Meat Composition and Quality Assessment of King Scallops (Pecten maximus) and Frozen Atlantic Sea Scallops (Placopecten magellanicus) on a Retail Level
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Procurement
2.2. Chemical Analyses
2.2.1. Proximate Composition and pH
2.2.2. Citric Acid
2.2.3. Condensed Phosphates
2.2.4. Total Volatile Basic Nitrogen (TVB-N)
2.2.5. Fatty Acid Profiles
2.2.6. Free Amino Acids
2.2.7. Mineral Element Analysis
2.3. DNA Analysis
2.4. Statistical Evaluation
3. Results and Discussion
3.1. Authentication
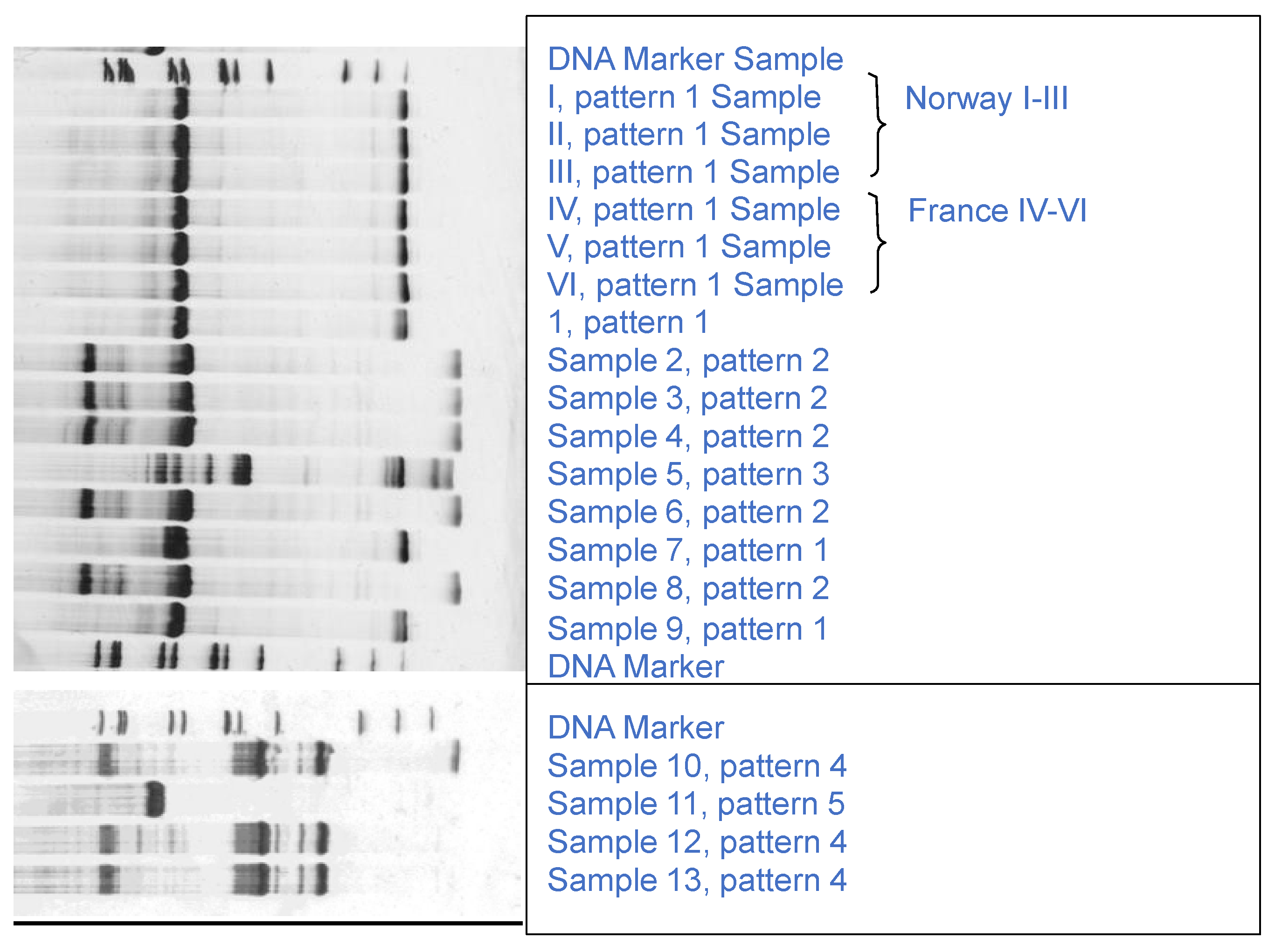
3.2. Chemical Results for Fresh King Scallop Meats (Pecten maximus)
3.2.1. Proximate Analyses, including Moisture to Protein Ratio, pH, TVB-N and Mineral Content
| Norway I (n = 20) | Norway II (n = 18) | Norway III (n = 20 + 10) | France I (n = 30 + 10) | France II (n = 20) | France III (n = 20) | |
|---|---|---|---|---|---|---|
| Muscle weight (g) | 27.0 | 38.3 | 24.7 | 17.3 | 26.1 | 23.7 |
| pH | 6.1 | 6.0 | 6.2 | 6.2 | 6.0 | 6.2 |
| TVB-N (mg/100 g) | 14.3 | 11.1 | 12.6 | 6.5 | (24.5) 1 | 9.9 |
| Moisture (%) | 78.2 | 78.0 | 77.3 (76.2 ± 0.9) | 76.5 (75.0 ± 1.1) | 74.9 | 74.6 |
| Protein (%) | 18.9 | 19.2 | 18.2 (18.6 ± 0.5) | 20.0 (18.9 ±0.8) | 18.0 | 18.4 |
| Moisture/Protein-Ratio | 4.1 | 4.1 | 4.3 | 3.8 | 4.2 | 4.1 |
| Lipid (%) | 1.1 | 1.3 | 1.1 | 1.0 | 0.4 | 1.1 |
| Carbohydrate (%) | 2.7 | 3.2 | 4.0 (3.9 ± 0.7) | 3.5 (3.5 ± 0.7) | 6.8 | 6.7 |
| Ash (%) | 1.4 | 1.4 | 1.4 (1.8 ± 0.1) | 1.5 (1.6 ± 0.2) | 1.5 | 1.4 |
| NaCl (%) | 0.48 | 0.34 | 0.44 | 0.37 | 0.47 | 0.34 |
| P2O5 (g kg−1) | 6.3 | 5.8 | 5.8 | 6.0 | 6.0 | 6.3 |
| Na (mg kg−1) | 1028 | 1096 | 994 | 916 | 1167 | 826 |
| K (mg kg−1) | 3976 | 4047 | 3937 | 4118 | 3860 | 4100 |
| Ca (mg kg−1) | 301 | 278 | 296 | 267 | 255 | 240 |
| Mg (mg kg−1) | 342 | 352 | 330 | 356 | 348 | 350 |
| Zn (mg kg−1) | 13.7 | 13.3 | 15.1 | 16.9 | 12.6 | 13.3 |
| Se (mg kg−1) | 0.32 | 0.33 | 0.26 | 0.29 | 0.22 | 0.20 |
| As (mg kg−1) | 2.23 | 1.54 | 2.05 | 1.78 | 1.52 | 1.51 |
| Citric acid (mg kg−1) | <5 | <5 | <5 | <5 | <5 | <5 |
| Most frequently free amino acids (FAA) | ||||||
| Alanine (mg/100 g) | 64 | 52 | 63 | 90 | 110 | 150 |
| Arginine (mg/100 g) | 180 | 265 | 280 | 365 | 240 | 325 |
| Glycine (mg/100 g) | 1520 | 1160 | 1640 | 1220 | 1580 | 1590 |
| Taurine (mg/100 g) | 795 | 890 | 975 | 1220 | 800 | 840 |
| Total FAA (mg/100 g) | 2666 | 2450 | 3053 | 3032 | 2840 | 2999 |
3.2.2. Citric Acid
3.2.3. Fatty Acid Profile
| King Scallops | Atlantic Sea Scallops | ||||
|---|---|---|---|---|---|
| Fresh | Frozen | Frozen | |||
| FA common name | FA Shorthand | Norway I-III n = 3 | France I-III n = 3 | n = 4 | n = 8 |
| Myristic acid | 14:0 | 2.4 ± 0.02 a | 2.5 ± 0.20 a | 3.2 ± 0.36 b | 2.1 ± 0.20 a |
| Pentadecanoic acid | 15:0 | 0.8 ± 0.01 a | 0.8 ± 0.03 a | 0.8 ± 0.06 a | 0.8 ± 0.09 a |
| Palmitic acid | 16:0 | 17.3 ± 0.20 a | 18.4 ± 0.29 b | 17.4 ± 0.88 a | 18.6 ± 0.55 b |
| Heptadecanoic acid | 17:0 | 1.0 ± 0.03 a | 1.1 ± 0.04 a | 1.1 ± 0.11 a | 0.7 ± 0.09 b |
| Stearic acid | 18:0 | 7.1 ± 0.18 a | 7.9 ± 0.25 b | 6.8 ± 0.35 a | 5.6 ± 0.31 c |
| ∑SFA | 28.5 ± 0.30 a | 30.7 ± 0.47 a | 29.3 ± 1.12 a | 27.8 ± 1.20 a | |
| Palmitoleic acid | 16:1n-7 | 1.6 ± 0.15 a | 1.2 ± 0.04 a | 1.4 ± 0.39 a | 2.0 ± 0.52 a |
| Elaidic acid | 18:1n-9t | n.d. | n.d | n.d | n.d |
| Oleic acid | 18:1n–9c | 1.0 ± 0.13 a | 1.0 ± 0.05 a | 1.3 ± 0.12 b | 1.2 ± 0.12 ab |
| Vaccenic acid | 18-1n-7 | 3.1 ± 0.32 a | 3.2 ± 0.30 a | 2.9 ± 0.25 a | 5.4 ± 0.24 b |
| Gondoic acid | 20:1n–9 | 1.7 ± 0.13 a | 1.5 ± 0.20 a | 1.8 ± 0.33 a | 0.9 ± 0.06 b |
| Erucic acid | 22:1n-9 | n.d. | n.d. | n.d. | n.d. |
| ∑MUFA | 7.3 ± 0.26 a | 6.8 ± 0.43 a | 7.4 ± 0.42 a | 9.4 ± 0.78 b | |
| Linoleic acid | 18:2n–6c | 0.4 ± 0.27 a | 0.4 ± 0.28 a | 0.5 ± 0.27 a | 0.4 ± 0.04 a |
| γ-Linolenic acid | 18:3n–6 | n.d | n.d | n.d | n.d |
| α-Linolenic acid | 18:3n–3 | 0.2 ± 0.21 a | 0.2 ± 0.27 a | 0.7 ± 0.04 b | 0.2 ± 0.18 a |
| Stearidonic acid | 18:4n–3 | 1.4 ± 0.13 a | 1.8 ± 0.12 ab | 2.1 ± 0.30 b | 1.7 ± 0.39 ab |
| Eicosadienic acid | 20:2n–6 | 0.7 ± 0.03 a | 0.7 ± 0.05 a | 0.3 ± 0.34 b | 0.3 ± 0.11 b |
| Arachidonic acid | 20:4n–6 | 3.4 ± 0.24 a | 3.4 ± 0.58 a | 2.2 ± 0.26 b | 1.8 ± 0.15 b |
| Eicosapentaenoic acid (EPA) | 20:5n–3 | 20.0 ± 0.79 ac | 18.0 ± 0.86 ac | 14.8 ± 0.95 b | 20.3 ± 0.98 c |
| Docosatetraneoic acid | 22:4n–6 | 0.1 ± 0.17 | n.d | n.d | n.d |
| Docosapentaenoic acid (DPA) | 22:5n–3 | 1.6 ± 0.14 a | 1.2 ± 0.23 ab | 1.0 ± 0.02 bc | 0.9 ± 0.06 c |
| Docosahexaenoic acid (DHA) | 22:6n–3 | 26.2 ± 0.68 a | 25.9 ± 0.46 a | 26.1 ± 1.84 a | 21.3 ± 1.78 b |
| ∑PUFA | 53.9 ± 0.45 a | 51.6 ± 1.20 a | 47.8 ± 2.56 b | 47.0 ± 1.08 b | |
| unidentified | 10.4 ± 0.60 a | 10.9 ± 0.30 a | 15.5 ± 2.16 b | 15.8 ± 0.63 b | |
| ∑n–3 | 49.3 ± 0.34 | 47.2 ± 0.85 | 44.7 ± 2.18 | 44.5 ± 1.05 | |
| ∑n–6 | 4.6 ± 0.17 | 4.4 ± 0.35 | 3.0 ± 0.59 | 2.5 ± 0.09 | |
| Ratio n-3/n-6 | 10.9 ± 0.38 | 10.7 ± 0.62 | 15.2 ± 2.52 | 18.2 ± 0.44 | |
| EPA + DHA | 46.2 ± 0.23 | 44.0 ± 0.72 | 40.9 ± 2.23 | 41.6 ± 1.05 | |
3.2.4. Free Amino Acids
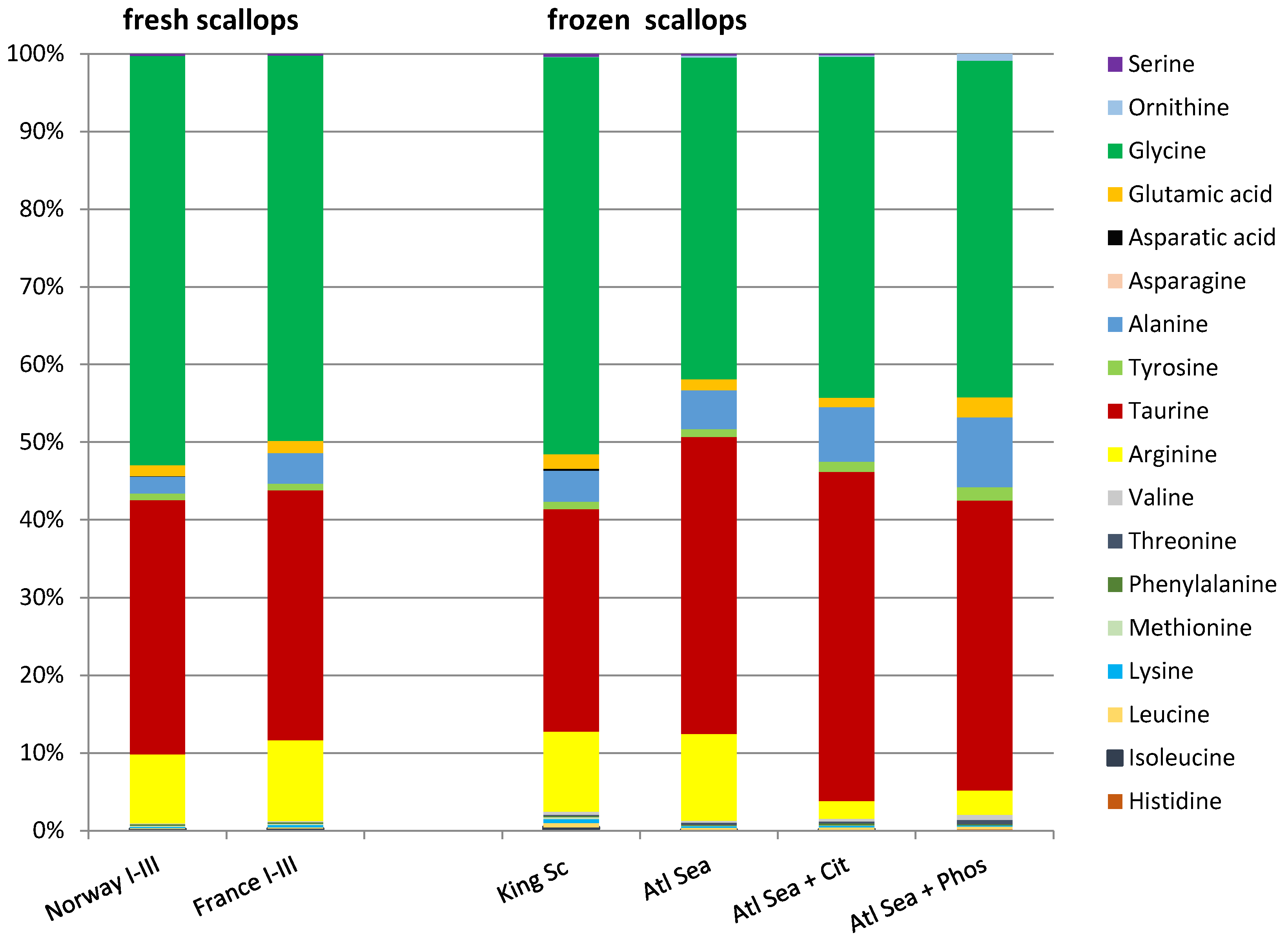
3.3. Products of Frozen King Scallops and Atlantic Sea Scallop Meats
3.3.1. Proximate Analyses, including pH, TVB-N-Values and Mineral Elements
| King Scallops | Atlantic Sea Scallops | |||
|---|---|---|---|---|
| Labelling (No of Products Analyzed) | NA (4) | NA (4) | Citrate (3) | Phosphate (1) |
| Glaze (%)/drip loss (%) | 8-27/20-34 | 7-15/1-8 | 7-13/6-10 | 12/6 |
| pH | 6.4 ± 0.5 (6.0–7.0) | 6.9 ± 0.6 (6.4–7.8) | 8.0 ± 0.6 (7.6–8.7) | 7.5 |
| TVB-N (mg/100 g) | 14.6 ± 1.5 (12.9–16.5) | 17.8 ± 2.9 (13.7–20.4) | 12.7 ± 3.2 (9.1–15.3) | 13.4 |
| Moisture (%) | 81.2 ± 5.1 (76.4–86.7) | 80.5 ± 4.3 (76.6–86.5) | 86.8 ± 2.3 (84.5–89.1) | 85.7 |
| Protein (%) | 14.1 ± 3.7 (10.2–16.6) | 15.0 ± 3.6 (10.0–17.8) | 10.5 ± 1.7 (9.2–12.4) | 7.8 |
| Moisture/Protein-Ratio | 6.2 ± 2.0 (4.4–8.6) | 5.7 ± 2.0 (4.3–8.6) | 8.3±1.3 (6.8–9.2) | 11.0 |
| Lipid (%) | 0.8 ± 0.1 (0.7–0.9) | 0.6 ± 0.1 (0.4–0.7) | 0.4 ± 0.1 (0.3–0.5) | 0.5 |
| Carbohydrate (%) | 3.5 ± 1.2 (2.4–4.8) | 2.9 ± 2.3 (0.4–5.8) | 1.9 ± 0.4 (1.4–2.3) | 4.6 |
| Citric acid (g·kg−1) | <0.005 | <0.005 -0.65 | 0.53–2.12 | <0.005 |
| Ash (%) | 1.35 ± 0.58 (0.65–1.42) | 1.69 ± 0.27 (1.42–2.05) | 1.82 ± 1.17 (0.70–3.03) | 1.93 |
| NaCl (%) | 0.29 ± 0.13 (0.16–0.42) | 0.46 ± 0.14 (0.31–0.63) | 0.25 ± 0.22 (0.11–0.50) | 0.21 |
| P2O5 (g·kg−1) | 6.0 ±.2.3 (3.5–9.1) | 6.9 ± 1.6 (5.9–9.3) | 4.78 ± 5.43 (1.03–11.00) | 9.7 |
| Diphosphate (g·kg−1) | <0.01 (3) 2 –2.39 | <0.01 | <0.01 | 3.61 |
| Triphosphate (g·kg−1) | <0.01 (3) 2 –0.93 | <0.01 | <0.01 | 1.88 |
| Na (mg·kg−1) | 1458 (240–3530) | 2797 (1513–5636) | 3760 (1658–5616) | 4907 |
| K (mg·kg−1) | 2298 (1181–3515) | 2902 (913–4135) | 1099 (602–2004) | 492 |
| Ca (mg·kg−1) | 450 (219–635) | 388 (311–483) | 486 (438–512) | 486 |
| Mg (mg·kg−1 | 268 (162–390) | 322 (138–430) | 140(106–205) | 105 |
| Zn (mg·kg−1) | 16.5 (12.3–21.6) | 10.3 (6.9–13.7) | 8.5 (7.5–9.6) | 5.3 |
| Se (mg·kg−1) | 0.2 | 0.2 (0.1–0.2) | 0.1 | 0.1 |
| As (mg·kg−1) | 1.0 (0.6–1.3) | 1.2 (0.5–1.9) | 1.0 (0.5–2.1) | 0.5 |
| Most frequently free amino acids (FAA) | ||||
| Alanine (mg/100 g) | 53 (38–82) | 75 (25–113) | 63 (39–100) | 52 |
| Arginine (mg/100 g) | 156 (64–350) | 249 (11–565) | 16 (6–33) | 18 |
| Glycine (mg/100 g) | 693 (453–1140) | 688 (168–905) | 381 (288–540) | 251 |
| Taurine (mg/100 g) | 395 (235–675) | 681 (144–1220) | 385 (225–620) | 216 |
| Total FAA (mg/100 g) | 1374 (842–2356) | 1761 (367–2854) | 883 (631–1321) | 579 |
3.3.2. NaCl, Phosphates and Citric Acid
Frozen King Scallops
Frozen Atlantic Sea Scallops
3.3.3. Fresh versus Frozen Scallops: Moisture to Protein Ratio
3.3.4. Fatty Acid Profile
3.3.5. Free Amino Acids
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Downey, R.; Marsh, L.; Flick, G.J. Shellfish-mollusks. In The Seafood Industry: Species, Products, Processing, and Safety, 2nd ed.; Granata, L.A., Flick, G.J., Martin, R.E., Eds.; Blackwell Publishing Ltd: Oxford, UK, 2012; pp. 136–150. [Google Scholar]
- FAO. Fisheries and Aquaculture Department Fact Sheets. 2014. Available online: http://www.fao.org/fishery/factsheets/en (accessed on 23 June 2015).
- Wilding, C.S.; Beaumont, A.R.; Latchford, J.W. Short communication. Are Pecten maximus and Pecten jacobaeus different species? J. Mar. Biol. Assoc. UK 1999, 79, 949–952. [Google Scholar] [CrossRef]
- Loreal, H.; Etienne, M. Added Water in Frozen Scallop Muscles. French Specifications and Methodology. 1990. Available online: http://www.archimer.ifremer.fr/doc/00099/20989/18615.pdf (accessed on 23 June 2015).
- DuPaul, W.D.; Otwell, W.S.; Rippen, T.E. An Evaluation of Processed Atlantic Sea Scallops (Placopecten magellanicus); Virginia Institute of Marine Science, College of William and Mary: Gloucester Point, VA, USA, 1993; Available online: http://www.vims.edu/GreyLit/VIMS/mrr93-1.pdf (accessed on 23 June 2015).
- Manthey-Karl, M.; Schröder, U.; Wagler, M. Wassergehalte tiefgefrorener Kammmuscheln (water content of frozen scallops). Inf. Fischereiforsch. 2012, 59, 61–69. [Google Scholar]
- Webb, N.B.; Thomas, F.B.; Busta, F.F.; Monroe, R.J. Variations in proximate composition of North Carolina scallop meats. J. Food Sci. 1969, 34, 471–474. [Google Scholar] [CrossRef]
- Kennedy, L. Investigating scallop processing practices. In EHOA Yearbook 10/11; Zahra Publishing Ltd.: Wicklow, Irland, 2011; pp. 52–55. Available online: http://www.ehai.ie/ (accessed on 23 June 2015).
- Chang, C.C.; Regenstein, J.M. Water uptake, protein solubility, and protein changes of cod mince stored on ice as affected by polyphosphates. J. Food Sci. 1997, 62, 305–309. [Google Scholar] [CrossRef]
- EU Commission. Regulation No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32011R1169 (accessed on 23 June 2015).
- Codex Alimentarius. Report of the 33rd Session of the Codex Committee on Fish and Fishery Products (REP14/FFP), 2014. Available online: http://www.ftp.fao.org/codex/Reports/Reports_2014/REP14_FFPe_rev.pdf (accessed on 23 June 2015).
- Botta, J.R.; Cahill, F.M. Moisture content of scallop meat: Effect of species, time and season and method of determining “added water”. In Proceedings of the Tropical and Subtropical Fisheries Technological Conference of the Americas, Williamsburg, VA, USA; 1992; pp. 43–50. [Google Scholar]
- Codex Alimentarius. Codex Standard 92-1981 for Quick Frozen Shrimps or Prawns. Codex Committee on Fish and Fishery Products (CCFFP). Available online: http://www.codexalimentarius.org/standards/list-of-standards/ (accessed on 23 September 2015).
- Antonacopoulos, N. Untersuchungsverfahren (Sensorik, Chemie). In Fische und Fischerzeugnisse, 2nd ed.; Ludorff, W., Meyer, V., Eds.; Paul Parey: Berlin/Hamburg, Germany, 1973; p. 219. [Google Scholar]
- AOAC. Method #968.06. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Smedes, F. Determination of total lipid using non-chlorinated solvents. Analyst 1999, 124, 1711–1718. [Google Scholar] [CrossRef]
- Karl, H.; Bekaert, K.; Berge, J.-P.; Cadun, A.; Duflos, G.; Oehlenschläger, J.; Poli, B.M.; Tejada, M.; Testi, S.; Timm-Heinrich, M. WEFTA interlaboratory comparison on total lipid determination in fishery products using the Smedes method. J. AOAC Int. 2012, 95, 1–5. [Google Scholar] [CrossRef]
- Karl, H.; Äkesson, G.; Etienne, M.; Huidobro, A.; Luten, J.; Mendes, R.; Tejada, M.; Oehlenschläger, J. WEFTA interlaboratory comparison on salt determination in fishery products. J. Aquat. Food Prod. Technol. 2002, 11, 215–228. [Google Scholar] [CrossRef]
- LFGB. Bestimmung des Gesamtphosphorgehaltes in Fleisch und Fleischerzeugnissen. Photometrisches Verfahren. L06.00–9. In Amtliche Sammlung von Untersuchungsverfahren Nach § 64 LFGB; Beuth-Verlag: Berlin, Germany, 2008. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kaufmann, A.; Pacciarelli, B. Determination of some ionic additives in meat products by ion chromatography. Mitt. Gebiete Hyg. 2000, 91, 581–596. [Google Scholar]
- Kaufmann, A.; Maden, K.; Leisser, W.; Mater, M.; Gude, T. Analysis of polyphosphates in fish and shrimps tissues by two different ion chromatography methods: Implications on false-negative and -positive findings. Food Addit. Contam. 2005, 22, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- EU Commission. Commission Regulation (EC) No 2074/2005 of 5 December 2005 Laying down Implementing Measures for Certain Products under Regulation (EC) No 853/2004 of the European Parliament and of the Council and for the Organisation of Official Controls under Regulation (EC) No 854/2004 of the European Parliament and of the Council and Regulation (EC) No 882/2004 of the European Parliament and of the Council, Derogating from Regulation (EC) No 852/2004 of the European Parliament and of the Council and Amending Regulations (EC) No 853/2004 and (EC) No 854/2004. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32005R2074 (accessed on 23 June 2015).
- DGF (Deutsche Gesellschaft für Fettwissenschaft, German Society for Fat Science). DGF-Einheitsmethode C-VI-10a. Gaschromatographie: Analyse der Fettsäuren und Fettsäureverteilung; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany, 2000. [Google Scholar]
- DGF (Deutsche Gesellschaft für Fettwissenschaft, German Society for Fat Science). DGF-Einheitsmethode C-VI-11d. Fettsäuremethylester (Alkalische Umesterung); Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany, 1998. [Google Scholar]
- Ostermeyer, U.; Molkentin, J.; Lehmann, I.; Rehbein, H.; Walte, H.-G. Suitability of instrumental analysis for the discrimination between wild-caught and conventionally and organically farmed shrimps. Eur. Food Res. Technol. 2014, 239, 1015–1029. [Google Scholar] [CrossRef]
- Antoine, F.R.; Wei, C.I.; Littell, R.C.; Marshall, M.R. HPLC Method for analysis of free amino acids in fish using o-phthaldialdehyde precolumn derivatization. J. Agric. Food Chem. 1999, 47, 5100–5107. [Google Scholar] [CrossRef] [PubMed]
- Manthey-Karl, M.; Ostermeyer, U.; Barth, J.; Rehbein, H. Quality aspects and species identification of cephalopod products on the German market. Arch. Lebensm. 2013, 64, 15–23. [Google Scholar]
- Karl, H.; Manthey-Karl, M.; Ostermeyer, U.; Lehmann, I.; Wagner, H. Nutritional composition and sensory attributes of Alaskan flatfishes compared to plaice (Pleuronectes platessa). Int. J. Food Sci. Technol. 2013, 48, 962–971. [Google Scholar] [CrossRef]
- Rehbein, H.; Kress, G. Detection of short mRNA sequences in fishery products. Deut. Lebensm.-Rundsch. 2005, 101, 333–337. [Google Scholar]
- Fernandez, A.; Garcia, T.; Gonzalez, I.; Asensio, L.; Rodriguez, M.A.; Hernandez, P.E.; Martin, R. Polymerase chain reaction-restriction fragment length polymorphism analysis of a 16S rRNA gene fragment for authentication of four clam species. J. Food Prot. 2002, 65, 692–695. [Google Scholar] [PubMed]
- Schiefenhövel, K.; Rehbein, H. Identification of tropical shrimp species by RFLP and SSCP analysis of mitochondrial genes. Arch. Lebensm. 2010, 61, 50–56. [Google Scholar]
- NCBI (National Center for Biotechnology Information). Available online: http://www.ncbi.nIm.nih.gov/ (accessed on 23 September 2015).
- Lopez-Piňon, M.J.; Insua, A.; Mendez, J. Identification of four scallop species using PCR and restriction analysis of the ribosomal DNA internal transcribed spacer region. Mar. Biotechnol. 2002, 4, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Näumann, G.; Stumme, B.; Rehbein, H. Differenzierung von Kammmuscheln durch DNA-Analyse. Inf. Fischereiforsch. 2012, 59, 1–7. [Google Scholar]
- Canapa, A.; Barucca, M.; Marinelli, A.; Olmo, E. Molecular data from the 16S rRNA gene for the phylogeny of Pectinidae (Mollusca: Bivalvia). J. Mol. Evol. 2000, 50, 93–97. [Google Scholar] [PubMed]
- Jiménez-Ruiz, E.I.; Ocaño-Higuera, V.M.; Maeda-Martínez, A.N.; Castillo-Yáñez, F.J.; Varela-Romero, A.; Graciano-Verdugo, A.Z.; Márquez Ríos, E. Quality and shelf life of the adductor muscle of lions paw scallop Nodipecten subnodosus transported and stored whole in refrigeration. Interciencia 2012, 37, 464–469. [Google Scholar]
- Comely, C.A. Seasonal variations in the flesh weights and biochemical content of the scallop Pecten maximus (L.) in the Clyde Sea Area. J. Cons. Int. Explor. Mer. 1974, 35, 281–295. [Google Scholar] [CrossRef]
- Rippen, T.E.; Sutton, H.C.; Lacey, P.F.; Lane, R.M.; Fisher, R.A.; DuPaul, W.D. Functional, microbial and sensory changes in ice-stored sea scallops (Placopecten magellanicus) treated with sodium tripolyphosphate. J. Muscle Foods 1996, 7, 93–108. [Google Scholar] [CrossRef]
- DuPaul, W.D.; Fisher, R.A.; Kirkley, J.E. Natural and ex-vessel moisture content of sea scallops (Placopecten magellanicus); Virginia Marine Resource Report 96–5; Virginia Institute of Marine Science, College of William and Mary: Gloucester Point, VA, USA, 1996; Available online: http://www.vims.edu/GreyLit/VIMS/mrr96-5.pdf (accessed on 23 June 2015).
- Pazos, A.J.; Román, G.; Acosta, C.P.; Aba, M.; Sánchez, J.L. Seasonal changes in condition and biochemical composition of the scallop Pecten maximus L. from suspended culture in the Ria de Arousa (Galicia, N.W. Spain) in relation to environmental conditions. J. Exp. Mar. Biol. Ecol. 1997, 211, 169–193. [Google Scholar] [CrossRef]
- Kimura, M. Studies on the quality control of the Japanese scallop adductor muscle. Sci. Rep. Hokkaido Fish. Exp. Stn. 2003, 65, 1–47. [Google Scholar]
- Holland, B.; Brown, J.; Buss, D.H. Fish and Fish Products, Third Supplement to the Fifth Edition of McCance and Widdowson’s “The Composition of Foods”; The Royal Society of Chemistry: Cambridge, UK, 1993. [Google Scholar]
- Sidwell, V.D.; Buzzell, D.H.; Foncannon, P.R.; Smith, A.L. Composition of the edible portion of raw (fresh or frozen) crustaceans, finfish, and mollusks. II. Macroelements: Sodium, potassium, chlorine, calcium, phosphorus, and magnesium. Mar. Fish. Rev. 1977, 39, 1–11. [Google Scholar]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Oehlenschläger, J. Minerals and trace elements. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M.L., Toldra, F., Eds.; CRC press: Boca Raton, FL, USA, 2010; pp. 351–375. [Google Scholar]
- Greig, R.A.; Wenzloff, D.R.; MacKenzie, C.L.; Merrill, A.S.; Zdanowicz, V.S. Trace metals in sea scallops, Placopecten magellanicus, from Eastern United States. Bull. Environ. Contam. Toxicol. 1978, 19, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; CAB International: Wallingford, UK, 2010; Chapter 15. [Google Scholar]
- He, M.; Wang, W.-X. Bioaccessibility of 12 trace elements in marine molluscs. Food Chem. Toxicol. 2013, 55, 627–636. [Google Scholar] [CrossRef]
- EFSA. EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Monographs on the Evaluation of Carcinogenic Risks to Humans; A Review of Human Carcinogens: Arsenic, Metals, Fibres, and Dusts, vol. 100 C; International Agency for Research on Cancer: Lyon, France, 2012; pp. 41–93. [Google Scholar]
- Fontcuberta, M.; Calderon, J.; Villalbí, J.R.; Centrich, F.; Portaña, S.; Espelt, A.; Duran, J.; Nebot, M. Total and inorganic arsenic in marketed food and associated health risks for the Catalan (Spain) population. J. Agric. Food Chem. 2011, 59, 10013–10022. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, K.A. Current perspectives in arsenic environmental and biological research. Environ. Chem. 2005, 2, 141–145. [Google Scholar] [CrossRef]
- Olmedo, P.; Pla, A.; Hernández, A.F.; Barbier, F.; Ayouni, L.; Gil, F. Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk assessment for the consumers. Environ. Int. 2013, 59, 63–72. [Google Scholar] [CrossRef]
- Chew, S.F.; Ip, Y.K. Biochemical adaptions of the mudskipper Boleophthalmus boddaerti to a lack of oxygen. Mar. Biol. 1992, 112, 567–571. [Google Scholar] [CrossRef]
- Piironen, J.; Hyvärinen, H. Composition of the milt of some teleost fishes. J. Fish. Biol. 1983, 22, 351–361. [Google Scholar]
- Jahresbericht 2005 des Kantonalen Laboratorium Bern; Annual Report; Kantonales Laboratorium Bern: Bern, Switzerland, 2005.
- Jahresbericht 2006 des Kantonalen Laboratoriums Bern; Annual Report; Kantonales Laboratorium Bern: Bern, Switzerland, 2006.
- Hardy, R.W.; Lee, C.-S. Aquaculture feed and seafood quality. Bull. Fish. Res. Agency 2010, 31, 43–50. [Google Scholar]
- Grahl-Nielsen, O.; Jacobsen, A.; Christophersen, G.; Magnesen, T. Fatty acid composition in adductor muscle of juvenile scallops (Pecten maximus) from five Norwegian populations reared in the same environment. Biochem. Syst. Ecol. 2010, 38, 478–488. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic Amines in Seafood Products. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M.L., Toldra, F., Eds.; CRC Press: Boca Raton, FL, US, 2010; pp. 833–850. [Google Scholar]
- Haard, N.F. Control of chemical composition and food quality attributes of cultured fish. Food Res. Int. 1992, 25, 289–307. [Google Scholar] [CrossRef]
- Otsuka, Y.; Tanaka, S.; Nishigaki, K.; Miyagawa, M. Change in the contents of arginine, ornithine, and urea in the muscle of marine invertebrates stored in ice. Biosci. Biotech. Biochem. 1992, 56, 863–866. [Google Scholar] [CrossRef]
- Wongso, S.; Yamanaka, H. Extractive components of the adductor muscle of Japanese baking scallop and changes during refrigerated storage. J. Food Sci. 1998, 63, 1–5. [Google Scholar] [CrossRef]
- Beltrán-Lugo, A.I.; Maeda-Martínez, A.N.; Pacheco-Aguilar, R.; Nolasco-Soria, H.G. Seasonal variations in chemical, physical, textural, and microstructural properties of adductor muscles of Pacific lions-paw scallop (Nodipecten subnodosus). Aquaculture 2006, 258, 619–632. [Google Scholar] [CrossRef]
- Undeland, I. Selected amino acids in fish. In Marine Functional Food; Luten, J.B., Ed.; Academic Publishers: Wageningen, The Netherlands, 2009; pp. 43–47. [Google Scholar]
- Mackie, I.M.; Pirie, L.; Ritchie, A.H.; Yamanaka, H. The formation of non-volatile amines in relation to concentrations of free basic amino acids during postmortem storage of the muscle of scallop (Pecten maximus), herring (Clupea harengus) and mackerel (Scomber scombrus). Food Chem. 1997, 60, 291–295. [Google Scholar] [CrossRef]
- Luten, J.; Schram, E.; Elvevoll, E. Tailor-made functional seafood for consumers: Dietary modulation of selenium and taurine in farmed fish. In Improving Farmed Fish Quality and Safety; Lie, Ø., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 343–362. [Google Scholar]
- Ferraro, V.; Cruz, I.; Jorge, R.F.; Malcata, F.X.; Pintado, M.E.; Castro, P.M.L. Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Res. Int. 2010, 43, 2221–2233. [Google Scholar]
- Karl, H.; Lehmann, I.; Rehbein, H.; Schubring, R. Composition and quality attributes of conventionally and organically farmed Pangasius fillets (Pangasius hypophthalmus) on the German market. Int. J. Food Sci. Technol. 2010, 45, 56–66. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Brouwer, E.; Koot, A.; Wijtten, M. Seafood and Water Management. Foods 2014, 3, 622–631. [Google Scholar] [CrossRef]
- Kołakowski, E.; Kołakowska, A.; Lachowicz, K.; Bortnowska, G.; Wianecki, M. The use of sodium carbonates to improve textural properties of cod minces. J. Sci. Food Agric. 1994, 66, 429–437. [Google Scholar] [CrossRef]
- Vyncke, W. Influence of sodium tripolyphosphate and citric acid on the shelf life of thornback ray (Raja clavata L.). Z. Lebensm. Unters. For. 1978, 166, 284–286. [Google Scholar] [CrossRef]
- Fernández-Segovia, I.; Escriche, I.; Gómez-Sintes, M.; Fuentes, A.; Serra, J.A. Influence of different preservation treatments on the volatile fraction of desalted cod. Food Chem. 2006, 98, 473–482. [Google Scholar] [CrossRef]
- Magnússon, H.; Sveinsdóttir, K.; Lauzon, H.L.; Thorkelsdóttir, Á.; Martinsdóttir, E. Keeping quality of desalted cod filets in consumer packs. J. Food Sci. 2006, 71, M69–M76. [Google Scholar]
- Sallam, K.I. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate and sodium citrate in refrigerated sliced salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.A.; Ribeiro, J.L.D. Do phosphates improve the seafood quality? Reality and legislation. Pan-Am. J. Aquat. Sci. 2008, 3, 237–247. [Google Scholar]
- Kuwahara, K.; Konno, K. Suppression of thermal denaturation of myosin and salt-induced denaturation of actin by sodium citrate in carp (Cyprinus carpio). Food Chem. 2010, 122, 997–1002. [Google Scholar]
- Lopkulkiaert, W.; Prapatsornwattana, K.; Rungsardthong, V. Effects of sodium bicarbonate containing traces of citric acid in combination with sodium chloride on yield and some properties of white shrimp (Penaeus vannamei) frozen by shelf freezing, air-blast and cryogenic freezing. LWT—Food Sci. Technol. 2009, 42, 768–776. [Google Scholar] [CrossRef]
- Ooizumi, T.; Kawase, M.; Akahane, Y. Permeation of sodium chloride into fish meat and its effect on moisture content as a function of the osmotic pressure of the soaking solution. Fish. Sci. 2003, 69, 830–835. [Google Scholar] [CrossRef]
- Fisher, R.A.; DuPaul, W.D.; Rippen, T.E. Nutritional, proximate, and microbial characteristics of phosphate processed sea scallops (Placopecten magellanicus) treated with sodium tripolyphosphate. J. Muscle Foods 1996, 7, 73–92. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manthey-Karl, M.; Lehmann, I.; Ostermeyer, U.; Rehbein, H.; Schröder, U. Meat Composition and Quality Assessment of King Scallops (Pecten maximus) and Frozen Atlantic Sea Scallops (Placopecten magellanicus) on a Retail Level. Foods 2015, 4, 524-546. https://doi.org/10.3390/foods4040524
Manthey-Karl M, Lehmann I, Ostermeyer U, Rehbein H, Schröder U. Meat Composition and Quality Assessment of King Scallops (Pecten maximus) and Frozen Atlantic Sea Scallops (Placopecten magellanicus) on a Retail Level. Foods. 2015; 4(4):524-546. https://doi.org/10.3390/foods4040524
Chicago/Turabian StyleManthey-Karl, Monika, Ines Lehmann, Ute Ostermeyer, Hartmut Rehbein, and Ute Schröder. 2015. "Meat Composition and Quality Assessment of King Scallops (Pecten maximus) and Frozen Atlantic Sea Scallops (Placopecten magellanicus) on a Retail Level" Foods 4, no. 4: 524-546. https://doi.org/10.3390/foods4040524
APA StyleManthey-Karl, M., Lehmann, I., Ostermeyer, U., Rehbein, H., & Schröder, U. (2015). Meat Composition and Quality Assessment of King Scallops (Pecten maximus) and Frozen Atlantic Sea Scallops (Placopecten magellanicus) on a Retail Level. Foods, 4(4), 524-546. https://doi.org/10.3390/foods4040524





