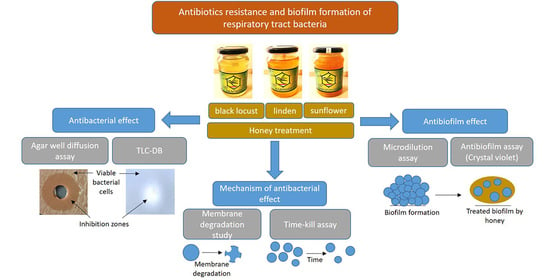
In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Melissopalynological Analysis
2.2. Thin Layer Chromatography–Direct Bioautography (TLC–DB)
2.3. Agar Well Diffusion Assay
2.4. Time-Kill Assay
2.5. Membrane Degradation Study
2.6. Microdilution Assay
2.7. Antibiofilm Activity
2.8. Statistical Analysis
3. Results
3.1. Melissopalynological Analysis
3.2. Antibacterial Activity of Honeys
3.2.1. Thin Layer Chromatography–Direct Bioautography (TLC–DB) Assay
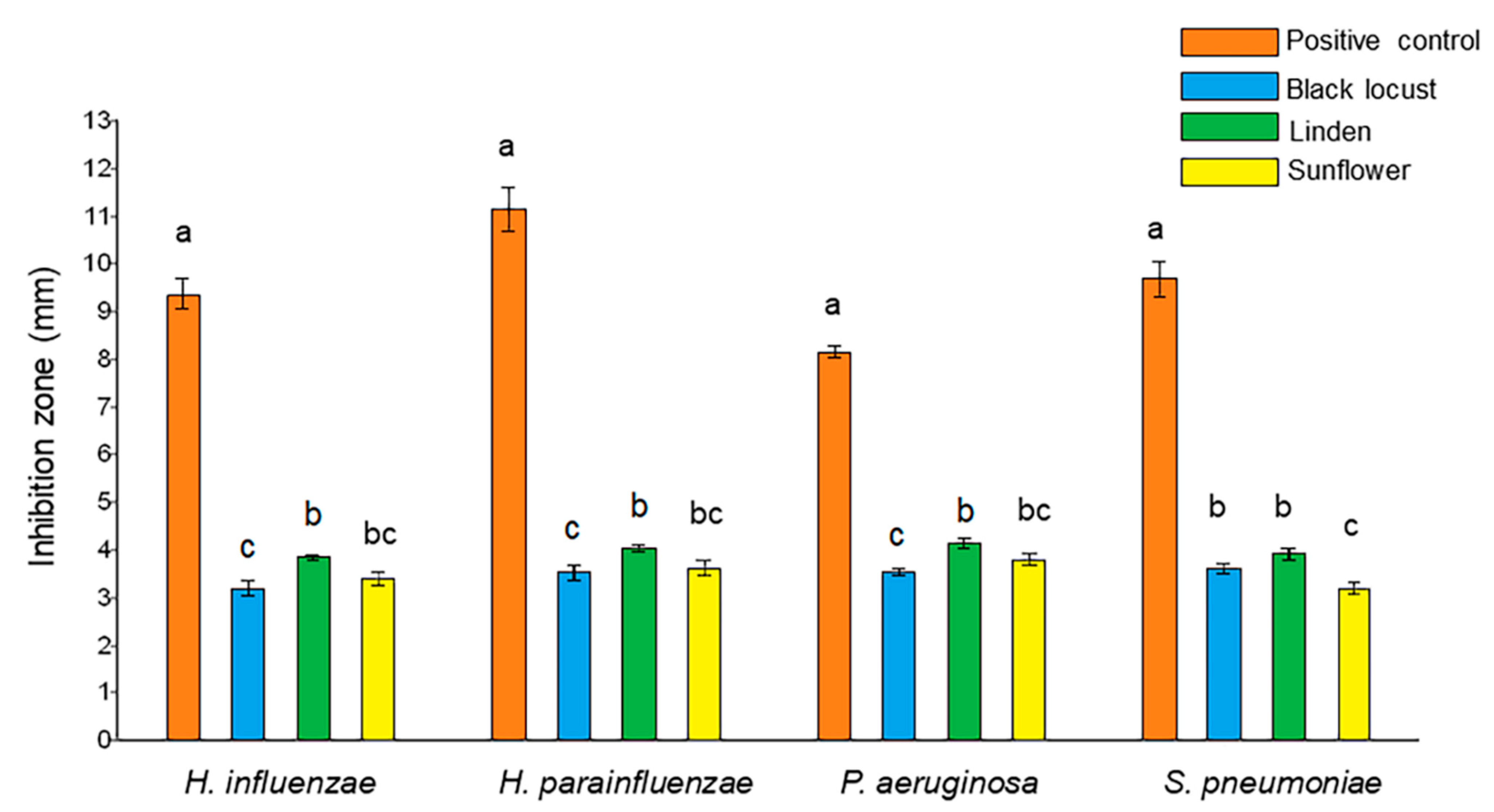
3.2.2. Agar Well Diffusion Assay
3.3. Kinetics and Mechanisms of Action of Antibacterial Activity
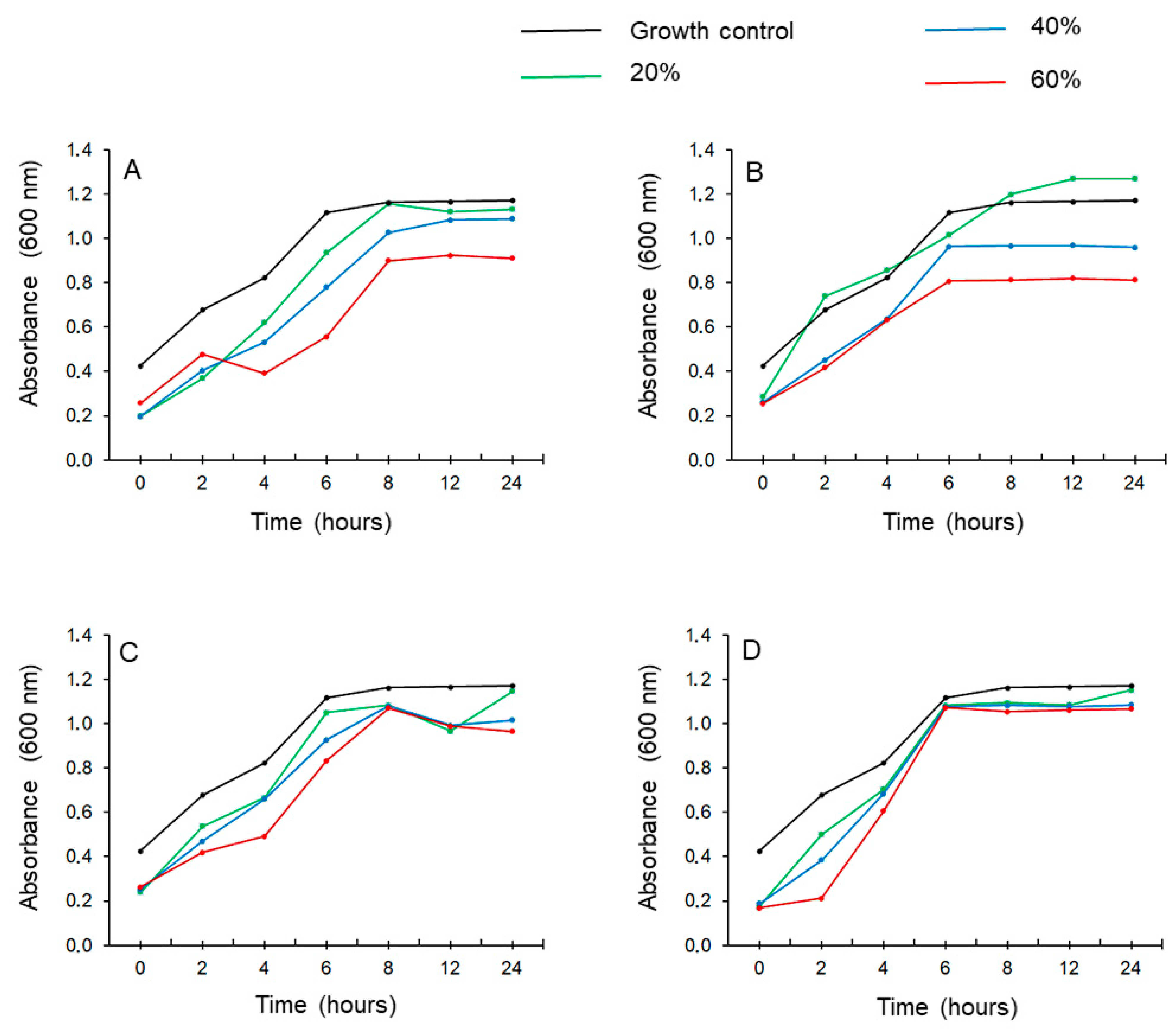
3.3.1. Time-Kill Assay
3.3.2. Membrane Degradation Study
3.4. Antibiofilm Activity
3.4.1. Broth Microdilution Test
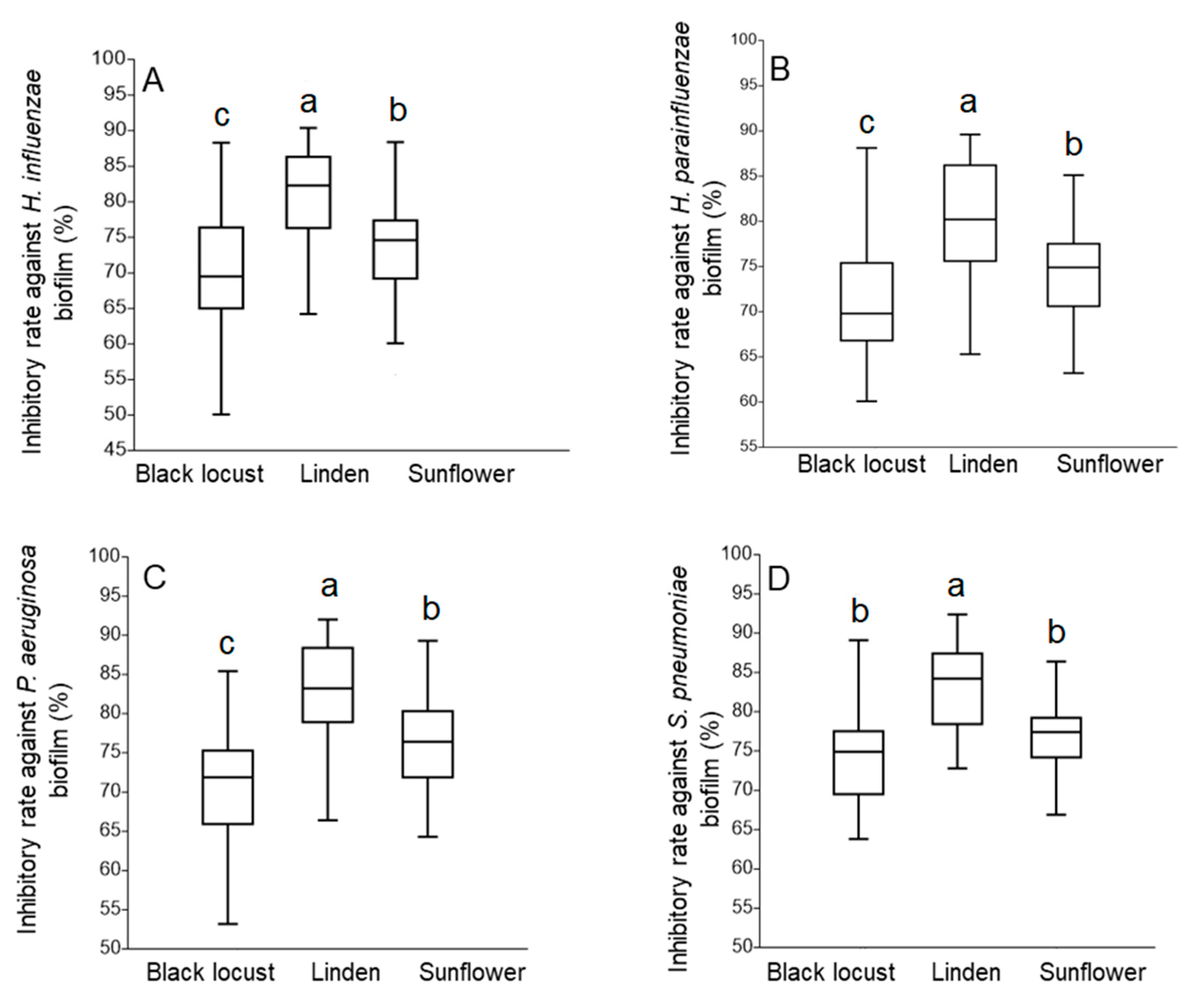
3.4.2. Antibiofilm Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EUR-Lex 32001L0110 EN EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32001L0110 (accessed on 6 July 2021).
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, S.M.; Jana, S.K.; Das, S.; Mandal, M. Studies on the Phenolic Profiling, Anti-Oxidant and Cytotoxic Activity of Indian Honey: In Vitro Evaluation. Nat. Prod. Res. 2010, 24, 1295–1306. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Hoszegi, T.K.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Melissopalynology, Antioxidant Activity and Multielement Analysis of Two Types of Early Spring Honeys from Hungary. Food Biosci. 2020, 35, 100587. [Google Scholar] [CrossRef]
- Tonks, A.; Cooper, R.; Jones, K.; Blair, S.; Parton, J. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.S.; Ghozy, S.; Minh, L.H.N.; Hashan, M.R.; Soliman, A.L.; Van, N.T.; Hirayama, K.; Huy, N.T. Honey in Bronchial Asthma: From Folk Tales to Scientific Facts. J. Med. Food 2019, 22, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Yangchan, J.; Ahmad, A.; Kumar, A.; Mishra, R.K.; Vyawahare, A.; Akhter, R.; Ashraf, G.M.; Shakil, S.; Khan, R. A Mechanistic Perspective on Chemopreventive and Therapeutic Potential of Phytochemicals in Honey. In Therapeutic Applications of Honey and Its Phytochemicals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 113–140. [Google Scholar]
- Al-Hatamleh, M.A.I.; Hatmal, M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.D.C.; Mohamud, R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules 2020, 25, 5017. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-Based Medicinal Prop-erties of Stingless Bee Products: Recent Progress and Future Directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Szweda, P. Antimicrobial Activity of Honey. Honey Anal. 2017. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, R.; Burton, N.; Cooper, R. Proteomic and genomic analysis of methicillin-resistant Staphylococcus aureus (MRSA) exposed to manuka honey in vitro demonstrated down-regulation of virulence markers. J. Antimicrob. Chemother. 2014, 69, 603–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal Activity of Different Honeys against Pathogenic Bacteria. Arch. Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.E.; Clarke, A.M.; Munzhelele, T.; Green, E.; Mkwetshana, N.F.; Ndip, R.N. Selected South African Honeys and Their Extracts Possess In Vitro Anti-Helicobacter pylori Activity. Arch. Med. Res. 2010, 41, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Irish, J.; Carter, D.A.; Shokohi, T.; Blair, S.E. Honey Has an Antifungal Effect against Candida Species. Med. Mycol. 2006, 44, 289–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P.; Kathju, S.; Høiby, N.; Moser, C.; William Costerton, J.; Moter, A.; Bjarnsholt, T. Towards Di-agnostic Guidelines for Biofilm-Associated Infections. FEMS Immunol. Med. Microbiol. 2012, 65, 127–145. [Google Scholar]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Gemmell, C.G.; Hunter, I.S. The association between biocide tolerance and the presence or absence of qac genes among hospital-acquired and community-acquired MRSA isolates. J. Antimicrob. Chemother. 2007, 61, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodis, N.; Tsapadikou, V.K.; Potsios, C.; Xaplanteri, P. Resistance Mechanisms in Bacterial Biofilm Formations: A Review. J. Emerg. Intern. Med. 2020, 4, 30. [Google Scholar]
- Otoole, A.G.; Kaplan, H.B.; Kolter, R. Biofilm Formation as Microbial Development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The microbiome and the respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [Green Version]
- Rytter, D.; Rask, C.U.; Vestergaard, C.H.; Andersen, A.-M.N.; Bech, B.H. Non-specific Health complaints and self-rated health in pre-adolescents; impact on primary health care use. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Chambers, S.T.; Murdoch, D.; Morris, A.; Holland, D.; Pappas, P.; Almela, M.; Fernández-Hidalgo, N.; Almirante, B.; Bouza, E.; Forno, D.; et al. HACEK Infective Endocarditis: Characteristics and Outcomes from a Large, Multi-National Cohort. PLoS ONE 2013, 8, e63181. [Google Scholar] [CrossRef]
- Feder, H.M., Jr.; Roberts, J.C.; Salazar, J.C.; Leopold, H.B.; Toro-Salazar, O. HACEK Endocarditis in Infants and Children: Two Cases and a Literature Review. Pediatric Infect. Dis. J. 2003, 22, 557–562. [Google Scholar] [CrossRef]
- Mohd-Zain, Z.; Kamsani, N.H.; Ismail, I.S.; Ahmad, N.; Mohd-Zain, Z.; Kamsani, N.H.; Ismail, I.S.; Ahmad, N. Antibiotic susceptibility profile of Haemophilus influenzae and transfer of co-trimoxazole resistance determinants. Trop. Biomed. 2012, 29, 372–380. [Google Scholar]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, T.-F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Vakulenko, S.B.; Mobashery, S. Versatility of Aminoglycosides and Prospects for Their Future. Clin. Microbiol. Rev. 2003, 16, 430–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chegini, Z.; Khoshbayan, A.; Moghadam, M.T.; Farahani, I.; Jazireian, P.; Shariati, A. Bacteriophage Therapy against Pseudomonas Aeruginosa Biofilms: A Review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, A.; Weiser, J.N.; Paton, J.C.; Andrew, P.W. The Role of Streptococcus Pneumoniae Virulence Factors in Host Respiratory Colonization and Disease. Nat. Rev. Microbiol. 2008, 6, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.; Slack, R.C.; Barer, M.R.; Irving, W.L. Medical Microbiology E-Book: A Guide to Microbial Infections: Pathogenesis, Immunity, Laboratory Diagnosis and Control. With STUDENT CONSULT Online Access; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Suaya, J.A.; Fletcher, M.A.; Georgalis, L.; Arguedas, A.; McLaughlin, J.M.; Ferreira, G.; Theilacker, C.; Gessner, B.D.; Verstraeten, T. Identification of Streptococcus Pneumoniae in Hospital-Acquired Pneumonia in Adults: A Systematic Review. J. Hosp. Infect. 2020, 108, 146–157. [Google Scholar] [CrossRef]
- Sanchez, C.J.; Shivshankar, P.; Stol, K.; Trakhtenbroit, S.; Sullam, P.M.; Sauer, K.; Hermans, P.W.; Orihuela, C.J. The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation in Vivo and in Biofilms. PLoS Pathog. 2010, 6, e1001044. [Google Scholar] [CrossRef]
- Domenech, M.; García, E.; Moscoso, M. Biofilm formation in Streptococcus pneumoniae. Microb. Biotechnol. 2012, 5, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan-Borrás, M.; Doménech, E.; Hellebrandova, M.; Escriche, I. Effect of country origin on physicochemical, sugar and volatile composition of acacia, sunflower and tilia honeys. Food Res. Int. 2014, 60, 86–94. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138, 148–156. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S. Romanian Honey Authentication Using Voltammetric Electronic Tongue. Correlation of Voltam-metric Data with Physico-Chemical Parameters and Phenolic Compounds. Comput. Electron. Agric. 2019, 157, 371–379. [Google Scholar] [CrossRef]
- Rusko, J.; Vainovska, P.; Vilne, B.; Bartkevics, V. Phenolic Profiles of Raw Mono-and Polyfloral Honeys from Latvia. J. Food Compos. Anal. 2021, 98, 103813. [Google Scholar] [CrossRef]
- Farkas, Á.; Zajácz, E. Nectar Production for the Hungarian Honey Industry. Eur. J. Plant Sci. Biotechnol. 2007, 1, 125–151. [Google Scholar]
- Sajtos, Z.; Herman, P.; Harangi, S.; Baranyai, E. Elemental analysis of Hungarian honey samples and bee products by MP-AES method. Microchem. J. 2019, 149, 103968. [Google Scholar] [CrossRef]
- Czipa, N.; Andrási, D.; Kovács, B. Determination of essential and toxic elements in Hungarian honeys. Food Chem. 2015, 175, 536–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.; Farkas, Á.; Kocsis, M. Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content. Molecules 2021, 26, 2825. [Google Scholar] [CrossRef]
- Von Der Ohe, W.; Oddo, L.P.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of Action of Melaleuca alternifolia (Tea Tree) Oil on Staphylococcus aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [Green Version]
- Bennis, S.; Chami, F.; Chami, N.; Bouchikhi, T.; Remmal, A. Surface alteration of Saccharomyces cerevisiae induced by thymol and eugenol. Lett. Appl. Microbiol. 2004, 38, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Guzelmeric, E.; Ciftci, I.; Yuksel, P.I.; Yesilada, E. Importance of Chromatographic and Spectrophotometric Methods in De-termining Authenticity, Classification and Bioactivity of Honey. LWT 2020, 132, 109921. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Zhang, C.; Liu, Y.; Ma, L.; Zhang, X. Effects of sub-minimum inhibitory concentrations of lemon essential oil on the acid tolerance and biofilm formation of Streptococcus mutans. Arch. Oral Biol. 2018, 87, 235–241. [Google Scholar] [CrossRef]
- Mandal, M.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Bizerra, F.C.; Da Silva, P.I.; Hayashi, M.A.F. Exploring the antibacterial properties of honey and its potential. Front. Microbiol. 2012, 3, 398. [Google Scholar] [CrossRef] [Green Version]
- Zainol, M.I.; Yusoff, K.M.; Yusof, M.Y.M. Antibacterial activity of selected Malaysian honey. BMC Complement. Altern. Med. 2013, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Israili, Z.H. Antimicrobial Properties of Honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Salom, K.; Butler, G.; Al Ghamdi, A.A. Honey and Microbial Infections: A Review Supporting the Use of Honey for Microbial Control. J. Med. Food 2011, 14, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of Physico-Chemical Properties and Antioxidant Capacities of Bioactive Honey Produced from Australian Grown Agastache rugosa and its Correlation with Colour and Poly-Phenol Content. Molecules 2018, 23, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cebrero, G.; Sanhueza, O.; Pezoa, M.; Báez, M.E.; Martínez, J.; Báez, M.; Fuentes, E. Relationship among the minor constituents, antibacterial activity and geographical origin of honey: A multifactor perspective. Food Chem. 2020, 315, 126296. [Google Scholar] [CrossRef]
- Silva, B.; Biluca, F.C.; Gonzaga, L.V.; Fett, R.; Dalmarco, E.M.; Caon, T.; Costa, A.C.O. In vitro anti-inflammatory properties of honey flavonoids: A review. Food Res. Int. 2021, 141, 110086. [Google Scholar] [CrossRef] [PubMed]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mama, M.; Teshome, T.; Detamo, J. Antibacterial Activity of Honey against Methicillin-Resistant Staphylococcus aureus: A Laboratory-Based Experimental Study. Int. J. Microbiol. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, A.C.; Aygin, D. Honey Dressing in Wound Treatment: A Systematic Review. Complement. Ther. Med. 2020, 51, 102388. [Google Scholar] [CrossRef]
- Balázs, V.L.; Horváth, B.; Kerekes, E.; Ács, K.; Kocsis, B.; Varga, A.; Böszörményi, A.; Nagy, D.U.; Krisch, J.; Széchenyi, A.; et al. Anti-Haemophilus Activity of Selected Essential Oils Detected by TLC-Direct Bioautography and Biofilm Inhibition. Molecules 2019, 24, 3301. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.; O’Brien, M.; Georges, K.; Suepaul, S. Physical characteristics and antimicrobial properties of Apis mellifera, Frieseomelitta nigra and Melipona favosa bee honeys from apiaries in Trinidad and Tobago. BMC Complement. Med. Ther. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Waili, N.S. Investigating the Antimicrobial Activity of Natural Honey and Its Effects on the Pathogenic Bacterial Infections of Surgical Wounds and Conjunctiva. J. Med. Food 2004, 7, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Newby, R.S.; Dryden, M.; Allan, R.N.; Salib, R.J. Antimicrobial activity of a novel bioengineered honey against non-typeable Haemophilus influenzae biofilms: An in vitro study. J. Clin. Pathol. 2018, 71, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Virjamo, V.; Tammela, P.; Fauch, L.; Julkunen-Tiitto, R. Screening bioactivity and bioactive constituents of Nordic unifloral honeys. Food Chem. 2017, 237, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Awan, U.A.; Ali, S.; Andleeb, S. A Comparative Study of Antibacterial and Antioxidant Activities of Wild Honey (Sunflower and Eucalyptus) and Commercial Honey. J. Pharm. Sci. Pharmacol. 2014, 1, 211–218. [Google Scholar] [CrossRef]
- Abeshu, M.; Geleta, B. Medicinal Uses of Honey. Biol. Med. 2016, 8, 279. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; da Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef]
- Moghadam, M.N.; Khaledi, E.M. Antibacterial activity and mechanism of action of some Iranian honeys compared to manuka honey against multidrug-resistant respiratory and urinary infections. Food Biosci. 2021, 41, 101003. [Google Scholar] [CrossRef]
- Alandejani, T.; Marsan, J.; Ferris, W.; Slinger, R.; Chan, F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Neck Surg. 2009, 141, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Majkut, M.; Kwiecińska-Piróg, J.; Wszelaczyńska, E.; Pobereżny, J.; Gospodarek-Komkowska, E.; Wojtacki, K.; Barczak, T. Antimicrobial activity of heat-treated Polish honeys. Food Chem. 2021, 343, 128561. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, I.; Tsadila, C.; Nikolaidis, M.; Tsavea, E.; Dimitriou, T.G.; Iliopoulos, I.; Amoutzias, G.D.; Mossialos, D. Tran-scriptomic Analysis of Pseudomonas Aeruginosa Response to Pine Honey via RNA Sequencing Indicates Multiple Mechanisms of Antibacterial Activity. Foods 2021, 10, 936. [Google Scholar] [CrossRef]
- Moussa, A.; Noureddine, D.; Mohamed, H.S.; Abdelmelek, M.; Saad, A. Antibacterial activity of various honey types of Algeria against Staphylococcus aureus and Streptococcus pyogenes. Asian Pac. J. Trop. Med. 2012, 5, 773–776. [Google Scholar] [CrossRef] [Green Version]
- Patel, H.R.; Krishnan, C.A.; Thanveer, K. Antimicrobial effect of honey on Streptococcus mutans—An in vitro study. Int. J. Dent. Sci. Res. 2013, 1, 46–49. [Google Scholar] [CrossRef]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef]
- Huttunen, S.; Riihinen, K.; Kauhanen, J.; Tikkanen-Kaukanen, C. Antimicrobial activity of different F innish monofloral honeys against human pathogenic bacteria. APMIS 2013, 121, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Otmani, A.; Amessis-Ouchemoukh, N.; Birinci, C.; Yahiaoui, S.; Kolayli, S.; Rodríguez-Flores, M.S.; Escuredo, O.; Seijo, M.C.; Ouchemoukh, S. Phenolic compounds and antioxidant and antibacterial activities of Algerian honeys. Food Biosci. 2021, 42, 101070. [Google Scholar] [CrossRef]
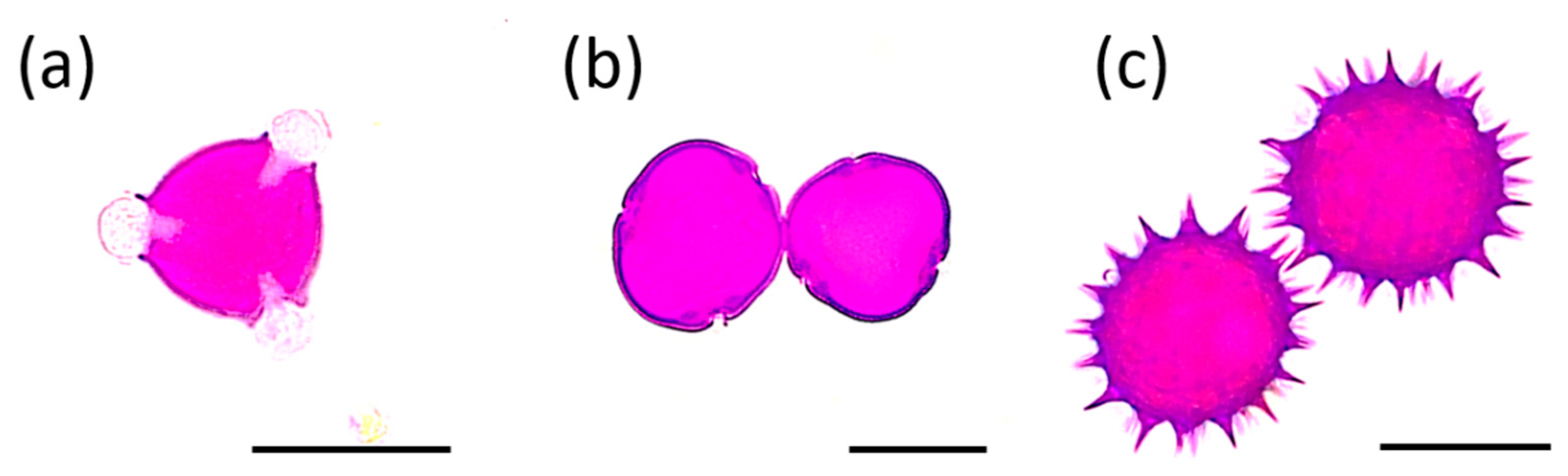

| Honey Type | Pollen Type—Relative Frequency (%) | ||||||
|---|---|---|---|---|---|---|---|
| Robinia | Tilia | Helianthus | Solidago | Brassica | Apiaceae | Other | |
| black locust R. pseudoacacia | 67.0 | 0.5 | - | 13.8 | 5.4 | 0.5 | 12.8 |
| linden Tilia sp. | 27.6 | 41.0 | 16.1 | - | - | - | 15.3 |
| sunflower H. annuus | 16.3 | 7.4 | 48.1 | 3.8 | - | 2.3 | 22.1 |
| Diameter of Inhibition Zones (cm) 1 | ||||
|---|---|---|---|---|
| Test Solution | H. influenzae | H. parainfluenzae | P. aeruginosa | S. pneumoniae |
| black locust 25% | 1.7 ± 0.2 a | 1.6 ± 0.3 a | 1.2 ± 0.1 ab | 1.6 ± 0.2 ab |
| linden 25% | 1.8 ± 0.2 a | 1.8 ± 0.2 a | 1.4 ± 0.2 a | 1.7 ± 0.2 a |
| sunflower 25% | 1.5 ± 0.1 ab | 1.6 ± 0.2 a | 1.0 ± 0.2 b | 1.3 ± 0.2 b |
| sugar solution 25% | 1.2 ± 0.2 b | 1.1 ± 0.1 b | 0.9 ± 0.2 b | 0.9 ± 0.3 c |
| antibiotic control | 3.5 ± 0.3 c | 3.5 ± 0.3 c | 3.4 ± 0.2 c | 3.6 ± 0.3 d |
| Diameter of Inhibition Zones (cm) 1 | ||||
|---|---|---|---|---|
| Test Solution | H. influenzae | H. parainfluenzae | P. aeruginosa | S. pneumoniae |
| black locust 50% | 1.9 ± 0.3 a | 1.9 ± 0.2 a | 1.7 ± 0.3 a | 1.9 ± 0.2 a |
| linden 50% | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 1.9 ± 0.3 a | 2.0 ± 0.1 a |
| sunflower 50% | 1.8 ± 0.2 a | 1.8 ± 0.2 a | 1.7 ± 0.2 a | 1.9 ± 0.2 a |
| sugar solution 50% | 1.4 ± 0.2 b | 1.3 ± 0.3 b | 1.2 ± 0.1 b | 1.3 ± 0.1 b |
| antibiotic control | 3.5 ± 0.3 c | 3.5 ± 0.3 c | 3.4 ± 0.2 c | 3.6 ± 0.3 c |
| Concentrations (%) | Lysis of P. aeruginosa Cells | Lysis of S. pneumoniae Cells | ||||||
|---|---|---|---|---|---|---|---|---|
| Sugar | Black Locust | Linden | Sunflower | Sugar | Black Locust | Linden | Sunflower | |
| A260 (%) | A260 (%) | |||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40 | 22.1 | 25.8 | 32.9 | 27.0 | 26.4 | 31.6 | 39.5 | 30.9 |
| 60 | 30.2 | 36.8 | 40.2 | 35.9 | 33.7 | 38.9 | 42.5 | 40.1 |
| 90 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Time (min) | Lysis of P. aeruginosa Cells | Lysis of S. pneumoniae Cells | ||||||
|---|---|---|---|---|---|---|---|---|
| Sugar | Black Locust | Linden | Sunflower | Sugar | Black Locust | Linden | Sunflower | |
| A260 (%) | A260 (%) | |||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 15.8 | 22.4 | 26.1 | 21.9 | 17.1 | 25.6 | 30.1 | 24.8 |
| 40 | 23.5 | 32.5 | 36.2 | 33.1 | 24.1 | 34.5 | 38.6 | 34.7 |
| 60 | 30.2 | 36.8 | 40.2 | 35.9 | 33.7 | 38.9 | 42.5 | 40.1 |
| 90 | 35.8 | 45.2 | 51.6 | 46.1 | 37.1 | 47.2 | 54.9 | 50.6 |
| Honey Samples | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| black locust | 42.0% | 42.0% | 52.5% | 44.0% | |
| MIC value | linden | 40.5% | 40.5% | 50.5% | 42.5% |
| sunflower | 42.0% | 42.0% | 52.5% | 45.0% | |
| black locust | 21.0% | 21.0% | 26.3% | 22.0% | |
| MIC/2 value | linden | 20.3% | 20.3% | 25.3% | 21.3% |
| sunflower | 21.0% | 21.0% | 26.3% | 22.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balázs, V.L.; Nagy-Radványi, L.; Filep, R.; Kerekes, E.; Kocsis, B.; Kocsis, M.; Farkas, Á. In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria. Foods 2021, 10, 1632. https://doi.org/10.3390/foods10071632
Balázs VL, Nagy-Radványi L, Filep R, Kerekes E, Kocsis B, Kocsis M, Farkas Á. In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria. Foods. 2021; 10(7):1632. https://doi.org/10.3390/foods10071632
Chicago/Turabian StyleBalázs, Viktória Lilla, Lilla Nagy-Radványi, Rita Filep, Erika Kerekes, Béla Kocsis, Marianna Kocsis, and Ágnes Farkas. 2021. "In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria" Foods 10, no. 7: 1632. https://doi.org/10.3390/foods10071632
APA StyleBalázs, V. L., Nagy-Radványi, L., Filep, R., Kerekes, E., Kocsis, B., Kocsis, M., & Farkas, Á. (2021). In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria. Foods, 10(7), 1632. https://doi.org/10.3390/foods10071632