Deacidification of Cranberry Juice Reduces Its Antibacterial Properties against Oral Streptococci but Preserves Barrier Function and Attenuates the Inflammatory Response of Oral Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cranberry Juice
2.1.1. Deacidification of Cranberry Juice
2.1.2. Analysis
Titratable Acidity
Organic Acid Content
Anthocyanin Content
Proanthocyanidin Content
Total Phenolic Compounds
Sugar Content
2.2. Bacteria and Growth Conditions
2.3. Bactericidal Activity against Planktonic Streptococci
2.4. Bactericidal Activity against Biofilm-Embedded Streptococci
2.5. Bacterial Adherence to Hydroxyapatite
2.6. Transepithelial Electrical Resistance of Oral Epithelial Barrier
2.7. Fluorescein Isothiocyanate-Conjugated Dextran (FD-4) Transport
2.8. Immunofluorescent Staining of Zonula Occludens−1 and Occludin
2.9. Production of IL-6 and IL-8 by Oral Epithelial Cells
2.10. Statistical Analysis
3. Results
3.1. Composition of the Raw and Deacidified CJs
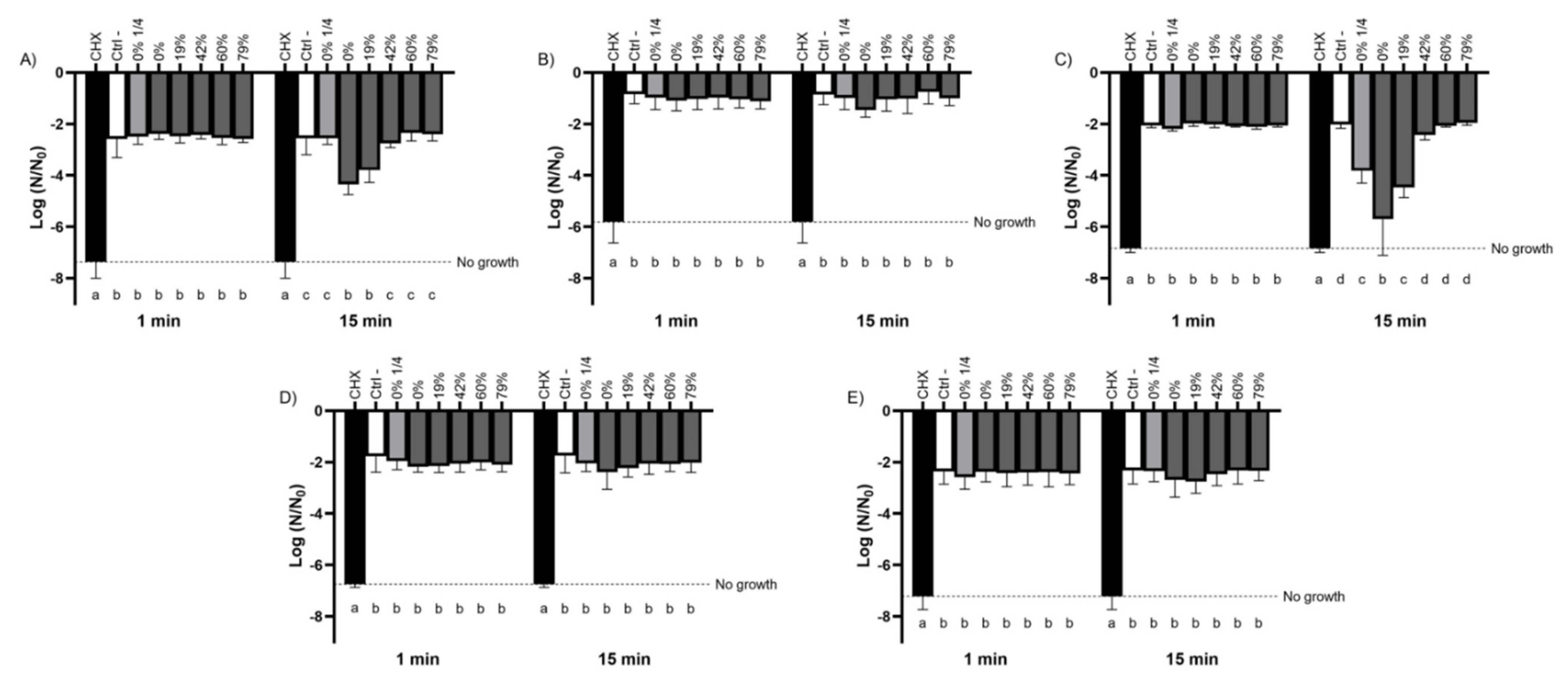
3.2. Bactericidal Activity of the Raw and Deacidified CJs against Planktonic Bacteria
3.3. Bactericidal Activity of the Raw and Deacidified CJs against Biofilm-Embedded Bacteria
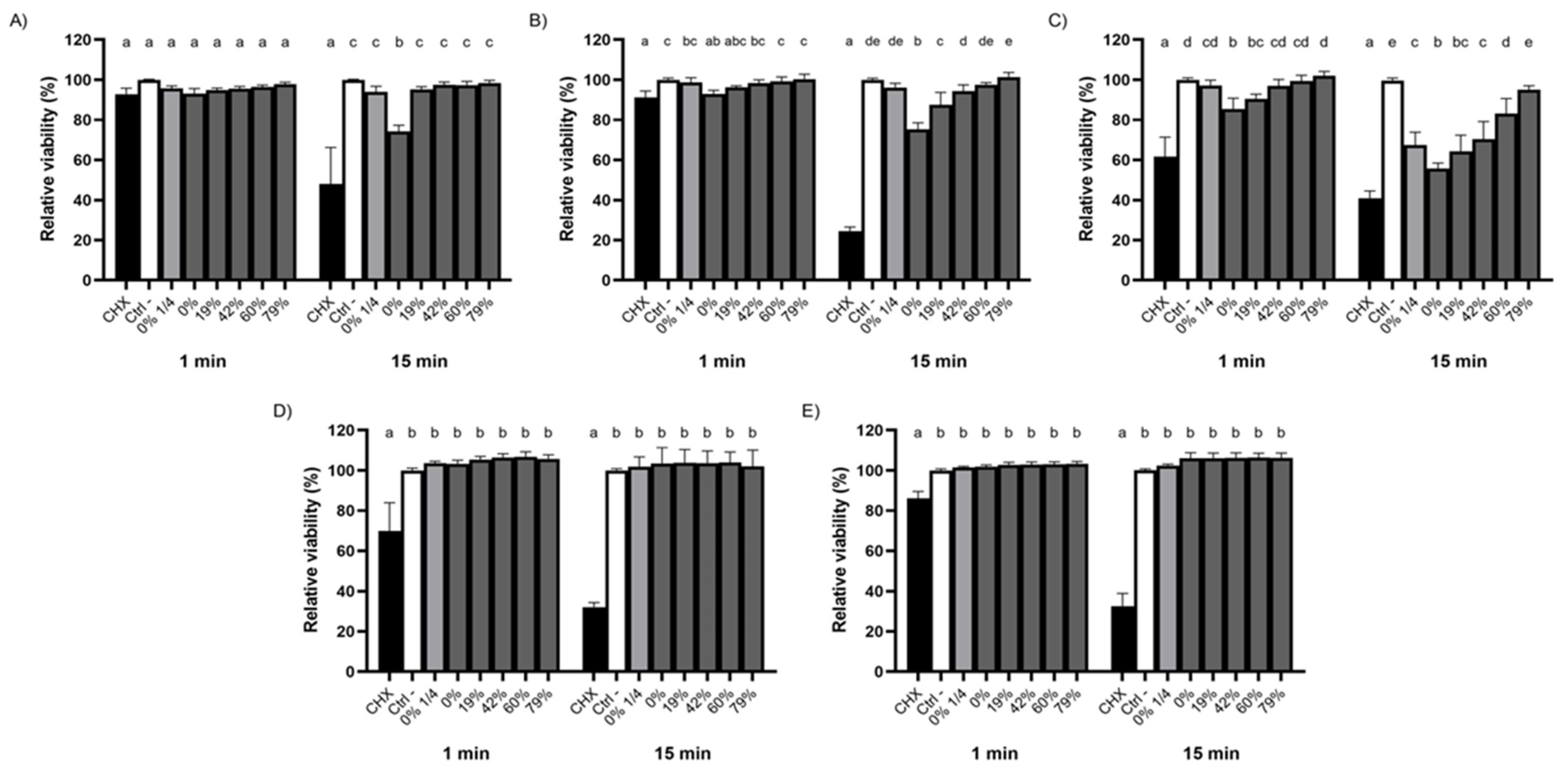
3.4. Impact of CJ Deacidification on Bacterial Adherence to Saliva-Coated HA
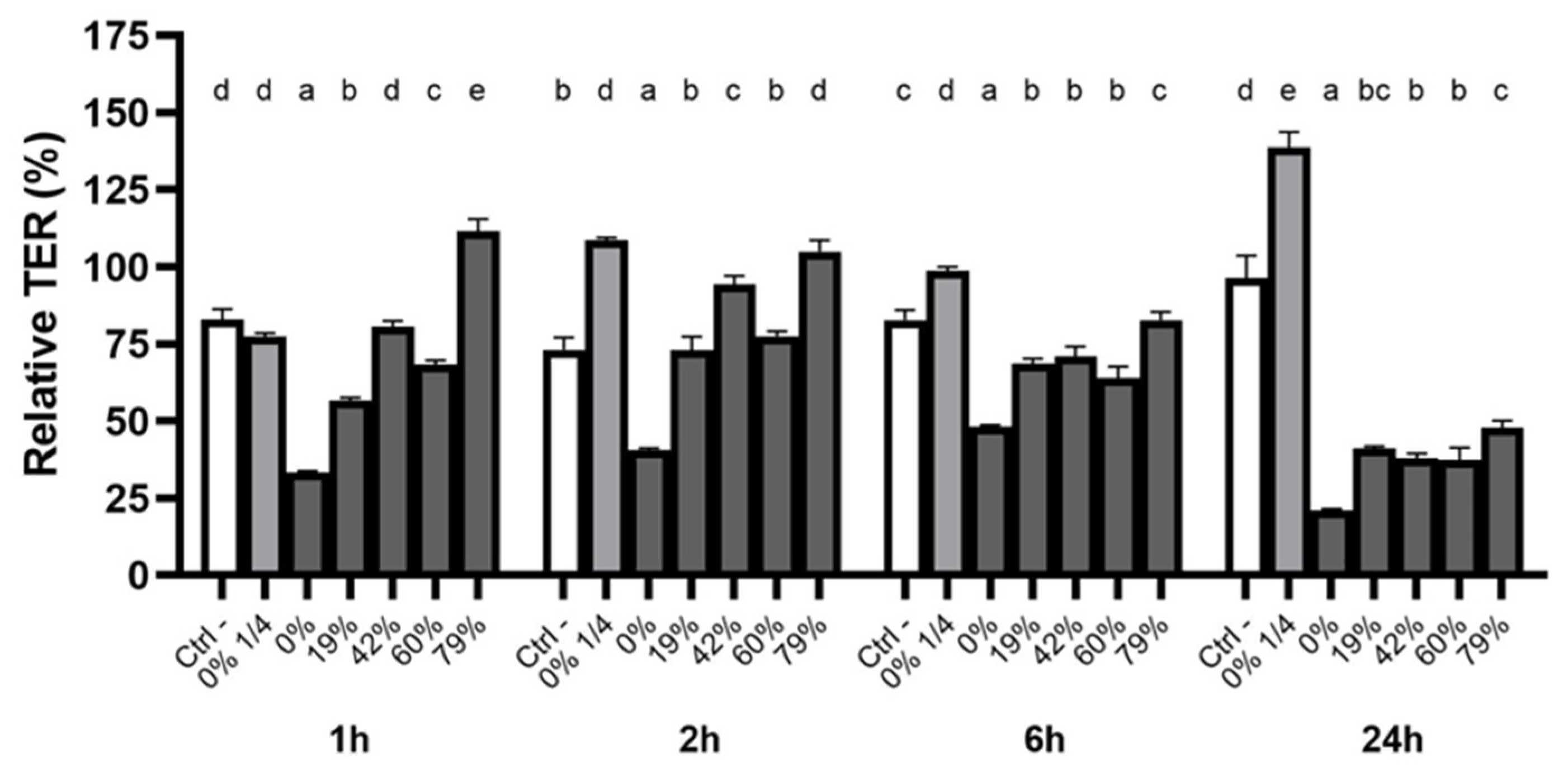
3.5. Effect of CJ Deacidification on Oral Epithelial Barrier Integrity
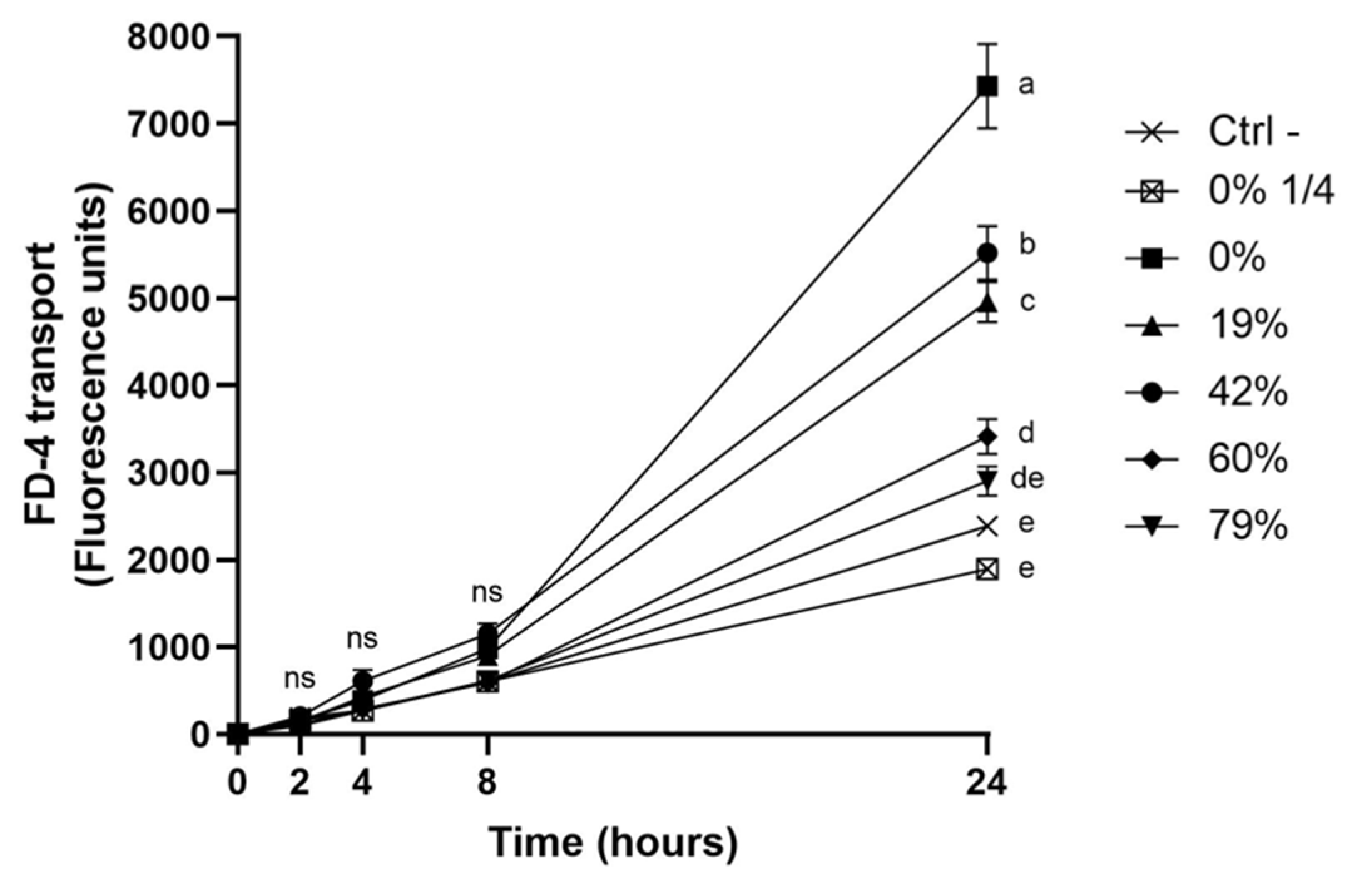
3.6. Impact of CJ Deacidification on the Paracellular Transport of FD-4
3.7. Immunofluorescence Staining of ZO-1 and Occludin Following Exposure to CJ Samples
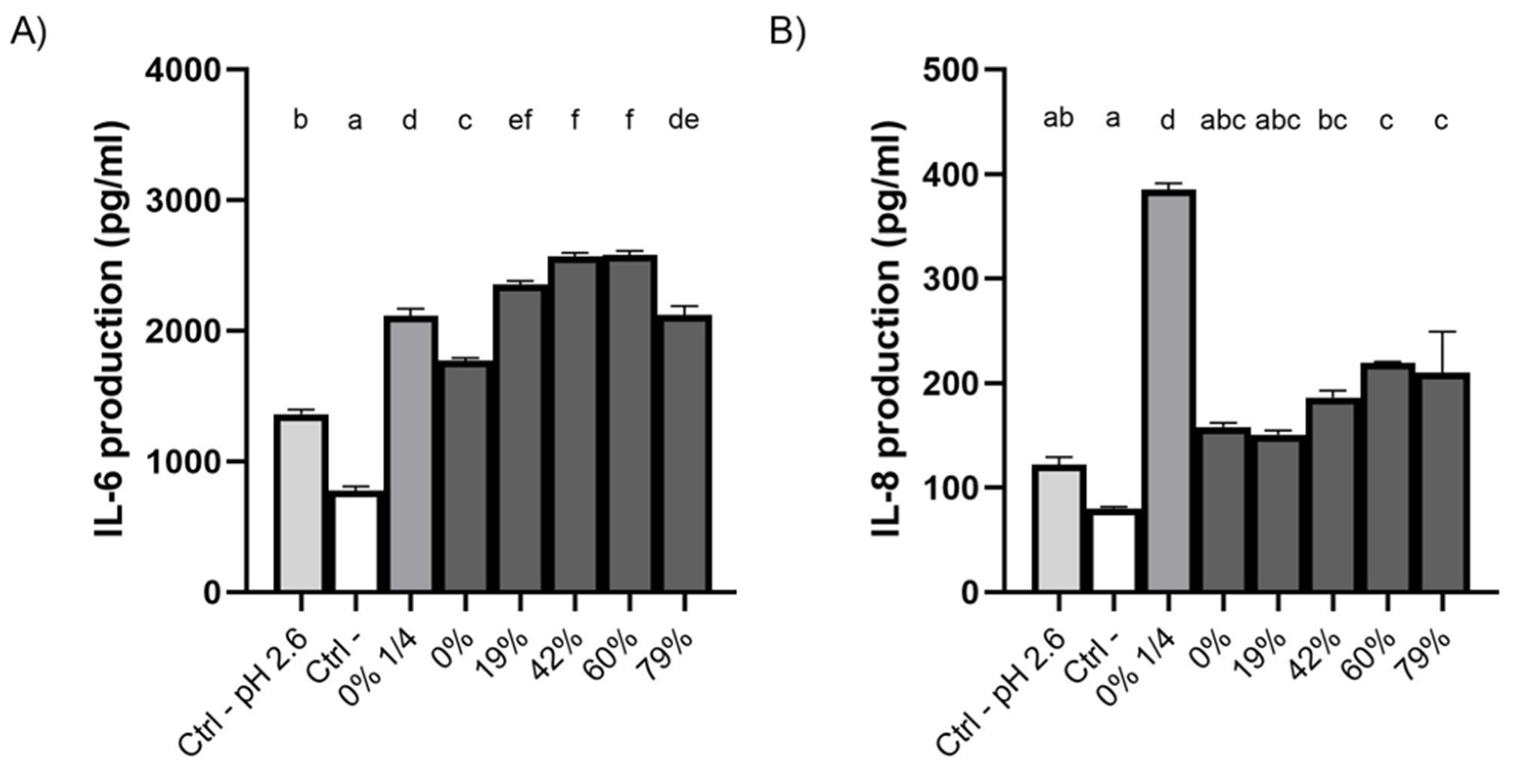
3.8. Effect of CJ Deacidification on IL-6 and IL-8 Production by Oral Epithelial Cells
4. Discussion
4.1. Bactericidal Activity of Raw and Deacidified CJs against Planktonic and Biofilm-Embedded Streptococci
4.2. Effect of CJ Deacidification on the Adherence of Streptococci to Saliva-Coated HA
4.3. Effect of CJ Deacidification on Oral Epithelial Barrier Function
4.4. Effect of CJ Deacidification on the Production of IL-6 and IL-8 by Oral Epithelial Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hojo, K.; Nagaoka, S.; Ohshima, T.; Maeda, N. Bacterial interactions in dental biofilm development. J. Dent. Res. 2009, 88, 982–990. [Google Scholar] [CrossRef]
- Ligtenberg, A.J.; Walgreen-Weterings, E.; Veerman, E.C.; de Soet, J.J.; de Graaff, J.; Amerongen, A.V. Influence of saliva on aggregation and adherence of Streptococcus gordonii HG 222. Infect. Immun. 1992, 60, 3878–3884. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.A.; Prakobphol, A.; Lee, T.; Hoover, C.I.; Fisher, S.J. Adherence of oral streptococci to salivary glycoproteins. Infect. Immun. 1992, 60, 31–38. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Zhang, Y.; Khammanivong, A.; Herzberg, M.C. Streptococcus gordonii Hsa environmentally constrains competitive binding by Streptococcus sanguinis to saliva-coated hydroxyapatite. J. Bacteriol. 2007, 189, 3106–3114. [Google Scholar] [CrossRef]
- Wang, S.-S.; Tang, Y.-L.; Pang, X.; Zheng, M.; Tang, Y.-J.; Liang, X.h. The maintenance of an oral epithelial barrier. Life Sci. 2019, 227, 129–136. [Google Scholar] [CrossRef]
- Peterson, S.N.; Snesrud, E.; Liu, J.; Ong, A.C.; Kilian, M.; Schork, N.J.; Bretz, W. The dental plaque microbiome in health and disease. PLoS ONE 2013, 8, e58487. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Yamashita, Y.; Shibata, Y.; Torigoe, H.; Tsuda, H.; Maeno, M. Effect of mixed mutans streptococci colonization on caries development. Oral Microbiol. Immunol. 2006, 21, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Hirose, H.; Hirose, K.; Isogai, E.; Miura, H.; Ueda, I. Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries Res. 1993, 27, 292–297. [Google Scholar] [CrossRef]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef]
- Banas, J.A.; Vickerman, M.M. Glucan-binding proteins of the oral streptococci. Crit Rev. Oral Biol. Med. 2003, 14, 89–99. [Google Scholar] [CrossRef]
- Hwang, G.; Liu, Y.; Kim, D.; Sun, V.; Aviles-Reyes, A.; Kajfasz, J.K.; Lemos, J.A.; Koo, H. Simultaneous spatiotemporal mapping of in situ pH and bacterial activity within an intact 3D microcolony structure. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ministère de l’Agriculture des Pêcheries et de l’Alimentation du Québec (MAPAQ). Portrait-Diagnostic Sectoriel de la Canneberge au Québec; Gouvernement du Québec: Quebec City, PQ, Canada, 2018.
- Feghali, K.; Feldman, M.; La, V.D.; Santos, J.; Grenier, D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J. Agric. Food Chem. 2011, 60, 5728–5735. [Google Scholar] [CrossRef]
- Rocha, D.M.U.P.; Caldas, A.P.S.; da Silva, B.P.; Hermsdorff, H.H.M.; Alfenas, R.D.C.G. Effects of blueberry and cranberry consumption on type 2 diabetes glycemic control: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Thimóteo, N.S.B.; Scavuzzi, B.M.; Simão, A.; Dichi, I. The impact of cranberry (Vaccinium macrocarpon) and cranberry products on each component of the metabolic syndrome: A review. Nutrire 2017, 42, 1–12. [Google Scholar] [CrossRef]
- Weh, K.M.; Clarke, J.; Kresty, L.A. Cranberries and cancer : An update of preclinical studies evaluating the cancer inhibitory potential of cranberry and cranberry derived constituents. Antioxidants 2016, 5, 27. [Google Scholar] [CrossRef]
- Duarte, S.; Gregoire, S.; Singh, A.P.; Vorsa, N.; Schaich, K.; Bowen, W.H.; Koo, H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol. Lett. 2006, 257, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, S.; Singh, A.P.; Vorsa, N.; Koo, H. Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity. J. Appl. Microbiol. 2007, 103, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; de Guzman, P.; Schobel, B.D.; Vacca Smith, A.V.; Bowen, W.H. Influence of cranberry juice on glucan-mediated processes involved in Streptococcus mutans biofilm development. Caries Res. 2006, 40, 20–27. [Google Scholar] [CrossRef]
- Philip, N.; Bandara, H.M.H.N.; Leishman, S.J.; Walsh, L.J. Effect of polyphenol-rich cranberry extracts on cariogenic biofilm properties and microbial composition of polymicrobial biofilms. Archives 2019, 102, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C.; Penndorf, K.A.; Feldman, M.; Steinberg, D.; Fridman, M.; Kashman, Y.; Ginsburg, I.; Ofek, I.; Weiss, E.I. Characterization of non-dialyzable constituents from cranberry juice that inhibit adhesion, co-aggregation and biofilm formation by oral bacteria. Food Funct. 2017, 8, 1955–1965. [Google Scholar] [CrossRef]
- Faucher, M.; Serre, E.; Langevin, M.-E.; Mikhaylin, S.; Lutin, F.; Bazinet, L. Drastic energy consumption reduction and ecoefficiency improvement of cranberry juice deacidification by electrodialysis with bipolar membranes at semi-industrial scale: Reuse of the recovery solution. J. Memb. Sci. 2018, 555, 105–114. [Google Scholar] [CrossRef]
- Faucher, M.; Henaux, L.; Chaudron, C.; Mikhaylin, S.; Margni, M.; Bazinet, L. Electromembrane approach to substantially improve the ecoefficiency of deacidified cranberry juice production: Physicochemical properties, life cycle assessment and ecoefficiency score. J. Food Eng. 2020, 273, 109802. [Google Scholar] [CrossRef]
- Serre, E.; Rozoy, E.; Pedneault, K.; Lacour, S.; Bazinet, L. Deacidification of cranberry juice by electrodialysis: Impact of membrane types and configurations on acid migration and juice physicochemical characteristics. Sep. Purif. Technol. 2016, 163, 228–237. [Google Scholar] [CrossRef]
- Stapleton, A.E.; Dziura, J.; Hooton, T.M.; Cox, M.E.; Yarova-Yarovaya, Y.; Chen, S.; Gupta, K. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: A randomized controlled trial. Mayo Clin. Proc. 2012, 87, 143–150. [Google Scholar] [CrossRef]
- Wing, D.A.; Rumney, P.J.; Preslicka, C.; Chung, J.H. Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: A randomized, controlled pilot study. J. Urol. 2009, 180, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Megert, B.; Shellis, R.P.; Wang, X. Analysis of the erosive effect of different dietary substances and medications. Br. J. Nutr. 2012, 107, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, F.; Qian, L.M.; Zhou, Z.R. Erosion behavior of human tooth enamel in citric acid solution. Tribiology Int. 2009, 42, 1558–1564. [Google Scholar] [CrossRef]
- Serre, E.; Boutin, Y.; Langevin, M.-E.; Lutin, F.; Pedneault, K.; Lacour, S.; Bazinet, L. Deacidification of cranberry juice protects against disruption of in-vitro intestinal cell barrier integrity. J. Funct. Foods 2016, 26, 208–216. [Google Scholar] [CrossRef]
- Renaud, V.; Faucher, M.; Perreault, V.; Serre, E.; Dubé, P.; Boutin, Y.; Bazinet, L. Evolution of cranberry juice compounds during in vitro digestion and identification of the organic acid responsible for the disruption of in vitro intestinal cell barrier integrity. J. Food Sci. Technol. 2020, 57, 2329–2342. [Google Scholar] [CrossRef]
- AOAC Acidity (Titratable) of Fruit Products. In AOAC Official Methods of Analysis; AOAC: Rockville City, MD, USA, 2000.
- AOAC Quinic, malic, and Citric Acids in Cranberry Juice Cocktail and Apple Juice. In AOAC Official Methods of Analysis; AOAC: Rockville City, MD, USA, 2010.
- Wu, X.; Prior, R.L. Systematic Identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States : Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Brownmiller, C.R.; Prior, R.L. Influence of extrusion processing on procyanidin composition and total anthocyanin contents of blueberry pomace. J. Food Sci. 2009, 74, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Fournier, E. Colorimetric quantification of carbohydrates. Curr. Protoc. Food Anal. Chem. 2001, E1.1.1–E1.1.8. [Google Scholar] [CrossRef]
- AFNOR NF EN 1040 Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Basic Bactericidal Activity of Chemical Disinfectants and Antiseptics—Test Method and Requirements (Phase 1); AFNOR: La PLaine Saint-Denis, France, 2006.
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Combet, E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015, 6, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Madhwani, T.; McBain, A.J. Bacteriological effects of a Lactobacillus reuteri probiotic on in vitro oral biofilms. Arch. Oral Biol. 2011, 56, 1264–1273. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Groeger, S.; Meyle, J.; Grenier, D. Green tea polyphenols enhance gingival keratinocyte integrity and protect against invasion by Porphyromonas gingivalis. Pathog. Dis. 2018, 76, 1–9. [Google Scholar] [CrossRef]
- Groeger, S.; Michel, J.; Meyle, J. Establishment and characterization of immortalized human gingival keratinocyte cell lines. J. Periodontal Res. 2008, 43, 604–614. [Google Scholar] [CrossRef]
- Gilchrist, E.P.; Moyer, P.; Shillitoe, E.J.; Clare, N.; Murrah, V.A. Establishment of a human polyclonal oral epithelial cell line. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 340–347. [Google Scholar] [CrossRef]
- Svensäter, G.; Larsson, U.-B.; Greif, E.C.G.; Cvitkovitch, D.G.; Hamilton, I.R. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 1997, 12, 266–273. [Google Scholar] [CrossRef]
- Sheng, J.; Marquis, R.E. Enhanced acid resistance of oral streptococci at lethal pH values associated with acid-tolerant catabolism and with ATP synthase activity. FEMS Microbiol. Lett. 2006, 262, 93–98. [Google Scholar] [CrossRef]
- Bender, G.R.; Sutton, S.V.; Marquis, R.E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 1986, 53, 331–338. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The biology of Streptococcus mutans. Microbiol. Spect. 2019, 7, 435–448. [Google Scholar] [CrossRef]
- Guo, L.; McLean, J.S.; Lux, R.; He, X.; Shi, W. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci. Rep. 2015, 5, 18015. [Google Scholar] [CrossRef] [PubMed]
- Korithoski, B.; Krastel, K.; Cvitkovitch, D.G. Transport and metabolism of citrate by Streptococcus mutans. J. Bacteriol. 2005, 187, 4451–4456. [Google Scholar] [CrossRef]
- Sheng, J.; Marquis, R.E. Malolactic fermentation by Streptococcus mutans. FEMS Microbiol. Lett. 2007, 272, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Sturr, M.G.; Marquis, R.E. Comparative acid tolerances and inhibitor sensitivities of isolated F-ATPases of oral lactic acid bacteria. Appl. Environ. Microbiol. 1992, 58, 2287–2291. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Guellmar, A.; Olschowsky, P.; Tonndorf-Martini, S.; Heyder, M.; Pfister, W.; Reise, M.; Sigusch, B. Antimicrobial effect of natural berry juices on common oral pathogenic bacteria. Antibiotics 2020, 9, 533. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Lemos, A.C.; Abranches, J.; Gonçalves, R.B.; Burne, R.A. Adaptive acid tolerance response of Streptococcus sobrinus. J. Bacteriol. 2004, 186, 6383–6390. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.R.; Abranches, J.; Kajfasz, J.K.; Lemos, J.A. Characterization of the Streptococcus sobrinus acid-stress response by interspecies microarrays and proteomics. Mol. Oral Microbiol. 2010, 25, 331–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burne, R.A.; Marquis, R.E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 2000, 193, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Casiano-Colón, A.; Marquis, R.E. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl. Environ. Microbiol. 1988, 54, 1318–1324. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Burne, R.A. Analysis of Streptococcus salivarius urease expression using continuous chemostat culture. FEMS Microbiol. Lett. 1996, 135, 223–229. [Google Scholar] [CrossRef]
- Gilmore, K.S.; Srinivas, P.; Akins, D.R.; Hatter, K.L.; Gilmore, M.S. Growth, development, and gene expression in a persistent Streptococcus gordonii biofilm. Infect. Immun. 2003, 71, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Feldman, M.; Ofek, I.; Weiss, E.I. Cranberry high molecular weight constituents promote Streptococcus sobrinus desorption from artificial biofilm. Int. J. Antimicrob. Agents 2005, 25, 247–251. [Google Scholar] [CrossRef] [PubMed]
- McNee, S.G.; Geddes, D.A.M.; Weetman, D.A. Diffusion of sugars and acids in human dental plaque in vitro. Arch. Oral Biol. 1982, 27, 975–979. [Google Scholar] [CrossRef]
- Stewart, P.S. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol. Bioeng. 1998, 59, 261–272. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.; Thompson, A.; Mansfield, J.M.; Grassmann, A.A.; Maas, K.; Caimano, M.J.; Barao, V.A.R.; Vickerman, M.M.; Dongari-Bagtzoglou, A. Role of glucosyltransferase R in biofilm interactions between Streptococcus oralis and Candida albicans. ISME J. 2020, 14, 1207–1222. [Google Scholar] [CrossRef]
- Hannig, C.; Sorg, J.; Spitzmüller, B.; Hannig, M.; Al-Ahmad, A. Polyphenolic beverages reduce initial bacterial adherence to enamel in situ. J. Dent. 2009, 37, 560–566. [Google Scholar] [CrossRef]
- Weiss, E.I.; Kozlovsky, A.; Steinberg, D.; Lev-dor, R.; Bar, R.; Greenstein, N.; Feldman, M.; Sharon, N.; Ofek, I. A high molecular mass cranberry constituent reduces mutans streptococci level in saliva and inhibits in vitro adhesion to hydroxyapatite. FEMS Microbiol. Lett. 2004, 232, 89–92. [Google Scholar] [CrossRef]
- Cowan, M.M.; Taylor, K.G.; Doyle, R.J. Role of sialic acid in the kinetics of Streptococcus sanguis adhesion to artificial pellicle. Infect. Immun. 1987, 55, 1552–1557. [Google Scholar] [CrossRef]
- Rozen, R.; Bachrach, G.; Bronshteyn, M.; Gedalia, I.; Steinberg, D. The role of fructans on dental biofilm formation by Streptococcus sobrinus, Streptococcus mutans, Streptococcus gordonii and Actinomyces viscosus. FEMS Microbiol. Lett. 2001, 195, 205–210. [Google Scholar] [CrossRef]
- Singh, A.K.; Woodiga, S.A.; Grau, M.A.; King, S.J. Streptococcus oralis neuraminidase modulates adherence to multiple carbohydrates on platelets. Infect. Immun. 2017, 85, e00774-16. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, S.; Sandberg, A.L.; Cisar, J.O. Salivary receptors for the proline-rich protein-binding and lectin-like adhesins of oral actinomyces and streptococci. J. Dent. Res. 2004, 83, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Couvigny, B.; Kulakauskas, S.; Pons, N.; Quinquis, B.; Abraham, A.-L.; Meylheuc, T.; Delorme, C.; Renault, P.; Briandet, R.; Lapaque, N.; et al. Identification of new factors modulating adhesion abilities of the pioneer commensal bacterium Streptococcus salivarius. Front. Microbiol. 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Yamazaki, I.; Yashiki, T.; Mima, H. Vaginal absorption of a potent luteinizing hormone-releasing hormone analogue (leuprolide) in rats II: Mechanism of absorption enhancement with organic acids. J. Pharm. Sci. 1983, 72, 75–78. [Google Scholar] [CrossRef]
- Cho, M.J.; Scieszka, J.F.; Burton, P.S. Citric acid as an adjuvant for transepithelial transport. Int. J. Pharm. 1989, 52, 79–81. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Goodenough, D.A. Localization of the tight junction protein, ZO-1, is modulated by extracellular calcium and cell-cell contact in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 1988, 107, 2389–2399. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Z.; Li, P.; Yang, Q. The effect of various absorption enhancers on tight junction in the human intestinal Caco-2 cell line. Drug Dev. Ind. Pharm. 2013, 39, 587–592. [Google Scholar] [CrossRef]
- Mongelli-Sabino, B.M.; Canuto, L.P.; Collares-Buzato, C.B. Acute and chronic exposure to high levels of glucose modulates tight junction-associated epithelial barrier function in a renal tubular cell line. Life Sci. 2017, 188, 149–157. [Google Scholar] [CrossRef]
- Oyama, Y.; Yamano, H.; Ohkuma, A.; Ogawara, K.; Higaki, K.; Kimura, T. Carrier-mediated transport systems for glucose in mucosal cells of the human oral cavity. J. Pharm. Sci. 1999, 88, 830–834. [Google Scholar] [CrossRef]
- Santaguida, S.; Janigro, D.; Hossain, M.; Oby, E.; Rapp, E.; Cucullo, L. Side by side comparison between dynamic versus static models of blood–brain barrier in vitro: A permeability study. Brain Res. 2006, 1109, 1–13. [Google Scholar] [CrossRef]
- Ramadan, Q.; Jing, L. Characterization of tight junction disruption and immune response modulation in a miniaturized Caco-2/U937 coculture-based in vitro model of the human intestinal barrier. Biomed. Microdevices 2016, 18, 11. [Google Scholar] [CrossRef]
- Dawes, C. Factors Influencing Salivary Flow Rate and Composition. In Saliva and Oral Health, 4th ed.; Edgar, W.M., Dawes, C., O’Mullane, D.M., Eds.; Stephen Hancocks Limited: London, UK, 2012; pp. 37–56. ISBN 978-0-9565668-3-6. [Google Scholar]
- Held, S.; Schieberle, P.; Somoza, V. Characterization of alpha-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J. Agric. Food Chem. 2007, 55, 8040–8046. [Google Scholar] [CrossRef]
- Grauso, M.; Lan, A.; Andriamihaja, M.; Bouillaud, F.; Blachier, F. Hyperosmolar environment and intestinal epithelial cells: Impact on mitochondrial oxygen consumption, proliferation, and barrier function in vitro. Sci. Rep. 2019, 9, 11360. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Wu, C.-S.; Huang, S.-M.; Wu, I.H.; Chen, G.-S. High-glucose environment enhanced oxidative stress and increased interleukin−8 secretion from keratinocytes: New insights into impaired diabetic wound healing. Diabetes 2013, 62, 2530–2538. [Google Scholar] [CrossRef] [PubMed]


| Deacidification Rate (%) | 0 (raw) | 19 | 42 | 60 | 79 |
|---|---|---|---|---|---|
| pH | 2.59 ± 0.03 a | 2.74 ± 0.01 b | 2.71 ± 0.01 b | 2.87 ± 0.01 c | 3.24 ± 0.02 d |
| Titrable acidity (g/L of citric acid monohydrate equivalents) | 9.25 ± 0.05 a | 7.48 ± 0.02 b | 5.40 ± 0.05 c | 3.72 ± 0.02 d | 1.91 ± 0.05 e |
| Sugar (g/L of glucose equivalents) | 44.4 ± 4.7 a | 44.1 ± 6.6 a | 44.9 ± 5.1 a | 47.8 ± 10.3 a | 53.3 ± 2.5 a |
| Organic acids (g/L) | |||||
| Quinic acid | 10.35 ± 0.31 a b | 10.72 ± 0.04 a | 10.49 ± 0.17 a b | 10.11 ± 0.14 b | 9.19 ± 0.11 c |
| Citric acid | 11.59 ± 0.20 a | 9.49 ± 0.15 b | 6.88 ± 0.15 c | 4.67 ± 0.09 d | 2.35 ± 0.06 e |
| Malic acid | 6.03 ± 0.10 a | 4.44 ± 0.04 b | 2.40 ± 0.11 c | 1.34 ± 0.06 d | 0.00 ± 0.00 e |
| Anthocyanins (mg/L of cyanidin−3-glucoside equivalents) | |||||
| Cyanidin−3-galactoside | 65.14 ± 0.51 a | 65.59 ± 0.67 a | 64.70 ± 0.32 a | 65.76 ± 1.22 a | 61.89 ± 0.61 b |
| Cyanidin−3-glucoside | 2.15 ± 0.12 a | 2.97 ± 0.06 b | 2.47 ± 0.10 c | 2.06 ± 0.06 a | 2.22 ± 0.19 a c |
| Cyanidin−3-arabinoside | 51.12 ± 0.69 a | 51.27 ± 0.15 a | 50.99 ± 0.37 a | 50.57 ± 0.89 a | 48.24 ± 0.22 b |
| Peonidin−3-galactoside | 84.74 ± 0.54 a | 85.91 ± 0.95 a | 83.76 ± 0.51 a | 85.02 ± 1.21 a | 80.74 ± 0.71 b |
| Peonidin−3-glucoside | 8.50 ± 0.10 a | 8.86 ± 0.09 b c | 9.01 ± 0.12 b | 8.71 ± 0.06 a b c | 8.53 ± 0.10 a c |
| Peonidin−3-arabinoside | 37.94 ± 0.62 a | 38.41 ± 0.39 a | 37.13 ± 0.36 a | 37.29 ± 0.34 a | 35.98 ± 0.30 b |
| Total | 249.58 ± 1.82 a | 253.11 ± 0.94 a | 248.05 ± 0.51 a | 249.40 ± 2.75 a | 237.60 ± 1.47 b |
| Proanthocyanidins (mg/L of epicatechin equivalents) | |||||
| Monomers | 39.35 ± 0.64 a | 40.43 ± 1.35 a | 36.05 ± 2.74 a | 36.67 ± 2.41 a | 37.43 ± 1.97 a |
| 2–3mers | 148.36 ± 1.80 a | 155.41 ± 6.66 a | 142.12 ± 18.47 a | 157.39 ± 3.86 a | 159.30 ± 9.67 a |
| 4–6mers | 59.92 ± 1.24 a | 62.94 ± 2.26 a | 57.64 ± 7.06 a | 62.55 ± 1.88 a | 62.29 ± 3.58 a |
| 7–10mers | 4.28 ± 0.27 a | 4.52 ± 0.35 a | 4.06 ± 0.54 a | 4.41 ± 0.48 a | 4.53 ± 0.50 a |
| Polymers | 5.55 ± 0.52 a | 5.60 ± 0.35 a | 5.90 ± 0.38 a | 5.88 ± 0.05 a | 5.78 ± 0.05 a |
| Total | 257.46 ± 2.36 a | 268.90 ± 10.34 a | 245.78 ± 29.00 a | 266.91 ± 8.35 a | 269.33 ± 15.61 a |
| Total phenolic compounds (mg/L of gallic acid equivalents) | 1074.79 ± 4.90 a | 1039.65 ± 28.27 a | 978.42 ± 47.79 a | 1075.52 ± 34.87 a | 984.21 ± 53.66 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellerin, G.; Bazinet, L.; Grenier, D. Deacidification of Cranberry Juice Reduces Its Antibacterial Properties against Oral Streptococci but Preserves Barrier Function and Attenuates the Inflammatory Response of Oral Epithelial Cells. Foods 2021, 10, 1634. https://doi.org/10.3390/foods10071634
Pellerin G, Bazinet L, Grenier D. Deacidification of Cranberry Juice Reduces Its Antibacterial Properties against Oral Streptococci but Preserves Barrier Function and Attenuates the Inflammatory Response of Oral Epithelial Cells. Foods. 2021; 10(7):1634. https://doi.org/10.3390/foods10071634
Chicago/Turabian StylePellerin, Geneviève, Laurent Bazinet, and Daniel Grenier. 2021. "Deacidification of Cranberry Juice Reduces Its Antibacterial Properties against Oral Streptococci but Preserves Barrier Function and Attenuates the Inflammatory Response of Oral Epithelial Cells" Foods 10, no. 7: 1634. https://doi.org/10.3390/foods10071634
APA StylePellerin, G., Bazinet, L., & Grenier, D. (2021). Deacidification of Cranberry Juice Reduces Its Antibacterial Properties against Oral Streptococci but Preserves Barrier Function and Attenuates the Inflammatory Response of Oral Epithelial Cells. Foods, 10(7), 1634. https://doi.org/10.3390/foods10071634







