound with potent anticoagulant and fibrinolytic activity from the clam Coelomactra antiquata. Mar. Drugs 2019, 18, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Liu, Y.; Jiang, P.; Xu, Y.; Zheng, W.; Song, S.; Ai, C. Effect of sulfate group on sulfated polysaccharides-induced improvement of metabolic syndrome and gut microbiota dysbiosis in high fat diet-fed mice. Int. J. Biol. Macromol. 2020, 164, 2062–2072. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Z.; Liu, Y.; Lou, Y. Optimization of extraction and purification of polysaccharides from Patinopecten yessoensis. Food Sci. 2017, 38, 208–213. [Google Scholar]
- Song, S.; Wang, L.; Wang, L.; Yu, Q.; Ai, C.; Fu, Y.; Yan, C.; Wen, C.; Zhu, Z. Structural characterization and anticoagulant activity of two polysaccharides from Patinopecten yessoensis viscera. Int. J. Biol. Macromol. 2019, 136, 579–585. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of Pro-Oxidant and Pro-Inflammatory Activities of M1 Macrophages by the Natural Dipeptide Carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, Y.; Song, C.; Hu, Y.; Dai, M.; Liu, B.; Pan, P. Neutrophil extracellular traps augmented alveolar macrophage pyroptosis via AIM2 inflammasome activation in LPS-induced ALI/ARDS. J. Inflamm. Res. 2021, 14, 4839–4858. [Google Scholar] [CrossRef]
- Dong, X.; Kim, Y.S.; Kim, E.K.; Shin, W.B.; Park, J.S.; Kim, S.J.; Go, E.A.; Park, P.J.; Kwon, S.C. Scallop extracts inhibited LPS-induced inflammation by suppressing MAPK and NF-κB activation in RAW264.7 macrophages. Adv. Exp. Med. Biol. 2019, 1155, 1069–1081. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Leng, P.-s.; Hu, Z. Feasible and effective reuse of municipal sludge for vegetation restoration: Physiochemical characteristics and microbial diversity. Sci. Rep. 2019, 9, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Zhou, W.; Wang, H.; Li, C.; Wang, L.; Li, Y.; Wang, J. Bacillus baekryungensis MS1 regulates the growth, non-specific immune parameters and gut microbiota of the sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2020, 102, 133–139. [Google Scholar] [CrossRef]
- Sun, H.; Ni, X.; Song, X.; Wen, B.; Zhou, Y.; Zou, F.; Yang, M.; Peng, Z.; Zhu, H.; Zeng, Y. Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl. Microbiol. Biotechnol. 2016, 100, 8105–8120. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Li, W.; Liu, Y.; Wu, T.; Zhang, M. Regulation of wheat germ polysaccharides in the immune response of mice from newborn to adulthood associated with intestinal microbiota. Food Funct. 2020, 11, 9662–9674. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.; Jian, S.; Bo, Z.; Jin, C.; Liu, J.; Kan, J.; Qian, C.; Zhang, N. Effects of polysaccharides from purple sweet potatoes on immune response and gut microbiota composition in normal and cyclophosphamide treated mice. Food Funct. 2018, 9, 937–950. [Google Scholar]
- Hu, T.; Huang, Q.; Wong, K.; Yang, H. Structure, molecular conformation, and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis sclerotia. Int. J. Biol. Macromol. 2017, 94 Pt A, 423–430. [Google Scholar] [CrossRef]
- Jackson, M.A.; Serena, V.; Maria-Emanuela, M.; Min, S.C.; Jonas, Z.; Bowyer, R.; Tiphaine, M.; Williams, F.; Cristina, M.; Bell, J.T. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yuan, Y.; Cao, J.; Shen, X.; Li, C. Beneficial Effects of Holothuria leucospilota Polysaccharides on Fermentability In Vivo and In Vitro. Foods 2021, 10, 1884. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Umemoto, E.; Fujita, S.; Hayashi, A.; Kikuta, J.; Kimura, I.; Haneda, T.; Imai, T.; Inoue, A.; Mimuro, H. GPR31-dependent dendrite protrusion of intestinal CX3CR1 + cells by bacterial metabolites. Nature 2019, 566, 1. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.B.; Chou, W.-C.; Conner, M.E.; Earl, A.M.; Knight, R.; Bjorkman, P.J.; et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaël, E.; Audebert, C.; Olivera-Ardid, S. The formation of glycan-specific natural antibodies repertoire in GalT-KO mice is determined by gut microbiota. Front. Immunol. 2019, 10, 342. [Google Scholar]
- Fabbiano, S.; Suárez-Zamorano, N.; Chevalier, C.; Lazarević, V.; Kieser, S.; Rigo, D.; Leo, S.; Veyrat-Durebex, C.; Gaïa, N.; Maresca, M.; et al. Functional gut microbiota remodeling contributes to the caloric restriction-induced metabolic improvements. Cell Metab. 2018, 28, 907–921.e7. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, S.; Wang, H.; Xiao, S.; Li, C.; Li, Y.; Liu, B. Xanthophyllomyces dendrorhous-derived astaxanthin regulates lipid metabolism and gut microbiota in obese mice induced by a high-fat diet. Mar. Drugs 2019, 17, 337. [Google Scholar] [CrossRef] [Green Version]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Bioactivity of sulfated polysaccharides from the edible red seaweed Mastocarpus stellatus. Bioact. Carbohydr. Diet. Fibre 2014, 3, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Di, Y.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; van Baarlen, P.; Hooiveld, G.; Norin, E.; Muller, M.; de Vos, W. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila. Front. Microbiol. 2011, 2, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Miyake, T.; Nakashima, K.; Ito, Y.; Tanahashi, C.; Uchigata, Y. Insulin Autoimmune Syndrome Accompanied by Multiple Myeloma. Intern. Med. 2016, 55, 2219–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whary, M.T.; Baumgarth, N.; Fox, J.G.; Barthold, S.W. Biology and diseases of mice. In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 43–149. [Google Scholar]
- Rasmussen, M. Aerococcus: An increasingly acknowledged human pathogen. Clin. Microbiol. Infect. 2016, 22, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pan, X. Bacteria: Proteus. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 486–489. [Google Scholar]
- Narvhus, J.A.; Axelsson, L. Lactic acid bacteria. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 3465–3472. [Google Scholar]
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. 1), S130–S152. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.M.; Li, L.; Li, S.Y.; Yan, Q.; Li, F. Effect of water extract from Berberis heteropoda Schrenk roots on diarrhea-predominant irritable bowel syndrome by adjusting intestinal flora. J. Ethnopharmacol. 2019, 237, 182–191. [Google Scholar] [CrossRef]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]


| Group | Characterization |
|---|---|
| C | Control group, daily gavage of 0.35 mL water |
| L | Low-dose administration group, daily gavage of equivalent to 50 mg polysaccharides/kg body weight |
| H | High-dose administration group, daily gavage of equivalent to 100 mg polysaccharides/kg body weight |
| Gene | Primers (5′–3′) |
|---|---|
| Tlr2 | forward: GACTCTTCACTTAAGCGAGTCT reverse: AACCTGGCCAAGTTAGTATCTC |
| Tlr4 | forward: GCCATCATTATGAGTGCCAATT reverse: AGGGATAAGAACGCTGAGAATT |
| Tlr5 | forward: GCTTCGTGTTTTGGACATAACT reverse: GGTGGATATGTTGTAGAGGGAG |
| Tlr7 | forward: TGTGATGCTGTGTGGTTTGTCTGG reverse: TTTGACCTTTGTGTGCTCCTGGAC |
| Tlr9 | forward: GATCTGCCCAAACTCCACACTCTG reverse: TCTGACAAGTCCACAAAGCGAAGG |
| MyD88 | forward: CGGAACTTTTCGATGCCTTTAT reverse: CACACACAACTTAAGCCGATAG |
| Irak1 | forward: GTTATGTGCCGCTTCTACAAAG reverse: GATGTGAACGAGGTCAGCTAC |
| Irak4 | forward: CTTCGGCGTGGTTCTGTTGGAG reverse: CCGCATCGCTCATCTTCTCATCC |
| Tnfa | forward: ATGTCTCAGCCTCTTCTCATTC reverse: GCTTGTCACTCGAATTTTGAGA |
| Il1b | forward: TCGCAGCAGCACATCAACAAGAG reverse: AGGTCCACGGGAAAGACACAGG |
| Actb | forward: CTACCTCATGAAGATCCTGACC reverse: CACAGCTTCTCTTTGATGTCAC |
| Parameters | C14 | L14 | H14 |
|---|---|---|---|
| Spleen index (mg/g) | 2.77 ± 0.16 a | 2.87 ± 0.16 a,b | 3.33 ± 0.53 b |
| Thymus index (mg/g) | 4.83 ± 0.16 a | 5.06 ± 0.43 a | 5.52 ± 0.80 a |
| IgG (g/L) | 15.82 ± 0.96 a | 19.99 ± 1.18 b | 25.86 ± 1.40 c |
| IgM (g/L) | 2.24 ± 0.12 a | 2.86 ± 0.28 b | 3.72 ± 0.09 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qin, J.; Cheng, Y.; Ai, Y.; Han, Z.; Li, M.; Qi, Y.; Zhao, Q.; Li, Z. Polysaccharide from Patinopecten yessoensis Skirt Boosts Immune Response via Modulation of Gut Microbiota and Short-Chain Fatty Acids Metabolism in Mice. Foods 2021, 10, 2478. https://doi.org/10.3390/foods10102478
Li Y, Qin J, Cheng Y, Ai Y, Han Z, Li M, Qi Y, Zhao Q, Li Z. Polysaccharide from Patinopecten yessoensis Skirt Boosts Immune Response via Modulation of Gut Microbiota and Short-Chain Fatty Acids Metabolism in Mice. Foods. 2021; 10(10):2478. https://doi.org/10.3390/foods10102478
Chicago/Turabian StyleLi, Ying, Juan Qin, Yinghui Cheng, Yuqing Ai, Zhiyi Han, Meng Li, Yanxia Qi, Qiancheng Zhao, and Zhibo Li. 2021. "Polysaccharide from Patinopecten yessoensis Skirt Boosts Immune Response via Modulation of Gut Microbiota and Short-Chain Fatty Acids Metabolism in Mice" Foods 10, no. 10: 2478. https://doi.org/10.3390/foods10102478
APA StyleLi, Y., Qin, J., Cheng, Y., Ai, Y., Han, Z., Li, M., Qi, Y., Zhao, Q., & Li, Z. (2021). Polysaccharide from Patinopecten yessoensis Skirt Boosts Immune Response via Modulation of Gut Microbiota and Short-Chain Fatty Acids Metabolism in Mice. Foods, 10(10), 2478. https://doi.org/10.3390/foods10102478






