Probiotics, Prebiotics, Synbiotics and Dental Caries. New Perspectives, Suggestions, and Patient Coaching Approach for a Cavity-Free Mouth
Abstract
1. Introduction
2. Nomenclature
3. Modes of Action of Oral Probiotics
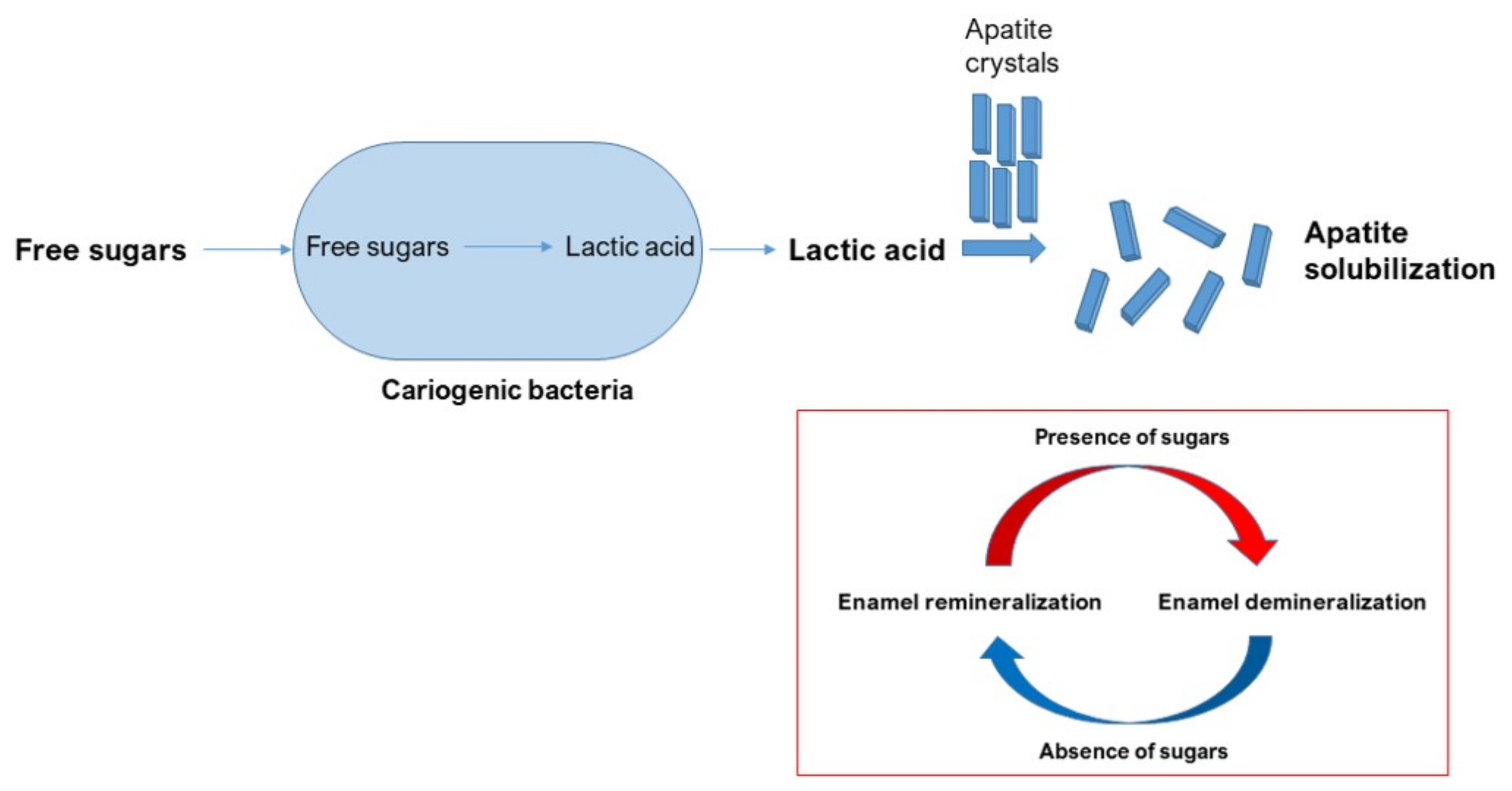
4. Caries Pathogenesis
5. Caries Management with Probiotics
6. Clinical Considerations on Probiotics’ Effectiveness
7. The Concept of the ‘Effector Strain’
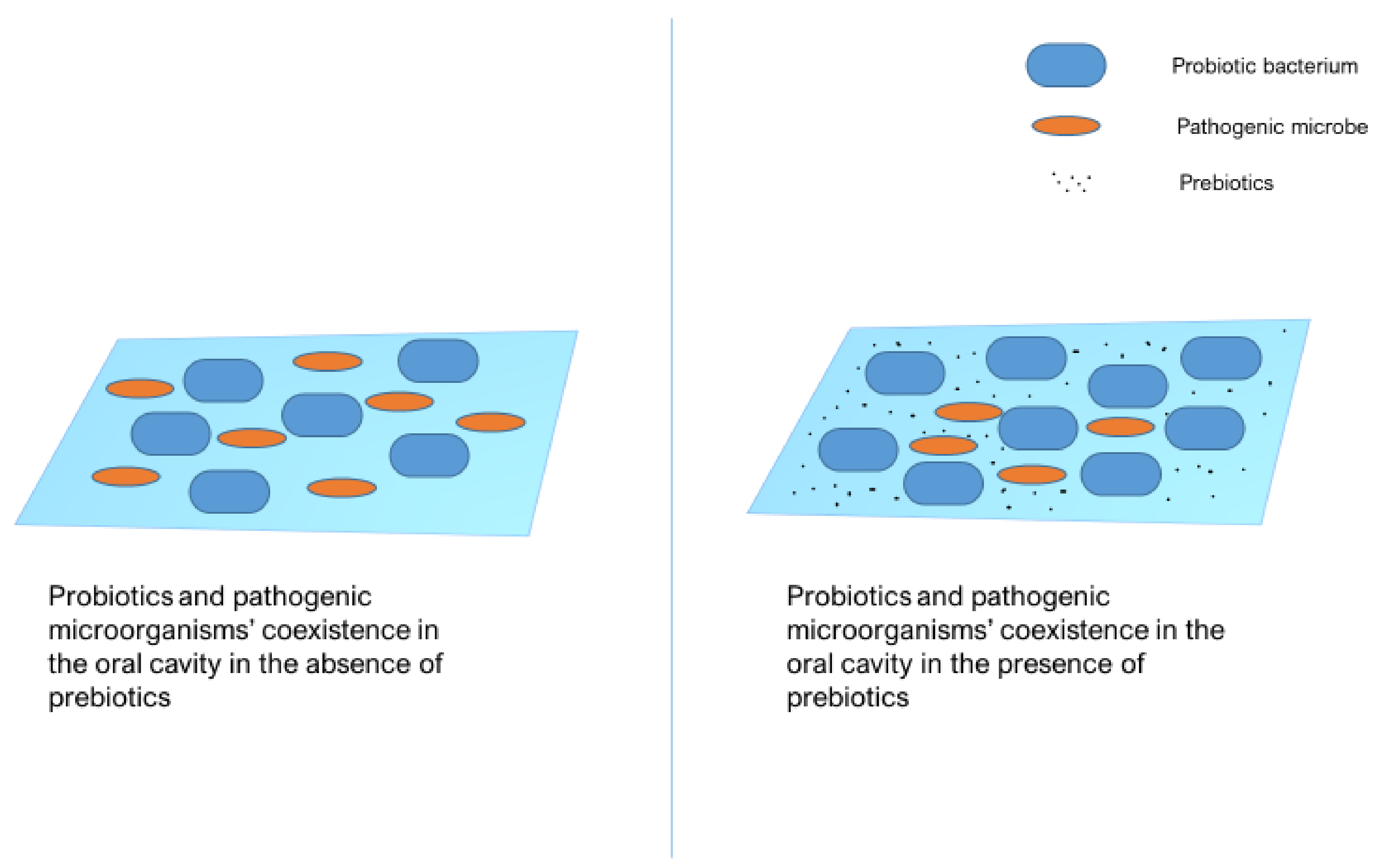
8. Synbiotics: A New Perspective in Caries Management
9. Patient Coaching Approach on the Use of Probiotics for Caries Prevention
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Conrads, G.; About, I. Pathophysiology of Dental Caries. Monogr. Oral Sci. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Luan, W.; Baelum, V.; Fejerskov, O.; Chen, X. Ten-Year Incidence of Dental Caries in Adult and Elderly Chinese. Caries Res. 2000, 34, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Beikler, T.; Flemmig, T.F. Oral biofilm-associated diseases: Trends and implications for quality of life, systemic health and expenditures. Periodontology 2000 2011, 55, 87–103. [Google Scholar] [CrossRef]
- De Amorim, R.G.; Figueiredo, M.J.; Leal, S.C.; Mulder, J.; Frencken, J.E. Caries experience in a child population in a deprived area of Brazil, using ICDAS II. Clin. Oral Investig. 2012, 16, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.A.; Thornton-Evans, G.; Li, X.; Iafolla, T. Dental Caries and Tooth Loss in Adults in the United States, 2011–2012; NCHS Data Brief, No. 197; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2015.
- Sweeney, L.C.; Dave, J.; Chambers, P.A.; Heritage, J. Antibiotic resistance in general dental practice—A cause for concern? J. Antimicrob. Chemother. 2004, 53, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zhou, Y.; Li, Z.; Huang, T.; Xiao, Y.; Cheng, L.; Peng, X.; Zhang, L.; Ren, B. Application of Antibiotics/Antimicrobial Agents on Dental Caries. Biomed. Res. Int. 2020, 2020, 5658212. [Google Scholar] [CrossRef]
- Teughels, W.; Van Essche, M.; Sliepen, I.; Quirynen, M. Probiotics and oral healthcare. Periodontology 2000 2008, 48, 111–147. [Google Scholar] [CrossRef]
- Caglar, E.; Kargul, B.; Tanboga, I. Bacteriotherapy and probiotics’ role on oral health. Oral Dis. 2005, 11, 131–137. [Google Scholar] [CrossRef]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2021, 10, 69. [Google Scholar] [CrossRef]
- Metchnikoff, I. Lactic acid as inhibiting intestinal putrefactions. In The Prolongation of Life: Optimistic Studies; Metchnikoff, I., Mitchel, P.C., Eds.; Heinemann: London, UK, 1907; pp. 161–183. ISBN 978-0-5989-8207-0. [Google Scholar]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-promoting factors produced by microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar]
- Havenaar, R.; Huis In’t Veld, J.H.J. Probiotics: A general view. In The Lactic Acid Bacteria in Health and Disease, 1st ed.; Wood, B.J.B., Ed.; Springer: Boston, MA, USA, 1992; Volume 1, pp. 151–170. ISBN 978-185-166-720-8. [Google Scholar]
- Naidu, A.S.; Bidlack, W.R.; Clemens, R.A. Probiotic spectra of lactic acid bacteria (LAB). Crit. Rev. Food Sci. Nutr. 1999, 39, 13–126. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361S–364S. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food. In Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002; Available online: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 15 December 2020).
- Roberfroid, M. Prebiotics: The Concept Revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbioata: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Meurman, J.H.; Antila, H.; Salminen, S. Recovery of Lactobacillus Strain GG (ATCC 53103) from Saliva of Healthy Volunteers after Consumption of Yoghurt Prepared with the Bacterium. Microb. Ecol. Health Dis. 1994, 7, 295–298. [Google Scholar] [CrossRef][Green Version]
- Näse, L.; Hatakka, K.; Savilahti, E.; Saxelin, M.; Pönkä, A.; Poussa, T.; Korpela, R.; Meurman, J.H. Effect of Long-Term Consumption of a Probiotic Bacterium, Lactobacillus rhamnosus GG, in Milk on Dental Caries and Caries Risk in children. Caries Res. 2001, 35, 412–420. [Google Scholar] [CrossRef]
- Ahola, A.J.; Yli-Knuuttila, H.; Suomalainen, T.; Poussa, T.; Ahlström, A.; Meurman, J.H.; Korpela, R. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch. Oral Biol. 2002, 47, 799–804. [Google Scholar] [CrossRef]
- Aminabadi, N.A.; Erfanparast, L.; Ebrahimi, A.; Okouei, S.G. Effect of Chlorhexidine Pretreatment on the Stability of Salivary Latobacilli Probiotic in Six- to Twelve-Year-Old Children: A randomized Controlled Trial. Caries Res. 2011, 45, 148–154. [Google Scholar] [CrossRef]
- Juneja, A.; Kakade, A. Evaluating the Effect of Probiotic Containig Milk on Salivary mutans streptococci Levels. J. Clin. Pediatr. Dent. 2012, 37, 9–14. [Google Scholar] [CrossRef]
- Stecksen-Blicks, C.; Sjöström, I.; Twetman, S. Effect of Long-Term Consumption of Milk Supplemented with Probiotic Lactobacilli and Fluoride on Dental Caries and General Health in Preschool Children: A Cluster Randomized Study. Caries Res. 2009, 43, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Makihira, S.; Fukushima, H.; Nishimura, H.; Ozaki, Y.; Ishida, K.; Darmawan, S.; Hamada, T.; Hara, K.; Matsumoto, A.; et al. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int. J. Food Microbiol. 2004, 95, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Caglar, E.; Kavaloglu-Cildir, S.; Ergeneli, S.; Sandalli, N.; Twetman, S. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol. Scand. 2006, 64, 314–318. [Google Scholar] [CrossRef]
- Stensson, M.; Koch, G.; Coric, S.; Abrahamsson, T.R.; Jenmalm, M.C.; Birkhed, D.; Wendt, L.K. Oral Administration of Lactobacillus reuteri during the First Year of Life Reduces Caries Prevalence in the Primary Dentition at 9 Years of Age. Caries Res. 2014, 48, 111–117. [Google Scholar] [CrossRef]
- Kavaloglu-Cildir, S.; Sandalli, N.; Nazli, S.; Alp, F.; Caglar, E. A Novel Delivery System of Probiotic Drop and its Effect on Dental Caries Risk Factors in Cleft Lip/Palate Children. Cleft Palate Craniofac. J. 2012, 49, 369–372. [Google Scholar] [CrossRef]
- Keller, M.K.; Nohr Larsen, I.; Karlsson, I.; Twetman, S. Effect of tablets containing probiotic bacteria (Lactobacillus reuteri) on early caries lesions in adolescents: A pilot study. Benef. Microbes 2014, 5, 403–407. [Google Scholar] [CrossRef]
- Gizani, S.; Petsi, G.; Twetman, S.; Caroni, C.; Makou, M.; Papagianoulis, L. Effect of the probiotic bacterium Lactobacillus reuteri on white spote lesion development in orthodontic patients. Eur. J. Orthod. 2016, 38, 85–89. [Google Scholar] [CrossRef]
- Alamoudi, N.M.; Almabadi, E.S.; El Ashiry, E.A.; El Derwi, D.A. Effect of Probiotic Lactobacillus reuteri on salivary Cariogenic Bacterial Counts among Groups of Preschool Children in Jeddah, Saudi Arabia: A randomized Clinical Trial. J. Clin. Pediatr. Dent. 2018, 42, 331–338. [Google Scholar] [CrossRef]
- Yadav, M.; Poornima, P.; Roshan, N.M.; Prachi, N.; Veena, M.; Neena, I.E. Evaluation of Probiotic Milk on Salivary Mutans Streptococci Count: An In Vivo Microbiological Study. J. Clin. Pediatr. Dent. 2014, 39, 23–26. [Google Scholar] [CrossRef]
- Hasslöf, P.; West, C.E.; Karlsson Videhult, F.; Brandelius, C.; Stecksen-Blicks, C. Early Intervention with Probiotic Lactobacillus paracasei F19 Has No Long-Term Effect on Caries Experience. Caries Res. 2013, 47, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.C.; Huang, C.S.; Ou-Yang, L.W.; Lin, S.Y. Probiotic Lactobacillus paracasei effect on cariogenic bacterial flora. Clin. Oral Investig. 2011, 15, 471–476. [Google Scholar] [CrossRef]
- Teanpaisan, R.; Piwat, S. Lactobacillus paracasei SD1, a novel probiotic, reduces mutans streptococci in human volunteers: A randomized placebo-controlled trial. Clin. Oral Investig. 2014, 18, 857–862. [Google Scholar] [CrossRef]
- Pahumunto, N.; Piwat, S.; Chankanka, O.; Akkarachaneeyakorn, N.; Rangsitsathian, K.; Teanpaisan, R. Reducing mutans streptococci and caries development by Lactobacillus paracasei SD1 in preschool children: A randomized placebo-controlled trial. Acta Odontol. Scand. 2018, 76, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Manmontri, C.; Nirunsittirat, A.; Piwat, S.; Wattanarat, O.; Pahumunto, N.; Makeudom, A.; Sastraruji, T.; Krisanaprakornkit, S.; Teanpaisan, R. Reduction of Streptococcus mutans by probiotic milk: A multicenter randomized controlled trial. Clin. Oral Investig. 2020, 24, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, E.; Mazaheri, R.; Tahmourespour, A. Effect of Probiotic Yogurt and Xylitol-Containing Chewing Gums on Salivary S Mutans Count. J. Clin. Pediatr. Dent. 2017, 41, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Damle, S.G.; Chawla, A. Salivary mutans streptococci and lactobacilli modulations in young children on consumption of probiotic ice-cream containing Bifidobacterium lactis Bb-12 and Lactobacillus La5. Acta Odontol. Scand. 2011, 69, 389–394. [Google Scholar] [CrossRef]
- Nishihara, T.; Suzuki, N.; Yoneda, M.; Hirofuji, T. Effects of Lactobacillus salivarius-containing tablets on caries risk factors: A randomized open-label clinical trial. BMC Oral Health 2014, 14, 110. [Google Scholar] [CrossRef]
- Lai, S.; Lingström, P.; Cagetti, M.G.; Cocco, F.; Meloni, G.; Arrica, M.A.; Campus, G. Effect of Lactobacillus brevis CD2 containing lozenges and plaque pH and cariogenic bacteria in diabetic children: A randomised clinical trial. Clin. Oral Investig. 2021, 25, 115–123. [Google Scholar] [CrossRef]
- Montalto, M.; Vastola, M.; Marigo, L.; Covino, M.; Graziosetto, R.; Curigliano, V.; Santoro, L.; Cuoco, L.; Manna, R.; Gasbarrini, G. Probiotic Treatment Increases Salivary Counts of Lactobacilli: A Double-Blind, Randomized, Controlled Study. Digestion 2004, 69, 53–56. [Google Scholar] [CrossRef]
- Caglar, E.; Kuscu, O.O.; Selvi Kuvvetli, S.; Kavaloglu Cildir, S.; Sandalli, N.; Twetman, S. Short-term effect of ice-cream containing Bifidobacterium lactis Bb-12 on the number of salivary mutans streptococci and lactobacilli. Acta Odontol Scand 2008, 66, 154–158. [Google Scholar] [CrossRef]
- Taipale, T.; Pienihäkkinen, K.; Salminen, S.; Jokela, J.; Söderling, E. Bifidobacterium animalis subsp. Lactis BB-12 Administration in Early Childhood: A randomized Clinical Trial of Effects on Oral Colonization by Mutans Streptococci and the Probiotic. Caries Res. 2012, 46, 69–77. [Google Scholar] [CrossRef]
- Javid, A.Z.; Amerian, E.; Basir, L.; Ekrami, A.; Haghighizadeh, M.H.; Maghsoumi-Norouzabad, L. Effects of the Consumption of Probiotic Yogurt Containing Bifidobacterium lactis Bb12 on the Levels of Streptococcus mutans and Lactobacilli in Saliva of Students with Initial Stages of Dental Caries: A Double-Blind Randomized Controlled Trial. Caries Res. 2020, 54, 68–74. [Google Scholar] [CrossRef]
- Caglar, E.; Sandalli, N.; Twetman, S.; Kavaloglu, S.; Ergeneli, S.; Selvi, S. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol. Scand. 2005, 63, 317–320. [Google Scholar] [CrossRef]
- Pinto, G.S.; Cenci, M.S.; Azevedo, M.S.; Epifanio, M.; Jones, M.H. Effect of Yogurt Containing Bifidobacterium animalis subsp. lactis DN-173010 Probiotic on Dental Plaque and Saliva in Orthodontic Patients. Caries Res. 2014, 48, 63–68. [Google Scholar] [CrossRef]
- Villavicencio, J.; Villegas, L.M.; Arango, M.C.; Arias, S.; Triana, F. Effects of a food enriched with probiotics on Streptococcus mutans and Lactobacillus spp. salivary counts in preschool children: A cluster randomized trial. J. Appl. Oral Sci. 2018, 26, e20170318. [Google Scholar] [CrossRef]
- Hillman, J.D.; Mo, J.; McDonell, E.; Cvitkovitch, D.; Hillman, C.H. Modification of an effector strain for replacement therapy of dental caries to enable clinical safety trials. J. Appl. Microbiol. 2007, 102, 1209–1219. [Google Scholar] [CrossRef]
- Zahradnik, R.T.; Magnusson, I.; Walker, C.; McDonell, E.; Hillman, C.H.; Hillman, J.D. Preliminary assessment of safety and effectiveness in humans of ProBiora3 TM, a probiotic mouthwash. J. Appl. Microbiol. 2009, 107, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Hedayati-Hajikand, T.; Lundberg, U.; Eldh, C.; Twetman, S. Effect of probiotic chewing tablets on early childhood caries—A randomized controlled trial. BMC Oral Health 2015, 15. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Lopez-Lopez, A.; Nicolescu, T.; Salavert, A.; Mendez, I.; Cune, J.; Llena, C.; Mira, A. A pilot study to assess oral colonization and pH buffering by the probiotic Streptococcus dentisani under different dosing regimes. Odontology 2020, 108, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.P.; Drummond, B.K.; Chilcott, C.N.; Tag, J.R.; Thomson, W.M.; Hale, J.D.F.; Wescombe, P.A. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: A randomized double-blind, placebo-controlled trial. J. Med. Microbiol. 2013, 62, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Koopaie, M.; Fatahzadeh, M.; Jahangir, S.; Bakhtiari, R. Comparison of the effect of regular and probiotic cake (Bacillus coagulans) on salivary pH and Streptococcus mutans count. Dent. Med. Probl. 2019, 56, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Loozen, G.; Quirynen, M. Do probiotics offer opportunities to manipulate the periodontal oral microbiota? J. Clin. Periodontol. 2011, 38, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Delcenserie, V.; Martel, D.; Lamoureux, M.; Amiot, K.; Boutin, Y.; Roy, D. Immunomodulatory Effects of Probiotics in the Intestinal Tract. Curr. Iss. Mol. Biol. 2008, 10, 37–54. [Google Scholar]
- Schiffrin, E.J.; Brassart, D.; Selvin, A.L.; Rochat, F.; Donnet-Hughes, A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: Criteria for strain selection. Am. J. Clin. Nutr. 1997, 66, 515S–520S. [Google Scholar] [CrossRef]
- Pelto, L.; Isolauri, E.; Lilius, E.M.; Nuutila, J.; Salminen, S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clin. Exp. Allergy 1998, 28, 1474–1479. [Google Scholar] [CrossRef]
- Schiffrin, E.J.; Rochat, F.; Link-Amster, H.; Aeschlimann, J.M.; Donnet-Hughes, A. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J. Dairy Sci. 1995, 78, 491–497. [Google Scholar] [CrossRef]
- Donnet-Hughes, A.; Rochat, F.; Serrant, P.; Aeschlimann, J.M.; Schiffrin, E.J. Modulaion of nonspecific mechanisms of defense by lactic acid bacteria: Effective dose. J. Dairy Sci. 1999, 82, 863–869. [Google Scholar] [CrossRef]
- Kaila, M.; Isolauri, E.; Saxelin, M.; Arvilommi, H.; Vesikari, T. Viable versus inactivated Lactobacillus strain GG in acute rotavirus diarrhea. Arch. Dis. Child. 1995, 72, 51–53. [Google Scholar] [CrossRef]
- Majamaa, H.; Isolauri, E.; Saxelin, M.; Vesikari, T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 333–338. [Google Scholar] [CrossRef]
- Fukushima, Y.; Kawata, Y.; Hara, H.; Terada, A.; Mitsuoka, T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int. J. Food. Microbiol. 1998, 42, 39–44. [Google Scholar] [CrossRef]
- Cosseau, C.; Devine, D.A.; Dullaghan, E.; Gardy, J.L.; Chikatamarla, A.; Gellatly, S.; Yu, L.L.; Pistolic, J.; Falsafi, R.; Tagg, J.; et al. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect. Immun. 2008, 76, 4163–4175. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, R.; Rudney, J.D. Streptococcus cristatus attenuates Fusobacterium nucleatum-induced interleukin-8 expression in oral epithelial cells. J. Periodontal. Res. 2008, 43, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, Ι.; Van Damme, J.; Van Essche, M.; Loozen, G.; Quirynen, M.; Teughels, W. Microbial interactions influence inflammatory host cell responses. J. Dent. Res. 2009, 88, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Della Riccia, D.N.; Bizzini, F.; Perilli, M.G.; Polimeni, A.; Trinchieri, V.; Amicosante, G.; Cifone, M.G. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007, 13, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Wattanarat, O.; Makeudom, A.; Sastraruji, T.; Piwat, S.; Tianviwat, S.; Teanpaisan, R.; Krisanaprakornkit, S. Enhancement of salivary human neutrophil peptide 1-3 levels by probiotic supplementation. BMC Oral Health 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Etzion, A.; Riley, M.A. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 2008, 81, 591–606. [Google Scholar] [CrossRef]
- Gordon, D.M. The potential of bacteriocin-producing probiotics and associated caveats. Future Microbiol. 2009, 4, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Jacobus, N.V.; Deneke, C.; Gorbach, S.L. Antimicrobial Substance from a human Lactobacillus Strain. Antimicrob. Agents Chemother. 1987, 31, 1231–1233. [Google Scholar] [CrossRef]
- Axelsson, L.T.; Chung, T.C.; Dobrogosz, W.J.; Lindgren, S.E. Production of a Broad Spectrum Antimicrobial Substance by Lactobacillus reuteri. Microb. Ecol. Health Dis. 1989, 2, 131–136. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Höltzel, A.; Walter, J.; Jung, G.; Hammes, W.P. Characterization of Reutericyclin Produced by Lactobacillus reuteri LTH2584. Appl. Environ. Microbiol. 2000, 66, 4325–4333. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Auchtung, T.A.; Hermans, K.E.; Whitehead, D.; Borhan, B.; Britton, R.A. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 2010, 156, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G.; Vogel, R.F. Studies on the Mode of Action of Reutericyclin. Appl. Environ. Microbiol. 2003, 69, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Elli, M.; Zink, R.; Rytz, A.; Reniero, R.; Morelli, L. Iron requirement of Lactobacillus spp. in completely chemically defined growth media. J. Appl. Microbiol. 2000, 88, 695–703. [Google Scholar] [CrossRef]
- Teughels, W.; Kinder Haake, S.; Sliepen, I.; Pauwels, M.; Van Eldere, J.; Cassiman, J.J.; Quirynen, M. Bacteria interfere with A. actinomycetemcomitans colonization. J. Dent. Res. 2007, 86, 611–617. [Google Scholar] [CrossRef]
- Sliepen, I.; Hofkens, J.; Van Essche, M.; Quirynen, M.; Teughels, W. Aggregatibacter actinomycetemcomitans adhesion inhibited in a flow cell. Oral Microbiol. Immunol. 2008, 23, 520–524. [Google Scholar] [CrossRef]
- Van Hoogmoed, C.G.; van der Kuijl-Booij, M.; van der Mei, H.C.; Busscher, H.J. Inhibition of Streptococcus mutans NS Adhesion to Glass with and without a Salivary Conditioning Film by Biosurfactant-Releasing Streptococcus mitis Strains. Appl. Environ. Microbiol. 2000, 66, 659–663. [Google Scholar] [CrossRef]
- Haukioja, A.; Loimaranta, V.; Tenovuo, J. Probiotic bacteria affect the composition of salivary pellicle and Streptococcal adhesion in vitro. Oral Microbiol. Immunol. 2008, 23, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Martin, M.V. Plaque-mediated diseases: Dental caries and periodontal diseases. In Marsh and Martin’s Oral Microbiology, 6th ed.; Marsh, P.D., Lewis, M.A.O., Rogers, H., Williams, D.W., Wilson, M., Eds.; Elsevier Ltd.: Amsterdam, Netherlands, 2016; pp. 112–129. ISBN 978-070-206-106-6. [Google Scholar]
- Marsh, P.D. Dental Biofilms in Health and Disease. In Understanding Dental Caries: From Pathogenesis to Prevention and Therapy, 1st ed.; Goldberg, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 41–52. ISBN 978-3-319-80837-6. [Google Scholar]
- Keyes, P.H. Research in dental caries. J. Am. Dent. Assoc. 1968, 76, 1357–1373. [Google Scholar] [CrossRef]
- Simon-Soro, A.; Belda-Ferre, P.; Cabrera-Rubio, R.; Alcaraz, L.D.; Mira, A. A Tissue-Dependent Hypothesis of Dental Caries. Caries Res. 2013, 47, 591–600. [Google Scholar] [CrossRef]
- Gedalia, I.; Dakuar, A.; Shapira, L.; Lewinstein, I.; Goultschin, J.; Rahamin, E. Enamel softening with Coca Cola and rehardening with milk or saliva. Am. J. Dent. 1991, 4, 120–122. [Google Scholar]
- Bowen, W.H.; Pearson, S.K. Effect of milk on cariogenesis. Caries Res. 1993, 27, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kashket, S.; Yaskell, T. Effectiveness of calcium lactate added to food in reducing intraoral demineralization of enamel. Caries Res. 1997, 31, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Gorbach, S.L.; Goldin, B.R. Lactobacillus strains and methods of selection. US Patent Application No. 4,839,281, 13 June 1989. [Google Scholar]
- Hatakka, K.; Savilahti, E.; Pönkä, A.; Meurman, J.H.; Poussa, T.; Näse, L.; Saxelin, M.; Korpela, R. Effect of long term consumption of probiotic milk on infections in children attending day care centres: Double blind, randomized trial. BMJ 2001, 322, 1327–1329. [Google Scholar] [CrossRef]
- Antoniadou, M.; Varzakas, T. Probiotics and Prebiotics and Their Effect on Food and Human Health: New Perspectives. In Probiotics, the Natural Microbiota in Living Organisms: Fundamental and Applications; El Enshasy, H.A., Yang, S.T., Eds.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-113-849-360-5. [Google Scholar]
- Grudianov, A.I.; Dmitrieva, N.A.; Fomenko, E.V. Use of probiotics Bifidumbacterin and Acilact in tablets in therapy of periodontal inflammations. Stomatol. Mosk. 2002, 81, 39–43. [Google Scholar]
- Van Houte, J. Role of Micro-organisms in Caries Etiology. J. Dent. Res. 1994, 73, 672–681. [Google Scholar] [CrossRef]
- Busscher, H.J.; Mulder, A.F.J.M.; van der Mei, H.C. In vitro Adhesion to Enamel and in vivo Colonization of Tooth Surfaces by Lactobacilli from a Bio-Yoghurt. Caries Res. 1999, 33, 403–404. [Google Scholar] [CrossRef]
- Petti, S.; Tarsitani, G.; D’Arca, A.S. A randomized clinical trial of the effect of yoghurt on the human salivary microflora. Arch. Oral Biol. 2001, 46, 705–712. [Google Scholar] [CrossRef]
- Yli-Knuuttila, H.; Snäll, J.; Kari, K.; Meurman, J.H. Colonization of Lactobacillus rhamnosus GG in the oral cavity. Oral Microbiol. Immunol. 2006, 21, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Devine, D.A.; Marsh, P.D. Prospects for the development of probiotics and prebiotics for oral applications. J. Oral Microbiol. 2009, 1, 1949. [Google Scholar] [CrossRef]
- Killian, M.; Frandsen, E.V.G.; Haubek, D.; Poulsen, K. The etiology of periodontal disease revisited by population genetic analysis. Periodontology 2000 2006, 42, 158–179. [Google Scholar] [CrossRef]
- Hillman, J.D.; Socransky, S.S. Replacement therapy for the prevention of dental disease. Adv. Dent. Res. 1987, 1, 119–125. [Google Scholar] [CrossRef]
- Hillman, J.D. Lactate Dehydrogenase Mutants of Streptococcus mutans: Isolation and Preliminary Characterization. Infect. Immun. 1978, 21, 206–212. [Google Scholar] [CrossRef]
- Ikeda, T.; Iwanami, T.; Hirasawa, M.; Watanabe, C.; McGhee, J.R.; Shiota, T. Purification and Certain Properties of a Bacteriocin from Streptococcus mutans. Infect. Immun. 1982, 35, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Hillman, J.D.; Johnson, K.P.; Yaphe, B.I. Isolation of a Streptococcus mutans Strain Producing a Novel Bacteriocin. Infect. Immun. 1984, 44, 141–144. [Google Scholar] [CrossRef]
- Brown, A.T.; Wittenberger, C.L. Fructose-1,6-Diphosphate-Dependent Lactate Dehydrogenase from a Cariogenic Streptococcus: Purification and Regulatory Properties. J. Bacteriol. 1972, 110, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P. The Bacteriocins. Bacteriol. Rev. 1965, 29, 24–45. [Google Scholar] [CrossRef]
- Hillman, J.D.; Novak, J.; Sagura, E.; Gutierrez, J.A.; Brooks, T.A.; Crowley, P.J.; Hess, M.; Azizi, A.; Leung, K.P.; Cvitkovitch, D.; et al. Genetic and Biochemical Analysis of Mutacin 1140, a Lantibiotic from Streptococcus mutans. Infect. Immun. 1998, 66, 2743–2749. [Google Scholar] [CrossRef] [PubMed]
- Hurst, A. Nisin. Adv. Appl. Microbiol. 1981, 27, 85–123. [Google Scholar] [CrossRef]
- Ghobrial, O.G.; Derendorf, H.; Hillman, J.D. Pharmacodynamic activity of the lantibiotic MU1140. Int. J. Antimicrob. Agents 2009, 33, 70–74. [Google Scholar] [CrossRef]
- Ghobrial, O.G.; Derendorf, H.; Hillman, J.D. Pharmacokinetic and Pharmacodynamic Evaluation of the Lantibiotic MU1140. J. Pharm. Sci. 2010, 99, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Hasper, H.E.; Kramer, N.E.; Smith, J.L.; Hillman, J.D.; Zachariah, C.; Kuipers, O.P.; de Kruijff, B.; Breukink, E. An Alternative Bactericidal Mechanism of Action for Lantibiotic Peptides That Target Lipid II. Science 2006, 313, 1636–1637. [Google Scholar] [CrossRef]
- Smith, L.; Hasper, H.; Breukink, E.; Novak, J.; Cerkasov, J.; Hillman, J.D.; Wilson-Stanford, S.; Orugunty, R.S. Elucidation of the Antimicrobial Mechanism of Mutacin 1140. Biochemistry 2008, 47, 3308–3314. [Google Scholar] [CrossRef] [PubMed]
- De Kruijff, B.; van Dam, V.; Breukink, E. Lipid II: A central component in bacterial cell wall synthesis and a target for antibiotics. Prostaglandins Leukot. Essent. Fatty Acids 2008, 79, 117–121. [Google Scholar] [CrossRef]
- Malin, J.J.; de Leeuw, E. Therapeutic compounds targeting Lipid II for antibacterial purposes. Infect. Drug. Resist. 2019, 12, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Hillman, J.D.; Yaphe, B.I.; Johnson, K.P. Colonization of the Human Oral Cavity by a Strain of Streptococcus mutans. J. Dent. Res. 1985, 64, 1272–1274. [Google Scholar] [CrossRef]
- Hillman, J.D.; Dzuback, A.L.; Andrews, S.W. Colonization of the Human Oral Cavity by a Streptococcus mutans Mutant Producing Increased Bacteriocin. J. Dent. Res. 1987, 66, 1092–1094. [Google Scholar] [CrossRef]
- Hillman, J.D.; Chen, A.; Duncan, M.; Lee, S.W. Evidence that L-(+)-Lactate Dehydrogenase Deficiency Is Lethal in Streptococcus mutans. Infect. Immun. 1994, 62, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Hillman, J.D.; Duncan, M. L-(+)-Lactate Dehyrdrogenase Deficiency is Lethal in Streptococcus mutans. J. Bacteriol. 1994, 176, 1542–1545. [Google Scholar] [CrossRef][Green Version]
- Hillman, J.D.; Chen, A.; Snoep, J.L. Genetic and Physiological Analysis of the Lethal Effect of L-(+)-Lactate Dehydrogenase Deficiency in Streptococcus mutans: Complementation by Alcohol Dehydrogenase from Zymomonas mobilis. Infect. Immun. 1996, 64, 4319–4323. [Google Scholar] [CrossRef]
- Hillman, J.D.; Brooks, T.A.; Michalek, S.M.; Harmon, C.C.; Snoep, J.L.; van der Weijden, C.C. Construction and Characterization of an Effector Strain of Streptococcus mutans for Replacement Therapy of Dental Caries. Infect. Immun. 2000, 68, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Hillman, J.D.; McDonell, E.; Cramm, T.; Hillman, C.H.; Zahradnik, R.T. A spontaneous lactate dehydrogenase deficient mutant of Streptococcus rattus for use as a probiotic in the prevention of dental caries. J. Appl. Microbiol. 2009, 107, 1551–1558. [Google Scholar] [CrossRef]
- Hillman, J.D.; McDonell, E.; Hillman, C.H.; Zahradnik, R.T.; Soni, M.G. Safety Assessment of ProBiora3, a Probiotic Mouthwash: Subchronic Toxicity Study in Rats. Int. J. Toxicol. 2009, 28, 357–367. [Google Scholar] [CrossRef]
- Sun, J.H.; Xu, Q.A.; Fan, M.W. A new strategy for the replacement therapy of dental caries. Med. Hypotheses 2009, 73, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Mao, T.; Xu, Q.A.; Shao, J.; Liu, C.; Fan, M. A New gcrR-Deficient Streptococcus mutans Mutant for Replacement Therapy of Dental Caries. Sci. World J. 2013, 2013, 460202. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Healy, H.P.; Dawson, K.A.; Merchen, N.R.; Fahey, G.C., Jr. Supplemental Fructooligosaccharides and Mannanoligosaccharides Influence Immune Function, Ileal and Total Tract Nutrient Digestibilities, Microbial Populations and Concentrations of Protein Catabolites in the Large Bowel of Dogs. J. Nutr. 2002, 132, 980–989. [Google Scholar] [CrossRef]
- Yamada, K.; Tokunaga, Y.; Ikeda, A.; Ohkura, K.; Kaku-Ohkura, S.; Mamiya, S.; Ou Lim, B.; Tachibana, H. Effect of Dietary Fiber on the Lipid Metabolism and Immune Function of Aged Sprague-Dawley Rats. Biosci. Biotechnol. Biochem. 2003, 67, 429–433. [Google Scholar] [CrossRef][Green Version]
- Forchielli, M.L.; Walker, W.A. The role of gut-associated lymphoid tissues and mucosal defence. Br. J. Nutr. 2005, 93, S41–S48. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Robaina, L.; Real, F.; Sweetman, J.; Tort, L.; Izquierdo, M.S. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 2007, 23, 969–981. [Google Scholar] [CrossRef]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective Stimulation of Bifidobacteria in the Human Colon by Oligofructose and Inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef]
- Gibson, G.R.; McCartney, A.L.; Rastall, R.A. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2005, 93, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Kleessen, B.; Blaut, M. Modulation of gut mucosal biofilms. Br. J. Nutr. 2005, 93, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazzewi, F.H.; Khanna, S.; Tester, R.F.; Piggott, J. The potential use of hydrolysed konjac glucomannan as a prebiotic. J. Sci. Food Agric. 2007, 87, 1758–1766. [Google Scholar] [CrossRef]
- Elamir, A.A.; Tester, R.F.; Al-Ghazzewi, F.H.; Kaal, H.Y.; Ghalbon, A.A.; Elmegrahai, N.A.; Piggott, J.R. Effects of konjac glucomannan hydrolysates on the gut microflora of mice. Nutr. Food. Sci. 2008, 38, 422–429. [Google Scholar] [CrossRef]
- Park, S.F.; Kroll, R.G. Expression of listeriolysin and phosphatidylinositol specific phospholipace C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol. Microbiol. 1993, 8, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.F.; Al-Ghazzewi, F.H. A preliminary study of the synbiotic effects of konjac glucomannan hydrolysates (GMH) and lactobacilli on the growth of oral bacterium Streptococcus mutans. Nutr. Food Sci. 2011, 41, 234–237. [Google Scholar] [CrossRef]
- Kojima, Y.; Ohshima, T.; Seneviratne, C.J.; Maeda, N. Combining prebiotics and probiotics to develop novel synbiotics that suppress oral pathogens. J. Oral. Biosci. 2016, 58, 27–32. [Google Scholar] [CrossRef]
- Nunpan, S.; Suwannachart, C.; Wayakanon, K. The Inhibition of Dental Caries Pathogen by Using Prebiotic and Probiotic Combination. J. Dent. Assoc. Thai. 2017, 67, 31–38. [Google Scholar]
- Nunpan, S.; Suwannachart, C.; Wayakanon, K. Effect of Prebiotics-Enhanced Probiotics on the Growth of Streptococcus mutans. Int. J. Microbiol. 2019, 2019, 4623807. [Google Scholar] [CrossRef]
- Bijle, M.N.; Neelakantan, P.; Ekambaram, M.; Lo, E.C.M.; Yiu, C.K.Y. Effect of a novel synbiotic on Streptococcus mutans. Sci. Rep. 2020, 10, 7951. [Google Scholar] [CrossRef] [PubMed]
- Burne, R.A.; Marquis, R.E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 2000, 193, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Weaver, C.A.; Burne, R.A. Dual functions of Streptococcus salivarius urease. J Bacteriol 2000, 182, 4667–4669. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, T.; Zhang, J.; Zhou, X. Characterization of the Actinomyces naeslundii ureolysis and its role in bacterial aciduricity and capacity to modulate pH homeostasis. Microbiol. Res. 2006, 161, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Morou-Bermundez, E.; Burne, R.A. Analysis of urease expression in Actinomyces naeslundii WVU45. Infect. Immun. 2000, 68, 6670–6676. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.M. The effect of urea in counteracting the influence of carbohydrates on the pH of dental plaques. J. Dent. Res. 1943, 22, 63–71. [Google Scholar] [CrossRef]
- Clancy, K.A.; Pearson, S.; Bowen, W.H.; Burne, R.A. Characterization of recombinant, ureolytic Streptococcus mutans demonstrates an inverse relationship between dental plaque ureolytic capacity and cariogenicity. Infect. Immun. 2000, 68, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Van Wuyckhuyse, B.C.; Perinpanayagam, H.E.R.; Bevacqua, D.; Raubertas, R.E.; Billings, R.J.; Bowen, W.H.; Tabak, L.A. Association of free arginine and lysin concentrations in human parotid saliva with caries experience. J. Dent. Res. 1995, 74, 686–690. [Google Scholar] [CrossRef]
- Nascimento, M.M. Potential Uses of Arginine in Dentistry. Adv. Dent. Res. 2018, 29, 98–103. [Google Scholar] [CrossRef]
- Antoniadou, M. Delivering Self-Management Health Outcomes Using the Patient Activation Measure Instrument—Prospects for the Dentistry Field. Adv. Dent. Oral Health 2020, 12, 555833. [Google Scholar]
- Antoniadou, M.; Varzakas, T. Health and Oral Health Coaching Issues. Encyclopedia 2020. Available online: https://encyclopedia.pub/1755 (accessed on 1 April 2021).
- Avila, W.M.; Pordeus, I.A.; Paiva, S.M.; Martins, C.C. Breast and Bottle Feeding as Risk Factors for Dental Caries: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0142922. [Google Scholar] [CrossRef]
- Reddy, A.; Norris, D.F.; Momeni, S.S.; Waldo, B.; Ruby, J.D. The pH of beverages in the United States. J. Am. Dent. Assoc. 2016, 147, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Blotstein, F.A.; Jansen, E.C.; Jones, A.D.; Marshall, T.A.; Foxman, B. Dietary patterns associated with dental caries in adults in the United States. Community Dent. Oral Epidemiol. 2019, 48, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, M.; Varzakas, T. Diet and Oral Health Coaching Methods and Models for the Independent Elderly. Appl. Sci. 2020, 10, 4021. [Google Scholar] [CrossRef]
- Antoniadou, M.; Varzakas, T. Breaking the vicious circle of diet, malnutrition and oral health for the independent elderly. Crit. Rev. Food Sci. Nutr. 2020, 1–23. [Google Scholar] [CrossRef]
- Wärnberg-Gerdin, E.; Einarson, S.; Jonsson, M.; Aronsson, K.; Johansson, I. Impact of dry mouth conditions on oral health-related quality of life in older people. Gerodontology 2005, 22, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Izutsu, K.T. Iatrogenic Causes of Salivary Gland Dysfunction. J. Dent. Res. 1987, 66, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Scully, C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003, 9, 165–176. [Google Scholar] [CrossRef]
- Ohara, Y.; Hirano, H.; Yoshida, H.; Obuchi, S.; Ihara, K.; Fujiwara, Y.; Mataki, S. Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology 2013, 33, 20–27. [Google Scholar] [CrossRef]
- Saleh, J.; Zancanaro Figueiredo, M.A.; Cherubini, K.; Goncalves Salum, F. Salivary hypofunction: An update on aetiology, diagnosis and therapeutics. Arch. Oral Biol. 2015, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Kudiyirickal, M.G.; Pappachan, J.M. Diabetes mellitus and oral health. Endocrine 2015, 49, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulos, A.; Papadopoulos, P.; Andreadis, D. Chemotherapy: Oral side effects and dental interventions. A review of the literature. Stomatol. Dis. Sci. 2017, 1, 35–49. [Google Scholar] [CrossRef]
- Arrifin, A.; Heidari, E.; Burke, M.; Fenlon, M.R.; Banerjee, A. The Effect of Radiotherapy for Treatment of Head and Neck Cancer on Oral Flora and Saliva. Oral Health Prev. Dent. 2018, 16, 425–429. [Google Scholar] [CrossRef]
- Kapila, Y.V.; Dodds, W.J.; Helm, J.F.; Hogan, W.J. Relationship between Swallow Rate and Salivary Flow. Dig. Dis. Sci. 1984, 29, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Tollosa, D.N.; Tavener, M.; Hure, A.; James, E.L. Adherence to multiple health behaviours in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2019, 13, 327–343. [Google Scholar] [CrossRef]
- Miller, W.D. Action of the Products of Fermentation on the Different Structures of the Mouth. In The Microorganisms of the Human Mouth. The Local and General Diseases Which Are Caused by Them; Miller, W.D., Ed.; Karger: Basel, Switzerland, 1973; pp. 119–145. ISBN 978-3-8055-1614-3. [Google Scholar]
- Lingström, P.; Birkhed, D.; Ruben, J.; Arends, J. Effect of frequent consumption of starchy food items on enamel and dentin demineralization and on plaque pH in situ. J. Dent. Res. 1994, 73, 652–660. [Google Scholar] [CrossRef]
- Lingström, P.; van Houte, J.; Kashket, S. Food starches and dental caries. Crit. Rev. Oral Biol. Med. 2000, 11, 366–380. [Google Scholar] [CrossRef]
- Sheiham, A.; James, W.P.T. Diet and Dental Caries: The Pivotal Role of Free Sugars Reemphasized. J. Dent. Res. 2015, 94, 1341–1347. [Google Scholar] [CrossRef]
- Toverud, G. The influence of war and post-war conditions on the teeth of Norwegian school children. II. Caries in the permanent teeth of children aged 7–8 and 12–13 years. Millbank Mem. Fund Q. 1957, 35, 127–196. [Google Scholar] [CrossRef]
- Takeuchi, M. Epidemiological study on dental caries in Japanese children before, during and after World War II. Int. Dent. J. 1961, 11, 443–457. [Google Scholar]
- Caglar, E.; Kuscu, O.O. The Role of Diet in Caries Prevention. In Evidence-Based Caries Prevention, 1st ed.; Eden, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 87–106. [Google Scholar]
- Lingström, P.; Holm, A.K.; Mejare, I.; Twetman, S.; Söder, B.; Norlund, A.; Axelsson, S.; Lagerlöf, F.; Nordenram, G.; Petersson, L.G.; et al. Dietary factors in the prevention of dental caries: A systematic review. Acta Odontol. Scand. 2003, 61, 331–340. [Google Scholar] [CrossRef]
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Donohue, D.C.; Salminen, S. Safety of probiotic bacteria. Asia Pac. J. Clin. Nutr. 1996, 5, 25–28. [Google Scholar]
- Berkey, D.; Berg, R. Geriatric oral health issues in the United States. Int. Dent. J. 2001, 51, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.P. The role of medical care in contributing to health improvements within societies. Int. J. Epidemiol. 2001, 30, 1260–1263. [Google Scholar] [CrossRef]
- Mishra, S. Does modern medicine increase life-expectancy: Quest for the Moon Rabbit? Indian Heart J. 2016, 68, 19–27. [Google Scholar] [CrossRef]
- Buxbaum, J.D.; Chernew, M.E.; Fendrick, A.M.; Cutler, D.M. Contributions of Public Health, Pharmaceuticals, and Other Medical Care to US Life Expectancy Changes, 1990–2015. Health Aff. 2020, 39, 1546–1556. [Google Scholar] [CrossRef]
- Ezeh, A.C.; Bongaarts, J.; Mberu, B. Global population trends and policy options. Lancet 2012, 380, 142–148. [Google Scholar] [CrossRef]
- Müller, F.; Naharro, M.; Carlsson, G.E. What are the prevalence and incidence of tooth loss in the adult and elderly population in Europe? Clin. Oral Implants Res. 2007, 18, 2–14. [Google Scholar] [CrossRef]
- Slade, G.D.; Akinkugbe, A.A.; Sanders, A.E. Projections of U.S. Edentulism prevalence following 5 decades of decline. J. Dent. Res. 2014, 93, 959–965. [Google Scholar] [CrossRef]
- Frencken, J. Caries Epidemiology and Its Challenges. In Caries Excavation: Evolution of Treating Cavitated Carious Lesions, Monographs in Oral Science; Schwendicke, F., Frencken, J., Innes, N., Eds.; Karger: Basel, Switzerland, 2018; Volume 27, pp. 11–23. [Google Scholar]
- Thomson, W.M.; Ma, S. An ageing population poses dental challenges. Singap. Dent. J. 2014, 35, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wilson-Stanford, S.; Cromwell, W.; Hillman, J.D.; Guerrero, A.; Allen, C.A.; Sorg, J.A.; Smith, L. Site-Directed Mutations in the Lanthipeptide Mutacin 1140. Appl. Environ. Microbiol. 2013, 79, 4015–4023. [Google Scholar] [CrossRef]
- Ghobrial, O.G.; Derendorf, H.; Hillman, J.D. Development and validation of a LC-MS quantification method for the lantibiotic MU1140 in rat plasma. J. Pharm. Biomed. Anal. 2009, 49, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, O.; Derendorf, H.; Hillman, J.D. Human serum binding and its effect on the pharmacodynamics of the lantibiotic MU1140. Eur. J. Pharm. Sci. 2010, 41, 658–664. [Google Scholar] [CrossRef]
- Kirichenko, K.; Hillman, J.D.; Handfield, M.; Park, J.H. Complete synthesis of the bicyclic ring of a mutacin analog with orthogonally protected lanthionine via solid-phase intracyclization. J. Pept. Sci. 2019, 25, e3214. [Google Scholar] [CrossRef] [PubMed]
- Declerck, D.; Leroy, R.; Martens, L.; Lesaffre, E.; Garcia-Zattera, M.J.; Vanden Broucke, S.; Debyser, M.; Hoppenbrouwers, K. Factors associated with prevalence and severity of caries experience in preschool children. Community Dent. Oral Epidemiol. 2008, 36, 168–178. [Google Scholar] [CrossRef]
| Genus | Species | Strain |
|---|---|---|
| Lactobacillus | rhamnosus | GG (ATCC 53103) [20,21,22,23], hct 70 [24], LB21 [25], LC 705 [22] |
| reuteri | ATCC 55,730 (SD2112) [26,27,28], ATCC PTA 5289 [29,30,31,32], DSM 17,938 [29,30,31,32] | |
| casei | Shirota [33] | |
| paracasei | F19 [34], GMNL-33 [35], SD1 [36,37,38] | |
| achidophilus | ATCC 4356 [39], La-5 [40] | |
| salivarius | TI 2711 [41], WB21 [41] | |
| brevis | CD2 [42] | |
| bifidum [43] | ||
| bulgaricus [43] | ||
| sporogens [43] | ||
| thermophilus [43] | ||
| Bifidobacterium | animalis lactis | BB-12 (ATCC 27536) [40,44,45,46], DN-173010 [47,48] |
| bifidum | ATCC 29,521 [39] | |
| longum [49] | ||
| Streptococci | mutans | A2JM [50] |
| rattus | JH145TM [51,52] | |
| oralis | KJ3TM [52] | |
| uberis | KJ2TM [52] | |
| dentisani | CECT7746 [53] | |
| salivarius | M18 [54] | |
| Bacillus | coagulans [55] |
| Baseline Condition | Type of Study | Patient Type | Baseline Condition | Study Groups | Treatment | Probiotic Strains | Strain Concentration | Results |
|---|---|---|---|---|---|---|---|---|
| Meurman et al. (1994) [20] | Cohort study | Dental students (mean age 25 years) | Healthy | 1 test group (n = 9) | 2 × 250 g of probiotic yoghurt/day | Lactobacillus rhamnosus GG ATCC 53103 | 1 × 108 CFU/mL | LGG showed distinct growth in 8 of 9 subjects 2 weeks after treatment discontinuation. |
| Näse et al. (2001) [21] | Randomized, double-blind, placebo-controlled | Daycare children (1–6 years old) | Healthy Caries | Test (n = 231) Control (n = 220) | 5 × (±250 mL) of probiotic milk/week during a 7-month period 5 × (±250 mL) of placebo milk/week for 7 months | Lactobacillus rhamnosus GG ATCC 53103 | 5–10 × 105 CFU/mL | No significant differences in caries and MS scores. Significantly reduced caries-risk in the probiotic group, especially in the 3- to 4-year-old children. |
| Ahola et al. (2002) [22] | Randomized, double-blind, placebo-controlled | Young adults (18–35 years old) | Healthy | Test (n = 38) Control (n = 36) | 5 × 15 g of probiotic cheese/day for 3 weeks 5 × 15 g of placebo cheese/day for 3 weeks | Lactobacillus rhamnosus GG ATC 53103 Lactobacillus rhamnosus LC 705 | 1.9 × 107 CFU/g 1.2 × 107 CFU/g | No significant difference in MS and yeast counts during intervention. Significantly reduced MS scores and a tendency toward fewer patients with high Lactobacilli counts in the probiotic group during the post-treatment period. |
| Montalto et al. (2004) [43] | Randomized, double-blind, placebo-controlled | Young adults (23–37 years old) | Healthy | Group A (n = 14) Group B (n = 16) Group C (n = 5) | (Capsuled probiotics + Liquid placebo)/day for 45 days (Liquid probiotics + capsuled placebo)/day for 45 days (Liquid and capsuled placebo)/day for 45 days | L. sporogens L. bifidum L. bulgaricus L. thermophilus L. acidophilus L. casei L. rhamnosus | 16% 12% 12% 18% 20% 10% 12% (1.88 × 109 total CFU/day) | Significant increase in Lactobacilli groups in both probiotic groups. No change in MS counts in all groups. |
| Nikawa et al. (2004) [26] | Double-blind, placebo-controlled | Female dental hygienist students (20 years old) | Healthy | 40 subjects in total | 95 g of placebo yoghurt/day for 2 weeks + 95 g of probiotic yoghurt/day for 2 weeks 95 g of probiotic yoghurt/day for 2 weeks + 95 g of placebo yoghurt/day for 2 weeks | L. reuteri SD2112 (ATCC55730) | Data not provided | Probiotic yoghurt compared to the placebo yoghurt significantly reduced MS counts. |
| Caglar et al. (2005) [47] | Randomized, double-blind crossover | Young adults (21–24 years old) | Healthy | Test (n = 21) Control (n = 21) | Periods 1,3: run-in and wash-out, respectively Periods 2,4 (2 weeks each): 1 × 200 g of probiotic or placebo yoghurt/day | Bifidobacterium animalis lactis DN-173 010 | 7 × 107 CFU/g | Significant decrease in MS counts and a tendency toward Lactobacilli reduction due to probiotic yoghurt consumption. |
| Caglar et al. (2006) [27] | Randomized, placebo-controlled with 4 parallel arms | Young adults (21–24 years old) | Healthy | Group A (n = 30) Group B (n = 30) Group C (n = 30) Group D (n = 30) | 200 mL of water/day through probiotic straw for 3 weeks 200 mL of water/day through placebo straw for 3 weeks 1 probiotic tablet/day for 3 weeks 1 placebo tablet/day for 3 weeks | L. reuteri ATCC 55730 | 108 CFU/straw 108 CFU/tablet | Significant decrease in MS counts and tendency toward reduction of Lactobacilli scores in both probiotic groups. |
| Caglar et al. (2008) [44] | Randomized, double-blind, placebo-controlled crossover | Young individuals (mean age 20 years old) | Healthy | Test (n = 23) Control (n = 24) | Periods 1,3: run-in and wash-out, respectively Periods 2,4 (10 days each): 1 × 53 g of probiotic or placebo ice-cream/day | B. animalis lactis Bb-12 | 1 × 107 CFU/g | Significant decrease in MS counts after probiotic ice-cream consumption. No change in Lactobacilli counts after both ice-creams intake. |
| Stecksen-Blicks et al. (2009) [25] | Clustered, double-blind, placebo-controlled | Preschool children (1–5 years old) | Healthy Caries | Probiotic (n = 110) Placebo (n = 76) | 1 × 150 mL probiotic milk (supplemented with 2.5 mg fluoride/L)/day for 21 months 1 × 150 mL standard milk fluoride)/day for 21 months | L. rhamnosus LB21 | 1 × 107 CFU/mL | Significant reduction in caries development after intervention milk intake. Lower, but not significant, MS proportion and no increase in total Lactobacilli counts in the probiotic group. |
| Singh et al. (2011) [40] | Randomized, double-blind, placebo-controlled, crossover | Children (12–14 years old) | Healthy | Group I (n = 20) Group II (n = 20) | 1-week run-in, 54 g of placebo ice-cream/day for 10 days, 2-week wash out, 54 g of probiotic ice-cream/day for 10 days 1-week run-in, 54 g of probiotic ice-cream/day for 10 days, 2-week wash out, 54 g of placebo ice-cream/day for 10 days | B. animalis lactis BB-12 ATCC27536 L. acidophilus La-5 | 1 × 106 CFU/g 1 × 106 CFU/g | Significant decrease in MS counts after probiotic ice-cream consumption. No change in Lactobacilli counts after both ice-creams intake. |
| Chuang et al. (2011) [35] | Randomized, double-blind, placebo-controlled | Young adults (20–26 years old) | Healthy | Test (n = 42) Control (n = 36) | 3 probiotic (+11% xylitol) tablets/day for 2 weeks 3 placebo (11% xylitol) tablets/day for 2 weeks | L. paracasei GMNL-33 | 3 × 108 cells/tablet | No change in MS and Lactobacilli levels in both groups during the experiment. Significant reduction in MS counts in the post-treatment period compared to the respective levels recorded immediately after treatment cessation. |
| Kavaloglu-Cildir et al. (2011) [29] | Randomized, double-blind, placebo-controlled, crossover | Children (4–12 years old) | Healthy Cleft Lip/Palate | Test (n = 19) Control (n = 19) | Periods 1,3: run-in and wash-out, respectively Periods 2,4 (25 days each): 5 probiotics or placebo drops/day | L. reuteri DSM 17938 L. reuteri ATCC PTA 5289 | ≥1 × 108 CFU/5 drops ≥1 × 108 CFU/5 drops | No change in MS and Lactobacilli counts after the consumption of both drops. |
| Juneja et al. (2012) [24] | Randomized, double-blind, placebo-controlled | Children (12–15 years old) | Healthy Caries | Group I (n = 18) Group II (n = 18) | 2 × 150 mL of standard milk/day for 3 weeks 2 × 150 mL of probiotic milk/day for 3 weeks | L. rhamnosus hct 70 | 2.34 × 109 CFU/day | Significant reduction of MS levels immediately after the intake of probiotic milk. |
| Burton et al. (2013) [54] | Randomized, double-blind, placebo-controlled | Schoolchildren (5–10 years old) | Healthy Caries | Test (n = 40) Control (n = 43) | 2 probiotic lozenges/day for 3 months 2 placebo lozenges/day for 3 months | S. salivarius M18 | 3.6 × 109 CFU/lozenge | Significant reduction of plaque scores in the probiotic group. Children who presented a distinct oral colonization by M18 tended to possess lower counts of MS. |
| Teanpaisan et al. (2013) [36] | Randomized, double-blind, placebo-controlled | Young adults (18–25 years old) | Healthy Caries | Group A (n = 20) Group B (n = 17) | 1 × 10 g reconstituted probiotic milk powder in 50 mL of water/day for 4 weeks 1 × 10 g reconstituted placebo milk powder in 50 mL of water/day for 4 weeks | L. paracasei SD1 | ≥107 CFU/g or mL | Significant decrease of MS levels and increase of Lactobacilli levels after probiotic milk powder consumption. The probiotic could be detected up to 4 weeks after the discontinuation of the intervention. |
| Yadav et al. (2014) [33] | Randomized, double-blind, placebo-controlled, crossover | Children (6–8 years old) | Healthy Caries | Test (n = 31) Control (n = 31) | Periods 1,3: run-in (7 days) and wash-out (30 days), respectively Periods 2,4 (10 days each): 1 × 10 mL of probiotic or placebo milk/day | L. casei Shirota | Data not provided | Significant reduction of MS counts after the intake of probiotic milk. |
| Pinto et al. (2014) [48] | Randomized, double-blind, placebo-controlled, crossover | Orthodontic patients (median age 15 years) | Healthy | Group 1 (n = 15) Group 2 (n = 15) | 1-week run-in, 200 g of probiotic yoghurt/day for 2 weeks, 4-week wash out, 1 × 200 g of placebo yoghurt/day for 2 weeks 1-week run-in, 200 g of placebo yoghurt/day for 2 weeks, 4-week wash out, 200 g of placebo ice-cream/day for 2 weeks | B. animalis lactis DN-173010 | Data not provided | No significant difference in MS, Lactobacilli and total cultivable microorganisms counts after both yoghurts. Both yoghurts were equally efficient at reducing total cultivable microorganisms isolated from dental plaque. |
| Nishihara et al. (2014) [41] | Randomized, double-blind, placebo-controlled with 4 parallel arms +Cohort study | Sixth-year dental students (mean age 24.8 years) Dentists (mean age 30.0 years) | Healthy Healthy | Group 1 (n = 17) Group 2 (n = 16) Group 3 (n = 13) Group 4 (n = 18) 1 test group (n = 8) | 1 probiotic (+280 mg xylitol) tablet for 1 month 1 probiotic (+450 mg xylitol) tablet for 1 month 1 Ovalgen (+100 mg xylitol) tablet for 1 month 1 xylitol (280 mg) tablet for 1 month 3 × 1 tablet/day for 2 weeks | L. salivarius WB21 L. salivarius TI 2711 L. salivarius WB21 | 6.7 × 108 CFU/tablet 2.8 × 108 CFU/tablet 2.0 × 109 CFU/tablet | No significant change in MS levels. Significant increase in Lactobacilli counts in the two probiotic groups and enhanced buffering capacity in L. salivarius TI 2711 and Ovalgen group. Significant decrease in salivary MS levels. |
| Keller et al. (2014) [30] | Randomized, double-blind, placebo-controlled | Adolescents (12–17 years old) | Healthy Caries | Test (n = 19) Control (n = 17) | 2 probiotic tablets/day for 12 weeks 2 placebo tablets/day for 12 weeks | L. reuteri DSM 17938 L. reuteri ATCC PTA 5289 | 1 × 108 CFU/tablet 1 × 108 CFU/tablet | Significant decrease of fluorescence in decayed teeth over time in the probiotic group. No significant differences in fluorescence between the two groups. |
| Gizani et al. (2016) [31] | Randomized, double-blind, placebo-controlled | Adolescents and young adults (mean age 15.9 years) | Healthy Orthodontic treatment Caries | Test (n = 42) Control (n = 43) | 1 probiotic lozenge/day for 17 months 1 placebo lozenge/day for 17 months | L. reuteri DSM 17938 L. reuteri ATCC PTA 5289 | ≥108 CFU/lozenge ≥108 CFU/lozenge | Significant reduction of Lactobacilli counts and no alteration of MS levels in both groups. No difference in the incidence of white spot lesions between the two groups. |
| Ghasemi et al. (2017) [39] | Randomized, double-blind, placebo-controlled | Female students (19–27 years old) | Healthy | Group 1 (n = 25) Group 2 (n = 25) | 200 g of probiotic yoghurt/day for 3 weeks 3 × 2 xylitol gums/day for 3 weeks | L. acidophilus ATCC 4356 B. bifidum ATCC 29521 | 1.5 × 108 total CFU/g | Significant reduction of MS counts in both groups with no significant difference between them. |
| Koopaie et al. (2019) [55] | Randomized, double-blind, placebo-controlled, crossover | Adolescents and adults (mean age 41.67 years) | Healthy | Group 1 (n = 20) Group 2 (n = 20) | 70 g of probiotic cake/day for 1 week, 4-week wash-out period, 70 g of regular cake/day for 1 week 70 g of regular cake/day for 1 week, 4-week wash-out period, 70 g of probiotic cake/day for 1 week | B. coagulans | Data not provided | No statistical difference in MS levels after probiotic cake intake. Significant increase of MS counts after regular cake consumption. No significant alteration in salivary pH after the consumption of both cakes. |
| Javid et al. (2020) [46] | Randomized, double-blind, placebo-controlled | Students (18–30 years old) | Healthy Caries | Test (n = 33) Control (n = 33) | 300 g of probiotic yoghurt/day for 2 weeks 300 g of placebo yoghurt/day for 2 weeks | B. lactis Bb-12 | 106 CFU/ml | Significant reduction in MS and Lactobacilli levels in the probiotic group. |
| Ferrer et al. (2020) [53] | Prospective, mechanistic pilot with two parallel follow-up groups | Adults (25–35 years old) | Healthy | Group 1 (n = 6) Group 2 (n = 5) | 7 vials (multidose) containing the probiotic strain 2 vial (monodose) containing the probiotic strain | S. dentisani CECT7746 | 5.5 × 109 CFU/vial 4 × 1010 CFU/vial | Significant decrease of MS and significant increase in S. dentisani levels and salivary pH. The latter was stronger in the multi-dose schedule. |
| Baseline Condition | Type of Study | Patient Type | Baseline Condition | Study Groups | Treatment | Probiotic Strains | Strain Concentration | Results |
|---|---|---|---|---|---|---|---|---|
| Aminabadi et al. (2011) [23] | Randomized, double-blind with 4 parallel arms | Children (6–12 years old) | Healthy | Group A (n = 35) Group B (n = 35) Group C (n = 35) | 2 × 5 mL of 0.12% chlorhexidine/day for 2 weeks 15–20 mL of probiotic yoghurt for 3 weeks 2 × 5 mL of 0.12% chlorhexidine/day for 2 weeks + 15–20 mL of probiotic yoghurt for 3 weeks | L. rhamnosus GG | 2 × 108 CFU/g | Significant decrease in MS counts in all groups; only in groups A and C it was persisted for 5 weeks after the end of treatment. In group C LGG levels were more prominent than in group B. |
| Taipale et al. (2012) [45] | Randomized, double-blind, placebo-controlled with 3 parallel arms | Infants (1–2 months old) | Healthy | Test (n = 32) Control 1 (n = 35) Control 2 (n = 29) | 2 probiotic-tablets/day 2 xylitol-tablets/day 2 sorbitol-tablets/day Until the age of 2 years old | B. animalis lactis BB-12 | 5 × 109 CFU/tablet | Significant decrease in MS counts in the probiotic and the sorbitol groups at the age of 2 years. No observed permanent oral colonization of BB-12. Lactobacilli were unaffected. |
| Hasslöf et al. (2013) [34] | Randomized, double-blind, placebo-controlled | Infants (4 months old) | Healthy | Test (n = 56) Control (n = 62) | At least 1 serving of probiotic-cereals/day At least 1 serving of placebo-cereals/day | L. paracasei F19 | 1 × 108 CFU/serving | No significant difference in MS counts and caries experience between the two groups. No permanent establishment of LF19. |
| Stensson et al. (2014) [28] | Randomized, single-blind, placebo-controlled | Mothers (during the last month of gestation) + Infants (through the 1st year of life) | Healthy | Test (n = 60) Control (n = 53) | 5 drop of probiotic-oil/day (last month of gestation and 1st year of life) 5 drops of placebo-oil/day (last month of gestation and 1st year of life) | L. reuteri ATCC 55730 | 108 CFU/5 drops | Significant decrease in caries prevalence in the probiotic group. No significant intergroup differences in L. reuteri, MS, Lactobacilli and sIgA counts. |
| Hedayati-Hajikand et al. (2015) [52] | Randomized, double-blind, placebo-controlled | Preschool children (2–3 years old) | Healthy Caries | Test (n = 54) Control (n = 56) | 1 chewing probiotic-tablet/day 1 chewing placebo-tablet/day | S. uberis KJ2 TM S. oralis KJ3 TM S. rattus JH145TM | ≥1 × 108 total CFU/ tablet | Significantly lower caries increment in the probiotic group. |
| Villavicencio et al. (2017) [49] | Randomized, triple-blind, placebo-controlled | Preschool children (3–4 months old) | Healthy Caries | Test (n = 136) Control (n = 227) | 200 mL of reconstituted probiotic milk/day for 5 days a week during a 9-month period 200 mL of reconstituted standard reconstituted milk/day for 5 days a week during a 9-month period | L. rhamnosus B. longum | 5 × 106 CFU/g of powdered milk 3 × 106 CFU/g of powdered milk | Significantly lower counts of Lactobacilli count and higher buffering capacity in the test group. No significant difference in caries prevalence, MS counts, salivary pH and dental plaque between the groups. |
| Pahumunto et al. (2018) [37] | Randomized, double-blind, placebo-controlled | Preschool children (1.5–5 years old) | Healthy | Test (n = 62) Control (n = 62) | 5 g of probiotic milk powder in 50 mL of water/day for 3 months 5 g of standard milk powder in 50 mL of water/day for 3 months | L. paracasei SD1 | 1 × 107 CFU/g | Significantly lower risk of MS levels increases and of caries development in the test group. |
| Alamoudi et al. (2018) [32] | Randomized, double-blind, placebo-controlled | Children (3–6 years old) | Healthy | Test (n = 90) Control (n = 88) | 2 probiotic lozenges/day for 28 days 2 placebo lozenges/day for 28 days | L. reuteri DSM 17938 L. reuteri ATCC PTA 5289 | ≥2 × 108 total CFU/lozenge | Significant decrease in MS and Lactobacilli counts in the probiotic group. No statistical difference in plaque accumulation and buffer capacity between the groups. |
| Manmontri et al. (2020) [38] | Randomized, double-blind, placebo-controlled with 3 parallel arms | Preschool children (1–5 years old) | Healthy Caries | Group I (n = 86) Group II (n = 89) Group III (n = 93) | 1 × 3 g of placebo milk powder in 50 mL of milk for 7 days/week for 6 months 1 × 3 g of probiotic milk powder in 50 mL of milk for 7 days/week for 6 months 1 × 3 g of probiotic milk powder in 50 mL of milk for 3 days/week + 3 g of placebo milk powder in 50 mL of milk for 4 days/week for 6 months | L. paracasei SD1 | 1.8 × 107 total CFU/mL | Significantly lower counts of MS and higher levels of Lactobacilli in saliva in both probiotic groups than in the placebo group. No difference regarding these alterations between the probiotic groups |
| Lai et al. (2021) [42] | Randomized, double-blind, placebo-controlled | Children (4–14 years old) | Type 1 diabetes Caries | Test (n = 34) Control (n = 34) | 2 probiotic lozenges/day for 60 days 2 placebo lozenges/day for 60 days | L. brevis CD2 | 2 × 109 CFU/lozenge | Significant decrease in salivary MS and in maximum plaque pH fall and significant increase in lowest plaque pH. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amargianitakis, M.; Antoniadou, M.; Rahiotis, C.; Varzakas, T. Probiotics, Prebiotics, Synbiotics and Dental Caries. New Perspectives, Suggestions, and Patient Coaching Approach for a Cavity-Free Mouth. Appl. Sci. 2021, 11, 5472. https://doi.org/10.3390/app11125472
Amargianitakis M, Antoniadou M, Rahiotis C, Varzakas T. Probiotics, Prebiotics, Synbiotics and Dental Caries. New Perspectives, Suggestions, and Patient Coaching Approach for a Cavity-Free Mouth. Applied Sciences. 2021; 11(12):5472. https://doi.org/10.3390/app11125472
Chicago/Turabian StyleAmargianitakis, Markos, Maria Antoniadou, Christos Rahiotis, and Theodoros Varzakas. 2021. "Probiotics, Prebiotics, Synbiotics and Dental Caries. New Perspectives, Suggestions, and Patient Coaching Approach for a Cavity-Free Mouth" Applied Sciences 11, no. 12: 5472. https://doi.org/10.3390/app11125472
APA StyleAmargianitakis, M., Antoniadou, M., Rahiotis, C., & Varzakas, T. (2021). Probiotics, Prebiotics, Synbiotics and Dental Caries. New Perspectives, Suggestions, and Patient Coaching Approach for a Cavity-Free Mouth. Applied Sciences, 11(12), 5472. https://doi.org/10.3390/app11125472









