An In Vitro Study of Different Types of Greek Honey as Potential Natural Antimicrobials against Dental Caries and Other Oral Pathogenic Microorganisms. Case Study Simulation of Oral Cavity Conditions
Abstract
:1. Introduction
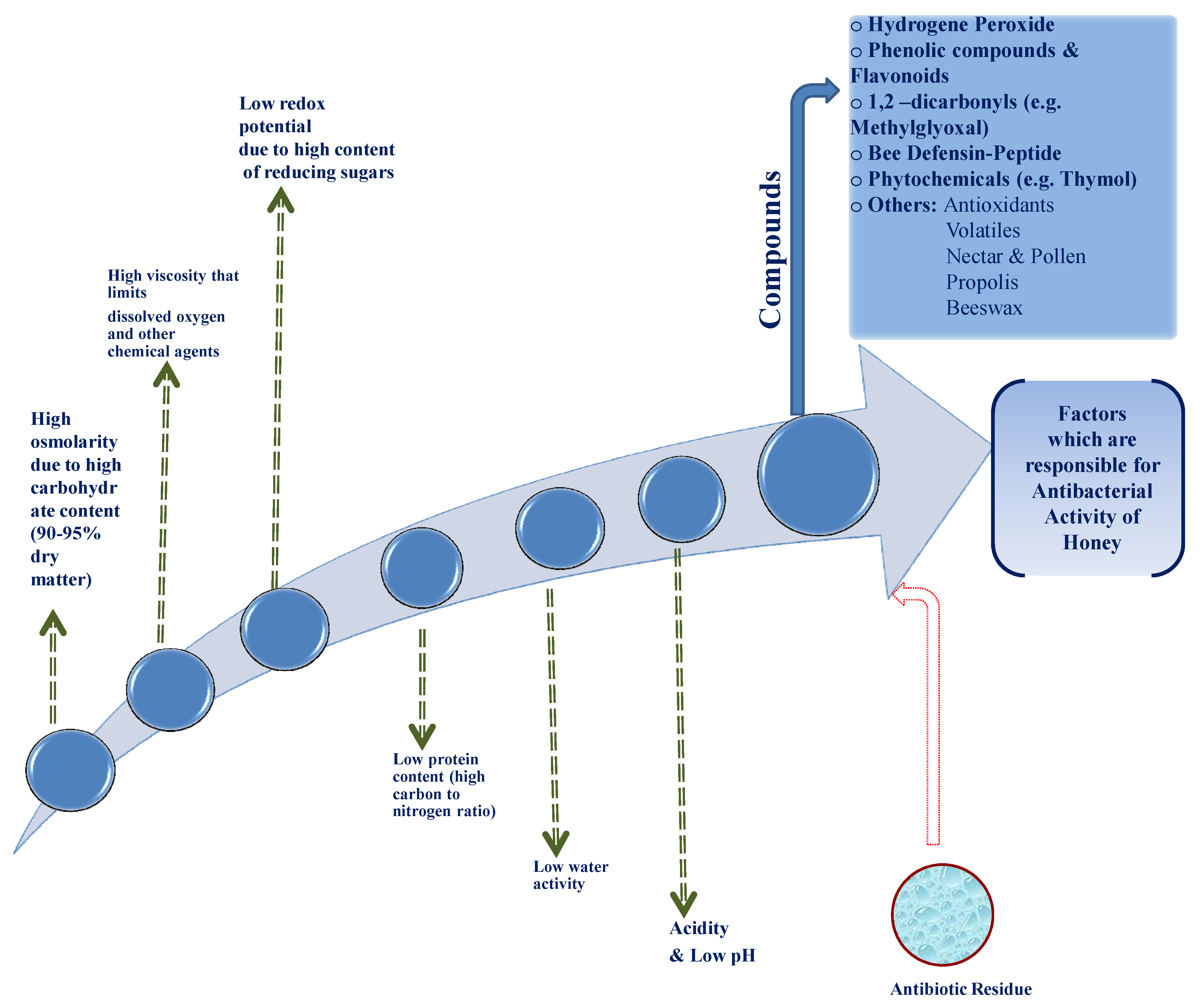
The Antibacterial Effectiveness of Honey
2. Materials and Methods
2.1. Honey Samples
2.1.1. Control Indexes of the Experimental Design
2.1.2. Determination of Physicochemical Parameters (All the Analyses Were Done in Triplicate)
2.2. Determination of the Antibacterial Activity
2.2.1. Tested Microbial Strains
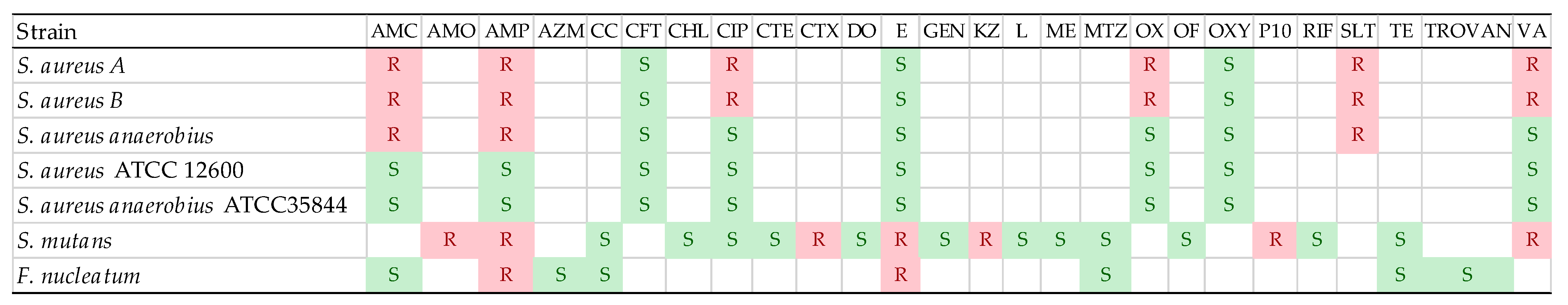
- Staphylococcusaureus subsp. aureus, methicillin, and vancomycin resistant (source: dental septicaemia)
- Staphylococcus aureus subsp. aureus, methicillin, and vancomycin resistant (source: tooth abscess)
- Staphylococcus aureus subsp. anaerobius (source: septicaemic gingivitis)
- Streptococcus mutans (source: oral cavity)
- Fusobacterium nucleatum (source: oral cavity)
- Staphylococcus aureus subsp. aureus, reference strain ATCC 12600
- Staphylococcus aureus subsp. αnaerobius, reference strain ATCC 35844
Antibiotic Susceptibility Assay
2.2.2. Study Design
Used Solvents
Introduction on the Methods of Assessment of Antimicrobial Potency of the Different Honey Types
Agar Well Diffusion Assay
Determination of Minimum Inhibitory Concentration (MIC)
Determination of Minimum Inhibitory Concentration (MIC) Performed After Enzymatic Treatment of Honey Samples with Catalase and Proteinase K
Statistical Analysis
3. Results
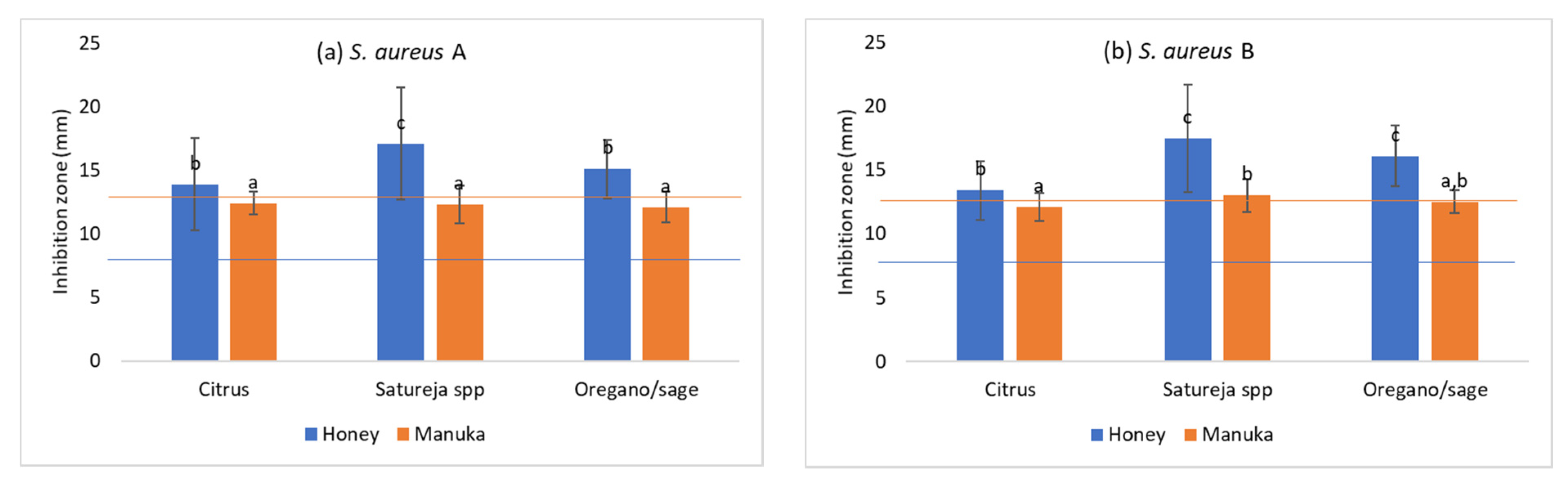
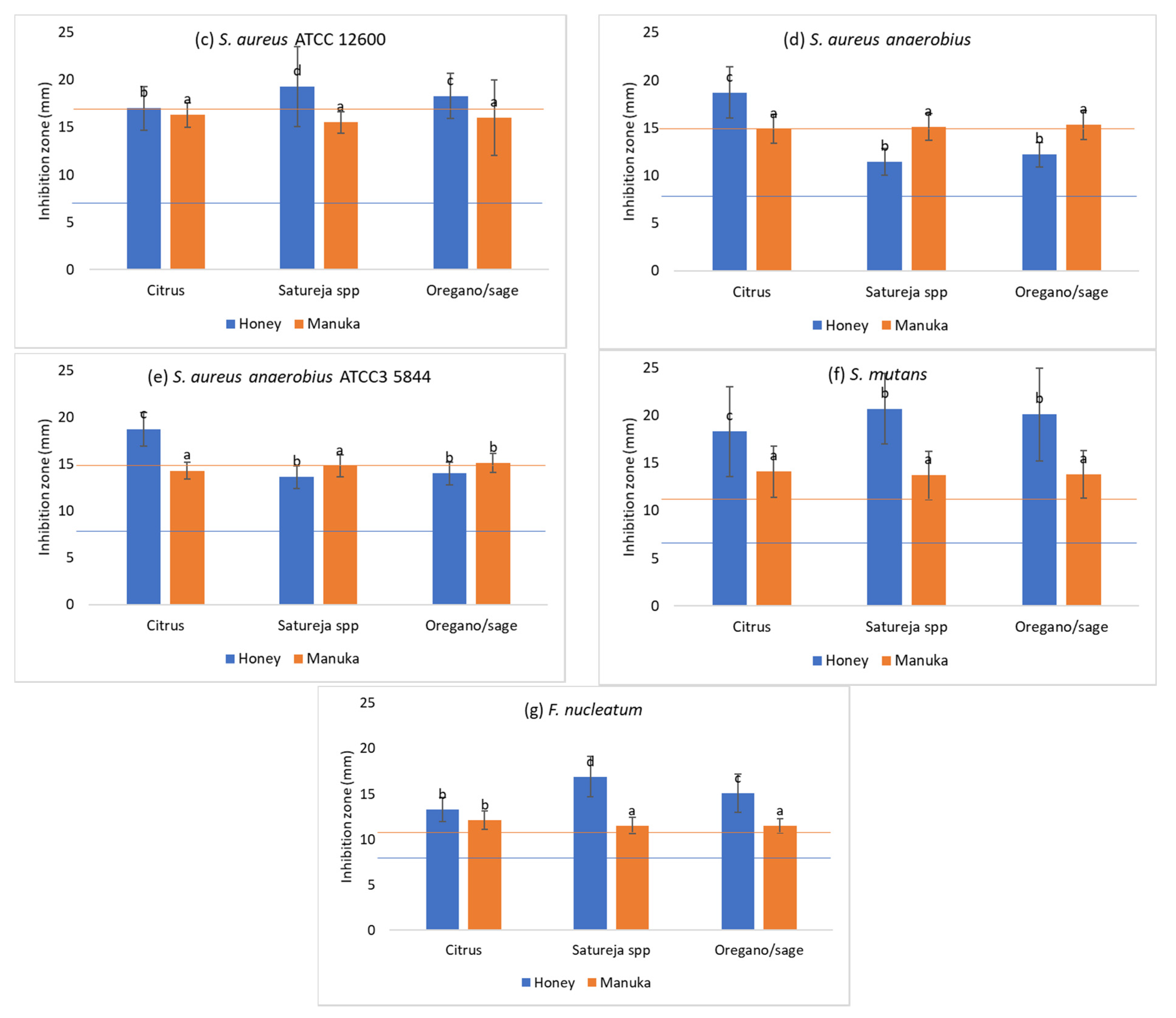
3.1. Antimicrobial Activity
3.2. Antibiotic Susceptibility of Used Strains
3.3. Effect of Physicochemical Characteristics in Antimicrobial Action
3.4. Effect of Honey pH in Antimicrobial Action
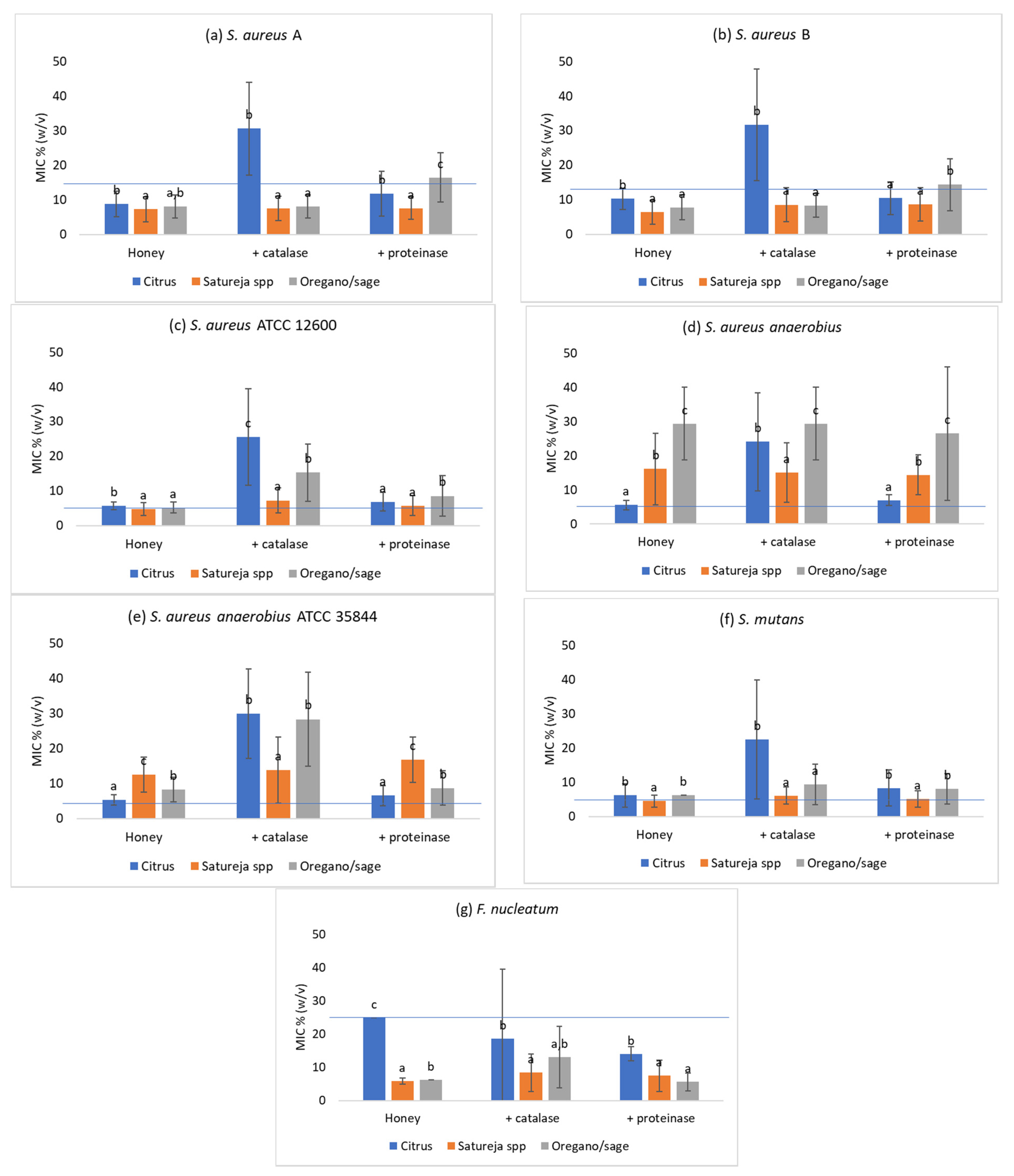
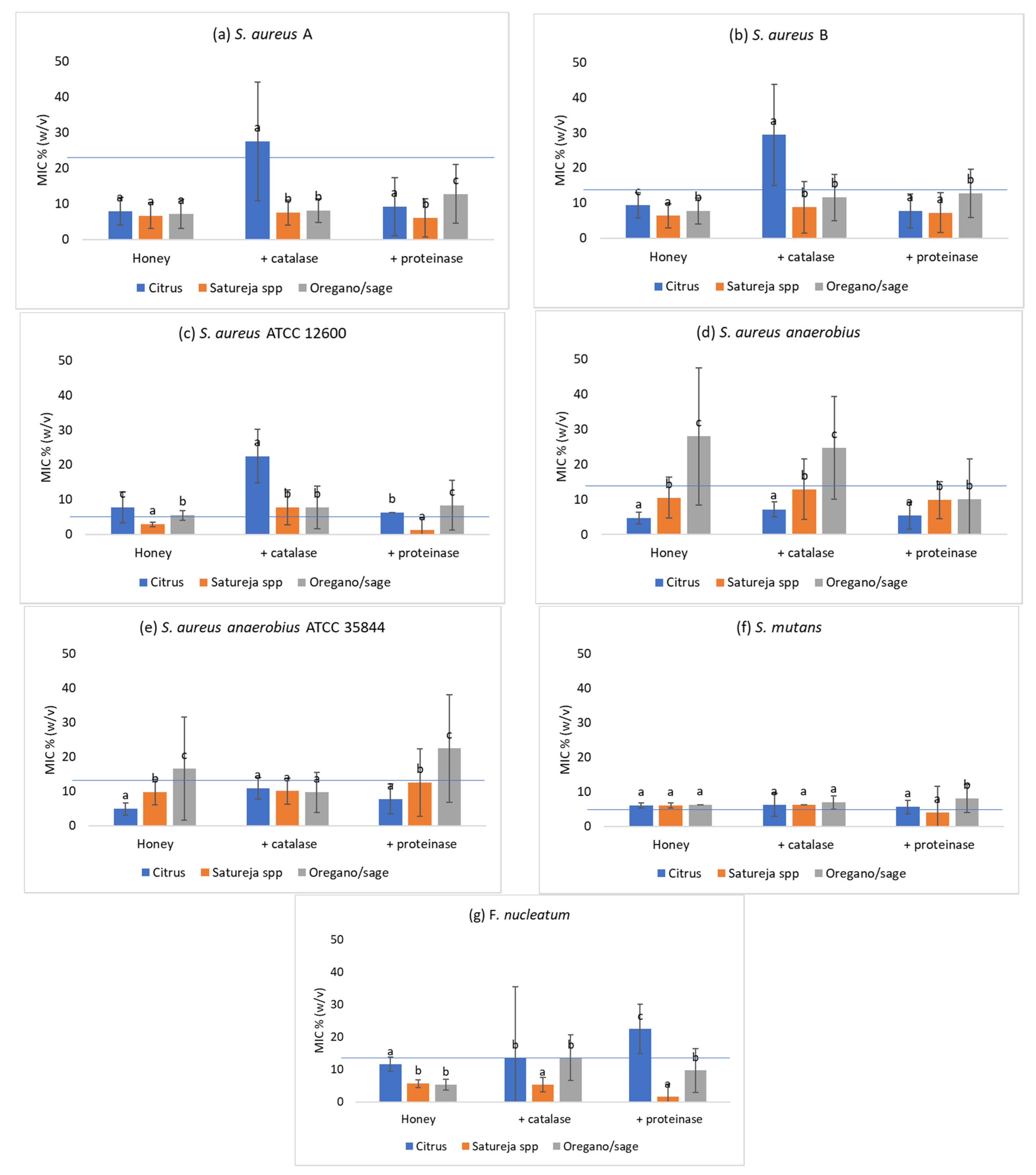
3.5. Effect of the Addition of Catalase
3.6. Effect of Proteinase K
4. Discussion
5. Conclusions
- The Greek honeys and particularly the citrus and the oregano and sage honey, showed an impressive antibacterial activity against all oral pathogens tested in this study as well as the reference strains.
- This antibacterial activity outperformed in most cases the one of manuka honey, which was used as control due to its well-studied and fully documented antibacterial activity.
- A significant part of the antibacterial activity was due to hydrogen peroxide. Further studies are needed for evaluating the effect of other compounds such as peptides and non-peptides (phenolic compounds, flavonoids, and others) in the antibacterial action.
- In in-vitro conditions, the antibacterial activity of honey is found to be enhanced in most cases when artificial saliva is used for its dilution.
- There is an indication that in a clinical environment, Greek honeys can be used as anti-cariogenic, anti-erosive and/or oral wound healing factor in patients with hyposalivation.
- Although further clinical research is needed, there is a strong indication that honey should be used in elderly patients through natural sources while propolis or other honey derivatives should be used in younger and healthy patients as extracts in oral hygiene products.
- Our results are promising, and a future project must include not «artificial saliva» but saliva from volunteers, and perhaps not only healthy volunteers but also volunteers with specific dental lesions. In this case, the interaction between the natural microflora of the oral cavity and the pathogenic bacteria in the presence of various types of honey should also be studied.
- The exact botanological profile of the various types of honeys should be investigated in order to classify them accurately and derive more specific clinical suggestions.
Author Contributions
Funding
Conflicts of Interest
References
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, N.; Garla, B.; Reddy, V.; Tandon, S.; Prasad, S. Antimicrobial Effect of Honey on Streptococcus Mutans of Dental Plaque. J. Oral Health Community Dent. 2014, 8, 72–75. [Google Scholar] [CrossRef]
- Mark Welch, J.L.; Dewhirst, F.E.; Borisy, G.G. Biogeography of the Oral Microbiome: The Site-Specialist Hypothesis. Annu. Rev. Microbiol. 2019, 73, 335–358. [Google Scholar] [CrossRef]
- Yeoh, S.C.; Hua, H.; Yepes, J.F.; Peterson, D.E. Oral Manifestations of Systemic Diseases and their Treatments. In Contemporary Oral Medicin; Farah, C., Balasubramaniam, R., McCullough, M., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Bakhtiari, S.; Sehatpour, M.; Mortazavi, H.; Bakhshi, M. Orofacial Manifestation of Adverse Drug Reactions: A Review Study. Med. Pharm. Rep. 2018, 91, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef]
- Stašková, A.; Nemcová, R.; Lauko, S.; Jenča, A. Oral Microbiota from the Stomatology Perspective. Bact. Biofilms 2020. [Google Scholar] [CrossRef] [Green Version]
- Shay, K. Infectious Complications of Dental and Periodontal Diseases in the Elderly Population. Clin. Infect. Dis. 2002, 34, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiol. Open 2020, 9, e1035. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, S.; Imtiyaz, Z.; Wali, A.F.; Khan, A.; Rashid, S.M.; Amin, I.; Ali, A.; Rehman, M.U.; Arafah, A. Honey: A Powerful Natural Antioxidant and Its Possible Mechanism of Action. In Therapeutic Applications of Honey and Its Phytochemicals; Rehman, M.U., Majid, S., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Zbuchea, A. Up-to-date use of honey for burns treatment. Ann. Burn. Fire Disasters 2014, 27, 22–30. [Google Scholar]
- Schencke, C.; Vasconcellos, A.; Sandoval, C.; Torres, P.; Acevedo, F.; Del Sol, M. Morphometric evaluation of wound healing in burns treated with Ulmo (Eucryphia cordifolia) honey alone and supplemented with ascorbic acid in guinea pig (Cavia porcellus). Burn. Trauma 2016, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Hussain, M.B.; Saeed, F.; Waheed, M.; Tufail, T. Phytochemistry, metabolism, and ethnomedical scenario of honey: A oncurrent review. Int. J. Food Prop. 2017, 20, S254–S269. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2018, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi-Motamayel, F.; Hendi, S.S.; Alikhani, M.Y.; Khamverdi, Z. Antibacterial activity of honey on cariogenic bacteria. J. Dent. (Tehran) 2013, 10, 10–15. [Google Scholar] [PubMed]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.; et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israili, Z.H. Antimicrobial properties of honey. Am J Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Voidarou, C.; Alexopoulos, A.; Tsinas, A.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. Effectiveness of Bacteriocin-Producing Lactic Acid Bacteria and Bifidobacterium Isolated from Honeycombs against Spoilage Microorganisms and Pathogens Isolated from Fruits and Vegetables. Appl. Sci. 2020, 10, 7309. [Google Scholar] [CrossRef]
- Voidarou, C.; Alexopoulos, A.; Plessas, S.; Karapanou, A.; Mantzourani, I.; Stavropoulou, E.; Fotou, K.; Tzora, A.; Skoufos, I.; Bezirtzoglou, E. Antibacterial activity of different honeys against pathogenic bacteria. Anaerobe 2011, 17, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Cooper, R.A.; Molan, P.C.; Harding, K.G. Antibacterial Activity of Honey against Strains of Staphylococcus aureus from Infected Wounds. J. R. Soc. Med. 1999, 92, 283–285. [Google Scholar] [CrossRef] [Green Version]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell Longev. 2017, 1259510. [Google Scholar] [CrossRef] [PubMed]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Molan, P. An explanation of why the MGO level in manuka honey does not show the antibacterial activity. N. Z. BeeKeeper 2008, 16, 11–13. [Google Scholar]
- Atrott, J.; Henle, T. Methylglyoxal in Manuka Honey—Correlation with Antibacterial Properties. Czech J. Food Sci. 2009, 27, S163–S165. [Google Scholar] [CrossRef] [Green Version]
- Tonks, A.J.; Cooper, R.A.; Jones, K.P.; Blair, S.; Parton, J.; Tonks, A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The Effect of New Zealand Kanuka, Manuka and Clover Honeys on Bacterial Growth Dynamics and Cellular Morphology Varies According to the Species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef] [Green Version]
- Cooper, R.A.; Molan, P.C.; Harding, K.G. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 2002, 93, 857–863. [Google Scholar] [CrossRef]
- Gkoutzouvelidou, M.; Panos, G.; Xanthou, M.N.; Papachristoforou, A.; Giaouris, E. Comparing the Antimicrobial Actions of Greek Honeys from the Island of Lemnos and Manuka Honey from New Zealand against Clinically Important Bacteria. Proceedings 2021, 70, 8. [Google Scholar] [CrossRef]
- Cunnif, P. Official Methods of Analysis of AOAC International, 16th ed.; 5th Revision, Volume II, Chapter 44, Subchapter 4; AOAC International: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucekova, M.; Bugarova, V.; Godocikova, J.; Majtan, J. Demanding New Honey Qualitative Standard Based on Antibacterial Activity. Foods 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Godocikova, J.; Bugarova, V.; Kast, C.; Majtan, V.; Majtan, J. Antibacterial potential of Swiss honeys and characterisation of their bee-derived bioactive compounds. J. Sci. Food Agric. 2020, 100, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Bang, L.M.; Buntting, C.; Molan, P. The Effect of Dilution on the Rate of Hydrogen Peroxide Production in Honey and Its Implications for Wound Healing. J. Altern. Complement. Med. 2003, 9, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Methods 954.11–979.21 and 977.20; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990; pp. 1028–1030. [Google Scholar]
- Terrab, A.; Recamales, A.F.; Hernanz, D.; Heredia, F.J. Characterisation of Spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chem. 2004, 88, 537–542. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al-Amri, A.; Al-Hadhrami, A.; Al-Belushi, S. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon 2018, 4, e00874. [Google Scholar] [CrossRef] [Green Version]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.; Piatti, E. Honey flavonoids as protection agents against oxidative damage to human red blood cells. Food Chem. 2017, 104, 1635–1640. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsì, F.; Ferrari, G.; Hamdi, S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. Available online: https://clsi.org/about/clsis-history/ (accessed on 12 January 2021).
- National Committee For Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals: Approved Standards; Document M31-A, 19; NCCLS: Wayne, PA, USA, 1999; pp. 9–10. [Google Scholar]
- Gal, J. About a synthetic saliva for in vitro studies. Talanta 2001, 53, 1103–1115. [Google Scholar] [CrossRef]
- Leung, V.W.; Darvell, B.W. Artificial salivas for in vitro studies of dental materials. J. Dent. 1997, 25, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Nordman, H.; Davies, J.R.; Lindell, G.; De Bolós, C.; Real, F.; Carlstedt, I. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem. J. 2002, 364, 191–200. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.T.; Singh, H. Colloidal stability and interactions of milk-protein-stabilized emulsions in an artificial saliva. Food Hydrocoll. 2009, 23, 1270–1278. [Google Scholar] [CrossRef]
- Malpass, G.E.; Arimilli, S.; Prasad, G.L.; Howlett, A.C. Complete artificial saliva alters expression of proinflammatory cytokines in human dermal fibroblasts. Toxicol. Sci. Off. J. Soc. Toxicol. 2013, 134, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Patton, T.; Barrett, J.; Brennan, J.; Moran, N. Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. J. Microbiol. Methods 2006, 64, 84–95. [Google Scholar] [CrossRef]
- Sindi, A.; Chawn, M.V.B.; Hernandez, M.E.; Green, K.; Islam, M.K.; Locher, C.; Hammer, K. Anti-biofilm effects and characterisation of the hydrogen peroxide activity of a range of Western Australian honeys compared to Manuka and multifloral honeys. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Mundo, M.A.; Padilla-Zakour, O.I.; Worobo, R.W. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int. J. Food Microbiol. 2004, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Walter, J. Loesche. In Microbiology of Dental Decay and Periodontal Disease. Medical Microbiolog, 4th ed.; The University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 99. [Google Scholar]
- Amargianitakis, M.; Antoniadou, M.; Rahiotis, C.; Varzakas, T. Probiotics, Prebiotics, Synbiotics and Dental Caries. New Perspectives, Suggestions, and Patient Coaching Approach for a Cavity-Free Mouth. Appl. Sci. 2021, 11, 5472. [Google Scholar] [CrossRef]
- Antoniadou, M.; Varzakas, T. Diet and Oral Health Coaching Methods and Models for the Independent Elderly. Appl. Sci. 2020, 10, 4021. [Google Scholar] [CrossRef]
- Antoniadou, M.; Varzakas, T. Breaking the vicious circle of diet, malnutrition and oral health for the independent elderly. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Łysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Bohova, J.; Prochazka, E.; Klaudiny, J. Methylglyoxal may affect hydrogen peroxide accumulation in manuka honey through the inhibition of glucose oxidase. J. Med. Food. 2014, 17, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med Sci. 2013, 16, 731–742. [Google Scholar]
- Bansal, V.; Medhi, B.; Pandhi, P. Honey -A remedy rediscovered and its therapeutic utility. Kathmandu Univ. Med. J. 2005, 3, 305–309. [Google Scholar]
- Molan, P.C. The Antibacterial Activity of Honey. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- Kinoo, M.S.; Mahomoodally, M.F.; Puchooa, D. Anti-Microbial and Physico-Chemical Properties of Processed and Raw Honeys of Mauritius. Adv. Infect. Dis. 2012, 2, 25–36. [Google Scholar] [CrossRef]
- Brown, E.; O’Brien, M.; Georges, K.; Suepaul, S. Physical characteristics and antimicrobial properties of Apis mellifera, Frieseomelitta nigra and Melipona favosa bee honeys from apiaries in Trinidad and Tobago. BMC Complement. Med. Ther. 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, A.; Traynor, K.; Santos, K.; Blaser, G.; Bode, U.; Molan, P. Medical honey for wound care—still the ‘latest resort’? Evid. Based Complement. Alternat. Med. 2009, 6, 165–173. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [Green Version]
- Al-Waili, N.S.; Haq, A. Effect of honey on antibody production against thymus-dependent and thymus-independent antigens in primary and secondary immune responses. J. Med. Food 2004, 7, 491–494. [Google Scholar] [CrossRef]
- Emsen, I.M. A different and safe method of split thickness skin graft fixation: Medical honey application. Burns 2007, 33, 782–787. [Google Scholar] [CrossRef]
- Olaitan, P.B.; Adeleke, O.E.; Ola, I.O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007, 7, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Szweda, P. Antimicrobial Activity of Honey. In Honey Analysis; de Alencar Arnaut de Toledo, V., Ed.; IntechOpen: London, UK, 2017; pp. 215–232. [Google Scholar] [CrossRef] [Green Version]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Fokt, H.; Pereira, A.; Ferreira, A.M.; Cunha, A.; Aguiar, C. How do bees prevent hive infections? The antimicrobial properties of Propolis. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 481–493. [Google Scholar]
- Mohsin, S.; Manohar, B.; Rajesh, S.; Asif, Y. The effects of a dentifrice containing propolis on Mutans Streptococci: A clinico-microbiological study. Ethiop. J. Health Sci. 2015, 25, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ophori, E.A.; Eriagbonye, B.N.; Ugbodaga, P. Antimicrobial activity of Propolis against Streptococcus Mutans. Afr. J. Biotechnol. 2010, 9, 4966–4969. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Ho, W.-L.; Chang, C.-M.; Chou, C.-C. Antibacterial activity of propolis ethanol extract against streptococcus mutans as influenced by concentration, temperature, pH and cell age. J. Food Drug Anal. 2007, 15. [Google Scholar] [CrossRef]
- Malhotra, N.; Rao, S.P.; Acharya, S.; Vasudev, B. Comparative in vitro evaluation of efficacy of mouthrinses against Streptococcus mutans, Lactobacilli and Candida albicans. Oral Health Prev. Dent. 2011, 9, 261–268. [Google Scholar] [CrossRef]
- Shin, H.; Ustunol, Z. Carbohydrate composition of honey from different floral sources and their influence on growth of selected intestinal bacteria. Food Res. Int. 2005, 38, 721–728. [Google Scholar] [CrossRef]
- Basualdo, C.; Sgroy, V.; Finola, M.S.; Marioli, J.M. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet. Microbiol. 2007, 124, 375–381. [Google Scholar] [CrossRef]
- Sela, M.; Shapira, L.; Grizim, I.; Lewinstein, I.; Steinberg, D.; Gedalia, I.; Grobler, S.R. Effects of honey consumption on enamel microhardness in normal versus xerostomic patients. J. Oral Rehabil. 1998, 25, 630–634. [Google Scholar] [CrossRef]
- Habluetzel, A.; Schmid, C.; Carvalho, T.S.; Lussi, A.; Eick, S. Impact of honey on dental erosion and adhesion of early bacterial colonizers. Sci Rep. 2018, 8, 10936. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. Not all honeys are the same for wound healing. Eur. Tissue Repair. Soc. Bull. 2002, 9, 5–6. [Google Scholar]
- Schubert, M.M.; Izutsu, K.T. Iatrogenic Causes of Salivary Gland Dysfunction. J. Dent. Res. 1987, 66, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Saleh, J.; ZancanaroFigueiredo, M.A.; Cherubini, K.; Salum, F.G. Salivary hypofunction: An update on aetiology, diagnosis and therapeutics. Arch. Oral Biol. 2015, 60, 242–255. [Google Scholar] [CrossRef]
- Kudiyirickal, M.G.; Pappachan, J.M. Diabetes mellitus and oral health. Endocrine 2015, 49, 27–34. [Google Scholar] [CrossRef]
- Poulopoulos, A.; Papadopoulos, P.; Andreadis, D. Chemotherapy: Oral side effects and dental interventions. A review of the literature. Stomatol. Dis. Sci. 2017, 1, 35–49. [Google Scholar] [CrossRef]
- Arrifin, A.; Heidari, E.; Burke, M.; Fenlon, M.R.; Banerjee, A. The Effect of Radiotherapy for Treatment of Head and Neck Cancer on Oral Flora and Saliva. Oral Health Prev. Dent. 2018, 16, 425–429. [Google Scholar] [CrossRef]
- Scully, C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003, 9, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ohara, Y.; Hirano, H.; Yoshida, H.; Obuchi, S.; Ihara, K.; Fujiwara, Y.; Mataki, S. Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology 2013, 33, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Küçük, M.; Kolaylı, S.; Karaoğlu, S.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C. Honey glycoproteins containing antimicrobial peptides, Jelleins of the Major Royal Jelly Protein 1, are responsible for the cell wall lytic and bactericidal activities of honey. PLoS ONE 2015, 10, e0120238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudzynski, K.; Sjaarda, C. Antibacterial compounds of Canadian honeys target bacterial cell wall inducing phenotype changes, growth inhibition and cell lysis that resemble action of β-lactam antibiotics. PLoS ONE 2014, 9, e106967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Shi, M.; Chen, X.X. Antimicrobial peptide evolution in the Asiatic honey bee Apiscerana. PLoS ONE 2009, 4, e4239. [Google Scholar] [CrossRef] [Green Version]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019, 67, 688–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Components in Artficial Saliva | Concetration (g/L) | |
|---|---|---|
| Sodium chloride | NaCl | 1.594 |
| Ammonium nitrate | NH4NO3 | 0.328 |
| Potassium phosphate | KH2PO4 | 0.636 |
| Potassium chloride | KCl | 0.202 |
| Potassium citrate | K3C6H5O7·H2O | 0.308 |
| Uric acid sodium salt | C5H3N4O3Na | 0.021 |
| Urea | H2NCONH2 | 0.198 |
| Lactic acid sodium salt | C3H5O3Na | 0.146 |
| Porcine gastric Mucin Type II | 1.35 | |
| D- (+) glucose | 0.1 | |
| α-amylase | 100,000 U | |
| Lysozyme | 750 U | |
| Water | Four times distilled H2O and 0.1 M NaOH used to achieve pH 6.8 | |
| Honey | pH | H2O2 (μg/g Unprocessed Honey) | Free Acidity | Lactonic Acidity | TPC (mg GAE/100 g of Honey) | TFCmg Quercetin Equivalents (CE)/100 g of Honey |
|---|---|---|---|---|---|---|
| 1 | 3.9 | 11 ± 0.4 a | 13.6 ± 0.2 de | 9.1 ± 0.2 f | 35.1 ± 0.4 d | 0.65 ± 0.2 ab |
| 2 | 3.5 | 37 ± 0.9 e | 12.1 ± 0.4 bc | 5.3 ± 0.4 d | 29.5 ± 0.2 b | 1.2 ± 0.2 abc |
| 3 | 3.7 | 11 ± 0.56 a | 22.1 ± 0.1 gh | 7.8 ± 0.2 e | 52.1 ± 0.7 h | 0.8 ± 0.7 a |
| 4 | 3.5 | 17 ± 0.8 b | 27 ± 0.6 i | 3.2 ± 0.2 a | 75.1 ± 0.8 j | 2.2 ± 0.3 d |
| 5 | 3.5 | 47 ± 2.54 g | 11.7 ± 0.1 abc | 4.5 ± 0.2 cd | 45.3 ± 0.7 f | 1.9 ± 0.4 cd |
| 6 | 3.6 | 32 ± 1.51 d | 12.8 ± 0.3 cd | 4.5 ± 0.4 cd | 29.2 ± 0.7 b | 0.55 ± 0.3 a |
| 7 | 3.8 | 52 ± 2.74 h | 15.2 ± 0.2 e | 7.1 ± 0.2 ce | 33.4 ± 0.2 c | 0.7 ± 0.2 ab |
| 8 | 3.9 | 41 ± 1.98 ef | 22.2 ± 0.3 h | 4.8 ± 0.1 d | 45.3 ± 0.5 f | 1.1 ± 0.3 abc |
| 9 | 3.8 | 39 ± 1.09 e | 17 ± 0.2 f | 4.1 ± 0.5 abc | 32.4 ± 0.7 c | 0.8 ± 0.2 ab |
| 10 | 3.9 | 40 ± 1.1 ef | 18.1 ± 0.3 f | 7.1 ± 0.3 e | 45.2 ± 0.3 f | 1.2 ± 0.2 ac |
| 11 | 3.5 | 39 ± 0.4 e | 11.2 ± 0.7 ab | 9.4 ± 0.3 fg | 33.7 ± 0.5 cdf | 0.7 ± 0.2 ab |
| 12 | 3.6 | 22 ± 1.04 c | 10.8 ± 0.2 a | 9.2 ± 0.1 f | 44.7 ± 0.2 f | 0.7 ± 0.3 ab |
| 13 | 3.9 | 59 ± 1.77 i | 14.2 ± 0.2 e | 4.2 ± 0.4 bc | 53.1 ± 0.7 h | 0.9 ± 0.1 ab |
| 14 | 4.1 | 55 ± 1.74 hi | 11.8 ± 0.3 abc | 3.5 ± 0.1 ab | 22.7 ± 0.4 a | 1.6 ± 0.2 cd |
| 15 | 4.2 | 24 ± 0.8 c | 14.2 ± 0.7 e | 3.7 ± 0.1 abc | 39.1 ± 0.1 e | 1.5 ± 0.7 abcd |
| 16 | 3.7 | 17 ± 0.8 b | 14.1 ± 0.5 e | 3.3 ± 0.2 a | 44.2 ± 0.2 f | 0.8 ± 0.2 ab |
| 17 | 3.6 | 14 ± 0.8 ab | 17.1 ± 0.4 f | 7.1 ± 0.6 be | 50.2 ± 0.4 g | 0.9 ± 0.1 ab |
| 18 | 4.1 | 29 ± 0.7 d | 21 ± 0.1 g | 8.9 ± 0.1 f | 55.1 ± 0.1 i | 1.2 ± 0.2 bc |
| 19 | 4.5 | 14 ± 2.1 ab | 14.3 ± 0.4 e | 10.2 ± 0.1 g | 29.7 ± 0.2 b | 1.5 ± 0.2 acd |
| 20 | 3.9 | 44 ± 1.11 fg | 11.7 ± 0.2 abc | 7.1 ± 0.7 e | 33.4 ± 0.7 c | 0.7 ± 0.2 ab |
| F = 349.7, p < 0.01 | F = 443.5, p < 0.01 | F = 163.9, p < 0.01 | F = 1800, p < 0.01 | F = 6.53, p < 0.01 |
| Cluster | pH | H2O2 (μg/g Unprocessed Honey) | Free Acidity | Lactonic Acidity | TPC (mg GAE/100 g of Honey) | TFCmg Quercetin Equivalents (CE)/100 g of Honey |
|---|---|---|---|---|---|---|
| 1 | 3.8 | 33.0 | 15.0 | 6.36 | 39.65 | 1.0 |
| 2 | 3.5 | 17.0 | 26.96 | 3.2 | 75.1 | 2.2 |
| Honey | pH | H2O2 (μg/g Unprocessed Honey) | Free Acidity | Lactonic Acidity | TPC (mg GAE/100 g of Honey) | TFC mg Quercetin Equivalents (CE)/100 g of Honey |
|---|---|---|---|---|---|---|
| 1 | 3.2 | 6 ± 0.7 cd | 22 ± 0.5 c | 16.8 ± 0.2 cde | 78.5 ± 0.1 g | 2.5 ± 0.4 bcde |
| 2 | 3.4 | 14 ± 0.4 g | 17.9 ± 0.2 a | 7.1 ± 0.3 abcd | 99.3 ± 2.1 h | 3.2 ± 0.3 e |
| 3 | 3.6 | 3 ± 0.6 a | 28 ± 0.3 d | 16.5 bcde | 117.1 ± 1.4 j | 1.7 ± 0.5 abcd |
| 4 | 4.1 | 3 ± 0.2 a | 17 ± 0.8 a | 1.7 ± 0.2 a | 65.2 ± 0.7 e | 2.9 ± 0.2 bcde |
| 5 | 3.9 | 5 ± 0.1 bc | 17.8 ± 0.9 a | 18.2 ± 0.3 de | 55.7 ± 0.3 d | 5.2 ± 0.1 f |
| 6 | 3.6 | 7 ± 0.4 de | 28 ± 0.4 d | 6.8 ± 0.1 abcd | 147.8 ± 1.7 l | 3.4 ± 0.4 de |
| 7 | 3.2 | 11 ± 0.3 f | 28 ± 0.1 d | 6.5 ± 0.3 abc | 130.1 ± 2.1 k | 1.4 ± 0.4 ab |
| 8 | 3.5 | 8 ± 0.4 e | 45.2 ± 0.4 f | 17.1 ± 0.2 cde | 47.8 ± 0.7 c | 0.9 ± 0.2 a |
| 9 | 3.1 | 8 ± 0.7 e | 55 ± 0.2 h | 8.5 ± 0.3 abcd | 55.2 ± 0.7 d | 3.6 ± 0.4 e |
| 10 | 3.0 | 10 ± 0.4 f | 28 ± 0.1 d | 7.8 ± 0.1 abcd | 65.7 ± 0.9 e | 1.7 ± 0.4 abc |
| 11 | 3.6 | 8 ± 0.1 e | 17 ± 0.3 a | 11.2 ± 0.3 abcd | 40.3 ± 0.5 b | 2.2 ± 0.4 bcd |
| 12 | 3.7 | 6 ± 0.1 cd | 72 ± 0.1 i | 5.3 ± 0.6 ab | 65.1 ± 0.8 e | 2.3 ± 0.1 bcd |
| 13 | 4.0 | 3 ± 0.4 a | 32.2 ± 0.4 e | 11.5 ± 0.3 abcde | 110.1 ± 3.2 i | 3.1 ± 0.4 de |
| 14 | 3.1 | 3 ± 0.3 a | 79.7 ± 0.2 j | 10.9 ± 0.1 abcd | 72.3 ± 1.1 f | 1.4 ± 0.4 ab |
| 15 | 3.3 | 15 ± 0.7 g | 32 ± 0.2 e | 16.2 ± 0.7 bcde | 58.1 ± 2.3 d | 5.2 ± 0.2 f |
| 16 | 3.0 | 5 ± 0.5 bc | 27.3 ± 0.1 d | 16.8 ± 0.7 bcde | 40.1 ± 3.1 b | 0.9 ± 0.2 a |
| 17 | 3.6 | 4 ± 0.1 b | 31 ± 0.2 e | 10.8 ± 0.4 abcd | 24.8 ± 0.9 a | 3.5 ± 0.9 de |
| 18 | 3.1 | 3 ± 0.4 a | 19.3 ± 0.4 b | 22.7 ± 0.2 e | 98.2 ± 3.1 h | 1.7 ± 0.2 abc |
| 19 | 3.2 | 14 ± 0.2 g | 32 ± 0.2 e | 17.5 ± 0.3 cde | 59.5 ± 0.4 d | 2.2 ± 0.4 bcd |
| 20 | 3.1 | 3 ± 0.1 a | 48.4 ± 0.3 g | 6.9 ± 0.2 abcd | 37.8 ± 2.1 b | 1.7 ± 0.7 abc |
| F = 286.6, p < 0.01 | F = 6444, p < 0.01 | F = 6.72, p < 0.01 | F = 1149, p < 0.01 | F = 28.23, p < 0.01 |
| Cluster | pH | H2O2 (μg/g Unprocessed Honey) | Free Acidity | Lactonic Acidity | TPC (mg GAE/100 g of Honey) | TFC mg Quercetin Equivalents (CE)/100 g of Honey |
|---|---|---|---|---|---|---|
| 1 | 3.4 | 6.9 | 33.9 | 11.4 | 72.66 | 2.5 |
| 2 | 3.6 | 3.6 | 28.3 | 33.3 | 115.9 | 1.5 |
| Honey | pH | H2O2 (μg/g Unprocessed Honey) | Free Acidity | Lactonic Acidity | TPC (mg GAE/100 g of Honey) | TFC mg Quercetin Equivalents (CE)/100 g of Honey |
|---|---|---|---|---|---|---|
| 1 | 3.1 | 11 ± 0.2 de | 52 ± 0.3 i | 11.5 ± 0.1 d | 38.2 ± 0.7 d | 5.1 ± 0.7 fgh |
| 2 | 3.6 | 9 ± 0.3 c | 38.2 ± 0.7 e | 15.2 ± 0.7 ef | 44.2 ± 0.2 e | 3.7 ± 0.5 cdef |
| 3 | 3.5 | 6 ± 0.7 ab | 99.1 ± 0.2 o | 9.1 ± 0.2 bc | 86.1 ± 3.1 k | 4.4 ± 0.6 defg |
| 4 | 3.2 | 5 ± 0.2 a | 87 ± 0.2 n | 4.5 ± 0.7 a | 45.3 ± 1.3 e | 4.1 ± 0.2 def |
| 5 | 3.5 | 5 ± 0.3 a | 65.1 ± 0.7 k | 11.2 ± 0.7 d | 55.7 ± 0.9 g | 2.8 ± 0.4 bcd |
| 6 | 4.1 | 11 ± 0.7 de | 55 ± 0.1 j | 14.2 ± 0.7 e | 62.7 ± 2.1 h | 2.9 ± 0.6 bcd |
| 7 | 3.6 | 13 ± 0.3 f | 47.8 ± 0.3 h | 5.6 ± 0.2 a | 33.9 ± 0.7 c | 3.3 ± 0.4 cd |
| 8 | 3.0 | 10 ± 0.4 cd | 38.2 ± 0.9 e | 7.8 ± 0.2 b | 61.4 ± 0.4 h | 4.7 ± 0.4 defg |
| 9 | 3.1 | 12 ± 0.1 ef | 71 ± 0.1 l | 11.3 ± 0.5 bd | 50.7 ± 2.7 f | 3.7 ± 0.2 cef |
| 10 | 3.2 | 5 ± 0.4 a | 41.2 ± 0.2 g | 11.1 ± 0.7 d | 42.7 ± 0.2 de | 3.1 ± 0.4 bcde |
| 11 | 3.5 | 13 ± 0.2 f | 32.7 ± 0.3 bc | 22.3 ± 01 h | 32.9 ± 3.5 bc | 2.3 ± 0.2 abc |
| 12 | 3.6 | 11 ± 0.2 de | 27.9 ± 0.7 a | 17.2 ± 0.7 g | 40.8 ± 0.8 de | 1.8 ± 0.3 ab |
| 13 | 3.0 | 7 ± 0.2 b | 32.1 ± 0.7 b | 10.3 ± 0.1 cd | 22.8 ± 0.2 a | 6.2 ± 0.8 h |
| 14 | 3.5 | 17 ± 0.4 h | 29.2 ± 0.7 a | 16.3 ± 0.4 fg | 85.3 ± 0.2 k | 3.1 ± 0.3 bcde |
| 15 | 4.2 | 11 ± 0.2 de | 33.2 ± 0.4 bc | 10.2 ± 0.7 cd | 37.8 ± 0.8 | 1.1 ± 0.5 a |
| 16 | 3.9 | 15 ± 0.3 g | 35.7 ± 0.2 d | 4.2 ± 0.4 a | 29.1 ± 0.7 b | 2.4 ± 0.3 abc |
| 17 | 3.1 | 7 ± 0.2 b | 40.2 ± 0.4 fg | 14.2 ± 0.1 e | 33.8 ± 0.7 c | 4.1 ± 0.8 def |
| 18 | 3.2 | 6 ± 0.1 ab | 33.7 ± 0.2 c | 7.8 ± 0.2 b | 41.5 ± 0.8 de | 3.3 ± 0.4 cd |
| 19 | 3.0 | 7 ± 0.3 b | 85.1 ± 0.6 m | 9.1 ± 0.7 c | 71.2 ± 1.7 j | 3.6 ± 0.2 bcd |
| 20 | 3.6 | 12 ± 0.2 ef | 39.7 ± 0.2 f | 11.7 ± 0.1 d | 37.5 ± 0.7 i | 5.8 ± 0.6 egh |
| F = 315.03, p < 0.01 | F = 6047, p < 0.01 | F = 243.8, p < 0.01 | F = 428.6, p < 0.01 | F = 21.4, p < 0.01 |
| Cluster | pH | H2O2 (μg/g Unprocessed Honey) | Free Acidity | Lactonic Acidity | TPC (mg GAE/100 g of Honey) | TFC mg Quercetin Equivalents (CE)/100 g of Honey |
|---|---|---|---|---|---|---|
| 1 | 3.2 | 9.21 | 50.21 | 10.92 | 45.65 | 3.55 |
| 2 | 3.5 | 16.9 | 29.13 | 16.23 | 85.23 | 3.03 |
| Honey samples (n) | pH | H2O2 (μg/g Unprocessed Honey) | Free Acidity | Lactonic Acidity | TPC (mg GAE/100 g of Honey) | TFC mg Quercetin Equivalents (CE)/100 g of Honey |
|---|---|---|---|---|---|---|
| Citrus (20) | 3.81 ± 0.3 a | 32.19 ± 15.0 a | 15.6 ± 4.3 a | 6.2 ± 2.3 a | 41.6 ± 11.9 a | 1.07 ± 0.5 a |
| Satureja spp. (20) | 3.41 ± 0.3 b | 6.89 ± 3.9 b | 33.8 ± 17.4 b | 11.8 ± 6.2 b | 73.4 ± 44.6 b | 2.48 ± 1.2 b |
| Oregano and sage | 3.42 ± 0.3 b | 9.59 ± 3.5 b | 49.1 ± 20.8 c | 11.2 ± 4.4 b | 47.6 ± 17.4 a | 3.52 ± 1.5 c |
| F = 30.02, p < 0.05 | F = 136.1, p < 0.05 | F = 66.9, p < 0.05 | F = 26.5, p < 0.05 | F = 34.0, p < 0.05 | F = 76.9, p < 0.05 |
| Bacterial Species | Honey Origin (N) | Well Diffusion Assay (mm) | MIC 75% (w/v) | MIC % (w/v) Using Artificial Saliva | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Honey | Manuka | Honey | Manuka | + Catalase | + Proteinase K | Honey | Manuka | + Catalase | + Proteinase K | ||
| S. aureus A | Citrus (20) | 13.9 ± 3.6 b1 | 12.4 ± 0.9 a2 | 8.9 ± 3.7 b1 | 12.5 ± 0 a2 | 30.6 ± 13.5 b3 | 11.8 ± 6.5 b2 | 7.9 ± 3.9 a1 | 21.9 ± 5.4 a2 | 27.5 ± 16.7 a3 | 9.2 ± 8.2 a1 |
| Satureja spp. (20) | 17.1 ± 4.4 c1 | 12.3 ± 1.5 a2 | 7.3 ± 3.6 a1 | 12.5 ± 0 a2 | 7.5 ± 3.5 a1 | 7.6 ± 3.3 a1 | 6.7 ± 3.6 a1 | 23.1 ± 4.5 a2 | 7.5 ± 3.5 b1 | 6.1 ± 5.4 b1 | |
| Oregano and sage (20) | 15.1 ± 2.3 b1 | 12.1 ± 1.2 a2 | 8.1 ± 3.4 ab1 | 12.5 ± 0 a2 | 8.1 ± 3.3 a1 | 16.5 ± 7.2 c2 | 7.2 ± 4.1 a1 | 25.0 ± 0 b3 | 8.1 ± 3.4 b1 | 12.8 ± 8.3 c2 | |
| Artificial honey | 8.0 ± 0 a1 | 13.0 ± 20 a2 | |||||||||
| S. aureus B | Citrus (20) | 13.4 ± 2.3 b1 | 12.1 ± 1.1 a2 | 10.3 ± 3.0 b1 | 12.2 ± 1.4 b1 | 31.6 ± 16.1 b2 | 10.5 ± 4.7 a1 | 9.4 ± 3.6 c1 | 14.1 ± 4.8 b2 | 29.4 ± 16.4 a3 | 7.8 ± 4.8 a1 |
| Satureja spp. (20) | 17.5 ± 4.2 c1 | 13.0 ± 1.3 b2 | 6.4 ± 3.4 a1 | 11.8 ± 1.9 b3 | 8.6 ± 5.0 a2 | 8.7 ± 4.9 a2 | 6.4 ± 3.4 a1 | 11.9 ± 1.9 a3 | 8.8 ± 7.3 b2 | 7.3 ± 5.6 a12 | |
| Oregano and sage (20) | 16.1 ± 2.4 c1 | 12.5 ± 0.9 ab2 | 7.8 ± 3.6 a1 | 10.9 ± 2.7 a2 | 8.4 ± 3.4 a1 | 14.4 ± 7.5 b3 | 7.8 ± 3.7 b1 | 10.9 ± 2.7 a2 | 11.6 ± 6.6 b2 | 12.8 ± 6.9 b2 | |
| Artificial honey | 8.0 ± 0 a1 | 13.0 ± 1.2 ab2 | |||||||||
| S. aureus ATCC 12600 | Citrus (20) | 17.0 ± 1.2 b1 | 16.3 ± 1.3 a1 | 5.8 ± 1.1 b1 | 6.25 ± 0 a1 | 25.6 ± 13.9 c2 | 6.9 ± 2.7 a1 | 7.8 ± 4.4 c1 | 6.25 ± 0 1 | 22.5 ± 7.6 a2 | 6.25 ± 0 b1 |
| Satureja spp. (20) | 19.3 ± 1.8 d1 | 15.5 ± 1.1 a2 | 4.8 ± 1.9 a1 | 6.1 ± 0.7 a2 | 7.3 ± 3.6 a3 | 5.8 ± 2.9 a12 | 2.9 ± 0.6 a1 | 6.25 ± 0 2 | 7.8 ± 5 b3 | 1.2 ± 3.7 a4 | |
| Oregano and sage (20) | 18.3 ± 1.6 c1 | 16.0 ± 4.0 a2 | 5.2 ± 1.6 ab1 | 6.25 ± 0 a2 | 15.3 ± 8.3 b4 | 8.6 ± 5.8 b3 | 5.5 ± 1.4 b1 | 6.25 ± 0 12 | 7.8 ± 6.1 b2 | 8.4 ± 7.2 c2 | |
| Artificial honey | 8.0 ± 0 a1 | 16.0 ± 2.0 a2 | |||||||||
| S. aureus anaerobius | Citrus (20) | 18.7 ± 2.7 c1 | 14.9 ± 1.5 a2 | 5.54 ± 1.4 a1 | 6.6 ± 1.4 a1 | 24.1 ± 14.4 b2 | 7.0 ± 2.4 a1 | 4.7 ± 1.6 a1 | 12.5 ± 0 a2 | 7.2 ± 2.2 a3 | 5.5 ± 3.9 a1 |
| Satureja spp. (20) | 11.4 ± 1.4 b1 | 15.1 ± 1.4 a2 | 16.1 ± 10.4 b2 | 6.25 ± 0 a1 | 15.1 ± 8.7 a2 | 14.4 ± 10.3 b2 | 10.5 ± 5.8 b12 | 11.9 ± 2.7 a12 | 12.9 ± 8.6 b2 | 9.8 ± 5.2 b1 | |
| Oregano and sage (20) | 12.2 ± 1.3 b1 | 15.3 ± 1.5 a2 | 29.4 ± 10.7 c2 | 6.6 ± 1.4 a1 | 29.4 ± 10.7 c2 | 26.5 ± 14.1 c2 | 25.0 ± 19.5 c2 | 11.9 ± 4.8 a1 | 24.7 ± 14.6 c2 | 10.0 ± 11.6 b1 | |
| Artificial honey | 8.0 ± 0 a1 | 15.0 ± 1.4 a2 | |||||||||
| S. aureus anaerobius ATCC 35844 | Citrus (20) | 18.7 ± 1.8 c1 | 14.3 ± 0.9 a2 | 5.4 ± 1.5 a1 | 6.25 ± 0 a1 | 30.0 ± 12.8 b2 | 6.6 ± 2.9 a1 | 4.9 ± 1.8 a1 | 12.5 ± 0 b2 | 10.9 ± 3.2 a3 | 7.8 ± 4.4 a4 |
| Satureja spp. (20) | 13.6 ± 1.2 b1 | 14.8 ± 1.2 ab | 12.2 ± 5.0 c2 | 6.25 ± 0 a1 | 13.9 ± 9.5 a23 | 16.8 ± 6.5 c3 | 9.8 ± 3.7 b1 | 11.25 ± 3.8 a1 | 10.1 ± 3.8 a1 | 12.5 ± 9.8 b1 | |
| Oregano and sage (20) | 14.0 ± 1.2 b1 | 15.1 ± 1.0 b2 | 8.3 ± 3.6 b1 | 6.25 ± 0 a1 | 28.4 ± 13.5 b2 | 8.7 ± 4.9 b1 | 16.6 ± 15 c2 | 11.9 ± 2.7 ab12 | 9.7 ± 5.8 a1 | 22.5 ± 15.7 c3 | |
| Artificial honey | 8.0 ± 0 a1 | 15.0 ± 2.0 ab1 | |||||||||
| Streptococcus mutans | Citrus (20) | 18.3 ± 4.7 b1 | 14.1 ± 2.7 a2 | 6.2 ± 3.4 b1 | 6.25 ± 0 a1 | 22.5 ± 17.4 b2 | 8.3 ± 5.3 b1 | 6.1 ± 0.7 a1 | 6.25 ± 0 a1 | 6.25 ± 3.4 a1 | 5.6 ± 1.9 a1 |
| Satureja spp. (20) | 20.7 ± 3.7 c1 | 13.7 ± 2.5 a2 | 4.5 ± 1.8 a1 | 6.25 ± 0 a2 | 6.1 ± 2.5 a2 | 5.1 ± 2.4 a1 | 6.1 ± 0.7 a1 | 6.25 ± 0 a1 | 6.25 ± 0 a1 | 4.0 ± 7.7 a2 | |
| Oregano and sage (20) | 20.1 ± 4.9 bc1 | 13.8 ± 2.5 a2 | 6.25 ± 0 b1 | 6.25 ± 0 a1 | 9.4 ± 6.0 a2 | 8.1 ± 4.5 b2 | 6.25 ± 0 a1 | 6.25 ± 0 a1 | 6.9 ± 1.9 a1 | 8.1 ± 4.0 b2 | |
| Artificial honey | 8.0 ± 0 a1 | 14.0 ± 2.0 a2 | |||||||||
| Fusobacteriumnucleatum | Citrus (20) | 11.3 ± 1.3 b1 | 12.1 ± 1.0 b2 | 25.0 ± 0 c1 | 25.0 ± 0 a1 | 18.7 ± 20.9 b2 | 14.1 ± 12.2 b2 | 11.6 ± 2.2 a1 | 12.5 ± 0 a1 | 13.7 ± 21.8 b1 | 22.5 ± 7.6 c2 |
| Satureja spp. (20) | 16.9 ± 2.2 d1 | 11.5 ± 0.9 a2 | 5.9 ± 0.9 a1 | 25.0 ± 0 a3 | 8.4 ± 5.7 a2 | 7.5 ± 4.7 a12 | 5.6 ± 1.2 b1 | 12.5 ± 0 a2 | 5.3 ± 2.2 a1 | 1.6 ± 3.9 a3 | |
| Oregano and sage (20) | 15.1 ± 2.1 c1 | 11.5 ± 0.8 a2 | 6.25 ± 0 b1 | 25.0 ± 0 a3 | 13.1 ± 9.3 ab2 | 5.6 ± 2.7 a1 | 5.3 ± 1.7 b1 | 12.5 ± 0 a3 | 13.7 ± 7 b3 | 9.7 ± 6.7 b2 | |
| Artificial honey | 8.0 ± 0 a1 | 10.9 ± 1.0 ab2 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voidarou, C.; Antoniadou, M.; Rozos, G.; Alexopoulos, A.; Giorgi, E.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. An In Vitro Study of Different Types of Greek Honey as Potential Natural Antimicrobials against Dental Caries and Other Oral Pathogenic Microorganisms. Case Study Simulation of Oral Cavity Conditions. Appl. Sci. 2021, 11, 6318. https://doi.org/10.3390/app11146318
Voidarou C, Antoniadou M, Rozos G, Alexopoulos A, Giorgi E, Tzora A, Skoufos I, Varzakas T, Bezirtzoglou E. An In Vitro Study of Different Types of Greek Honey as Potential Natural Antimicrobials against Dental Caries and Other Oral Pathogenic Microorganisms. Case Study Simulation of Oral Cavity Conditions. Applied Sciences. 2021; 11(14):6318. https://doi.org/10.3390/app11146318
Chicago/Turabian StyleVoidarou, Chrysoula (Chrysa), Maria Antoniadou, Georgios Rozos, Athanasios Alexopoulos, Elpida Giorgi, Athina Tzora, Ioannis Skoufos, Theodoros Varzakas, and Eugenia Bezirtzoglou. 2021. "An In Vitro Study of Different Types of Greek Honey as Potential Natural Antimicrobials against Dental Caries and Other Oral Pathogenic Microorganisms. Case Study Simulation of Oral Cavity Conditions" Applied Sciences 11, no. 14: 6318. https://doi.org/10.3390/app11146318
APA StyleVoidarou, C., Antoniadou, M., Rozos, G., Alexopoulos, A., Giorgi, E., Tzora, A., Skoufos, I., Varzakas, T., & Bezirtzoglou, E. (2021). An In Vitro Study of Different Types of Greek Honey as Potential Natural Antimicrobials against Dental Caries and Other Oral Pathogenic Microorganisms. Case Study Simulation of Oral Cavity Conditions. Applied Sciences, 11(14), 6318. https://doi.org/10.3390/app11146318










