Lutein and Brain Function
Abstract
:1. Introduction
2. Development of the Monkey Model for Lutein Studies

3. Roles of Lutein and Zeaxanthin in the Retina as a Guide to Studies of the Brain
4. What is Known about Lutein and Cognition/Brain Function in Humans?
5. Proposed Mechanisms of Lutein Action
6. Approaches for Evaluation of Lutein in Micro-Dissected Brains
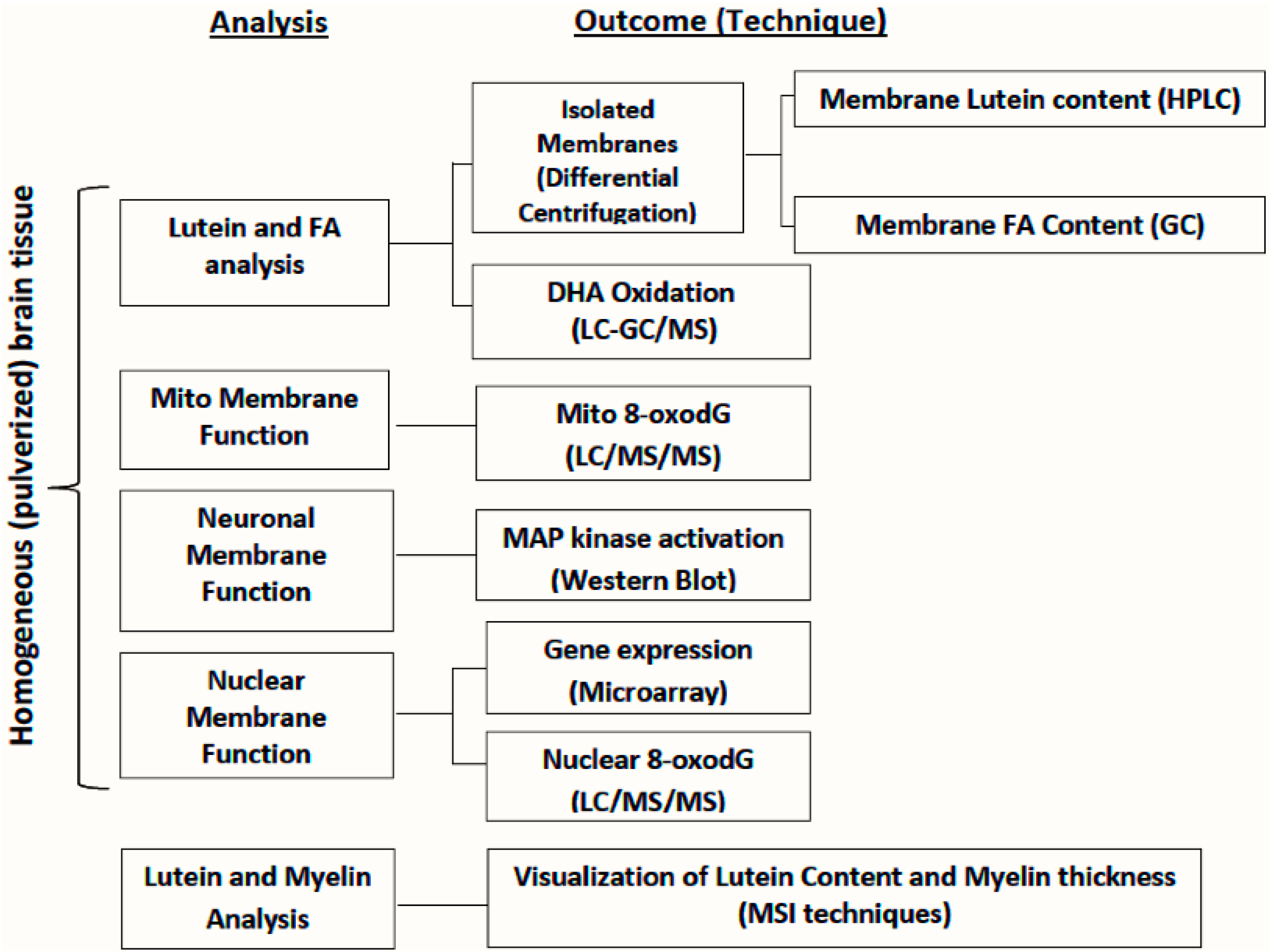
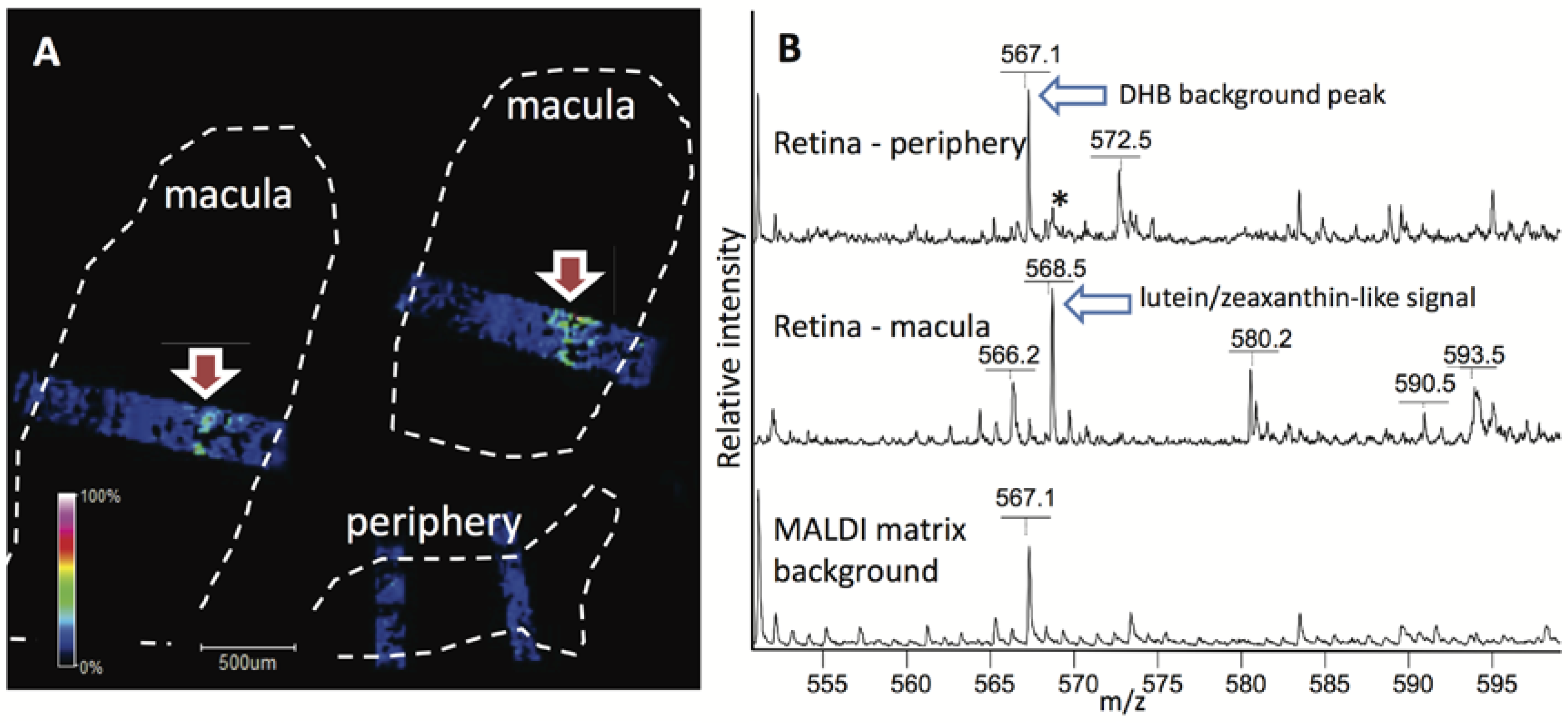
7. Approaches for Visualization of Lutein Localization in Monkey Macula/Brain
8. Approaches for Biosynthesis of 13C-Lutein
9. Conclusions and Future Research
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the georgia centenarian study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Iannaccone, A.; Scott, T.M.; Kritchevsky, S.B.; Jennings, B.J.; Carboni, G.; Forma, G.; Satterfield, S.; Harris, T.; Johnson, K.C.; et al. Macular pigment optical density is related to cognitive function in older people. Age Ageing 2014, 43, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Feeney, J.; Finucane, C.; Savva, G.M.; Cronin, H.; Beatty, S.; Nolan, J.M.; Kenny, R.A. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol. Aging 2013, 34, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Renzi, L.M.; Dengler, M.J.; Puente, A.; Miller, L.S.; Hammond, B.R. Relationships between macular pigment optical density and cognitive function in unimpaired and mildly cognitively impaired older adults. Neurobiol. Aging 2014, 35, 1695–1699. [Google Scholar] [PubMed]
- Vishwanathan, R.; Neuringer, M.; Snodderly, D.M.; Schalch, W.; Johnson, E.J. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr. Neurosci. 2013, 16, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Neuringer, M.; Russell, R.M.; Schalch, W.; Snodderly, D.M. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investig. Ophthalmol. Vis. Sci. 2005, 46, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Rubakhin, S.S.; Romanova, E.V.; Nemes, P.; Sweedler, J.V. Profiling metabolites and peptides in single cells. Nat. Methods 2011, 8, S20–S29. [Google Scholar] [CrossRef] [PubMed]
- Romanova, E.V.; Aerts, J.T.; Croushore, C.A.; Sweedler, J.V. Small-volume analysis of cell-cell signaling molecules in the brain. Neuropsychopharmacology 2014, 39, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Craft, N.E.; Haitema, T.B.; Garnett, K.M.; Fitch, K.A.; Dorey, C.K. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J. Nutr. Health Aging 2004, 8, 156–162. [Google Scholar] [PubMed]
- Li, B.; Vachali, P.; Frederick, J.M.; Bernstein, P.S. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 2011, 50, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Larson, A.J.; Frederick, J.M.; Southwick, K.; Thulin, C.D.; Bernstein, P.S. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem. 2004, 279, 49447–49454. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vachali, P.P.; Gorusupudi, A.; Shen, Z.; Sharifzadeh, H.; Besch, B.M.; Nelson, K.; Horvath, M.M.; Frederick, J.M.; Baehr, W.; et al. Inactivity of human β,β-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc. Natl. Acad. Sci. 2014, 111, 10173–10178. [Google Scholar] [CrossRef] [PubMed]
- Snodderly, D.M. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am. J. Clin. Nutr. 1995, 62, 1448–1461. [Google Scholar]
- SanGiovanni, J.P.; Neuringer, M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: Promise of molecular genetics for guiding mechanistic and translational research in the field. Am. J. Clin. Nutr. 2012, 96, 1223S–1233S. [Google Scholar] [CrossRef] [PubMed]
- Malinow, M.R.; Feeney-Burns, L.; Peterson, L.H.; Klein, M.L.; Neuringer, M. Diet-related macular anomalies in monkeys. Investig. Ophthalmol. Vis. Sci. 1980, 19, 857–863. [Google Scholar]
- Leung, I.Y.-F.; Sandstrom, M.M.; Zucker, C.L.; Neuringer, M.; Snodderly, D.M. Nutritional manipulation of primate retinas, II: Effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3244–3256. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.M.; Snodderly, D.M.; Johnson, E.J.; Schalch, W.; Koepcke, W.; Gerss, J.; Neuringer, M. Nutritional manipulation of primate retinas, V: Effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3934–3942. [Google Scholar] [CrossRef] [PubMed]
- Neuringer, M.; Sandstrom, M.M.; Johnson, E.J.; Snodderly, D.M. Nutritional manipulation of primate retinas, I: Effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Dou, H.-L.; Wu, Y.-Q.; Huang, Y.-M.; Huang, Y.-B.; Xu, X.-R.; Zou, Z.-Y.; Lin, X.-M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T.J.M. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L.; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar]
- Woo, S.J.; Park, K.H.; Ahn, J.; Choe, J.Y.; Jeong, H.; Han, J.W.; Kim, T.H.; Kim, K.W. Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology 2012, 119, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Schalch, W.; Johnson, E.J. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutr. Neurosci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Renzi, L.M.; Hammond, B.R. The relation between the macular carotenoids, lutein and zeaxanthin, and temporal vision. Ophthalmic Physiol. Opt. 2010, 30, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Bovier, E.R.; Renzi, L.M.; Hammond, B.R. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PLoS ONE 2014, 9, e108178. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.-Y.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Subczynski, W.K. Why has nature chosen lutein and zeaxanthin to protect the retina? J. Clin. Exp. Ophthamol. 2014, 5, 326. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Morel, A.; Saso, L.; Saluk, J. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.I. Carotenoid orientation: Role in membrane stabilization. In Carotenoids in Health and Disease; Krinsky, N.I., Mayne, S.T., Sies, H., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 151–164. [Google Scholar]
- Stahl, W.; Sies, H. Effects of carotenoids and retinoids on gap junctional communication. BioFactors 2001, 15, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A.; Gabrielska, J.; Grudziński, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, D.V.; Ojima, I.; Geng, X.; Adler, A.J. Tubulins in the primate retina: Evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site. Bioorg. Med. Chem. 2001, 9, 1967–1976. [Google Scholar] [CrossRef]
- Hulbert, A.J. The links between membrane composition, metabolic rate and lifespan. Comp. Biochem. Physiol. A 2008, 150, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Lipid compositions of different regions of the human brain during aging. J. Neurochem. 1990, 54, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Carrié, I.; Portoukalian, J.; Vicaretti, R.; Rochford, J.; Potvin, S.; Ferland, G. Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J. Nutr. 2004, 134, 167–172. [Google Scholar] [PubMed]
- Seger, C.A.; Cincotta, C.M. Dynamics of frontal, striatal, and hippocampal systems during rule learning. Cereb. Cortex 2006, 16, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Glisky, E.L. Changes in cognitive function in human aging. In Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sun, Y.; Sun, G.Y. Phospholipids and acyl groups of synaptosomal and myelin membranes isolated from the cerebral cortex of squirrel monkey (Saimiri sciureus). Biochim. Biophys. Acta 1972, 280, 306–315. [Google Scholar] [CrossRef]
- Tekpli, X.; Holme, J.A.; Sergent, O.; Lagadic-Gossmann, D. Role for membrane remodeling in cell death: Implication for health and disease. Toxicology 2013, 304, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T.; Farooqui, A.A. Aging: An important factor for the pathogenesis of neurodegenerative diseases. Mech. Ageing Dev. 2009, 130, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Pan, Y.; Kao, S.-Y.; Li, C.; Kohane, I.; Chan, J.; Yankner, B.A. Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008, 31, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Matthan, N.R.; Jalbert, S.M.; Resteghini, N.A.; Schaefer, E.J.; Ausman, L.M. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2006, 84, 497–504. [Google Scholar] [PubMed]
- Arneson, K.O.; Roberts, L.J. Measurement of products of docosahexaenoic acid peroxidation, neuroprostanes, and neurofurans. Methods Enzymol. 2007, 433, 127–143. [Google Scholar] [PubMed]
- Walter, M.F.; Blumberg, J.B.; Dolnikowski, G.G.; Handelman, G.J. Streamlined F2-isoprostane analysis in plasma and urine with high-performance liquid chromatography and gas chromatography/mass spectroscopy. Anal. Biochem. 2000, 280, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Gan, W.; Shi, F.; Hu, G.-X.; Chen, L.-G.; Hayakawa, H.; Sekiguchi, M.; Cai, J.-P. Age-dependent accumulation of 8-oxoguanine in the DNA and RNA in various rat tissues. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Zhao, D.-Y.; Sharifzadeh, M.; Ermakov, I.V.; Gellermann, W. Resonance Raman measurement of macular carotenoids in the living human eye. Arch. Biochem. Biophys. 2004, 430, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Trieschmann, M.; Heimes, B.; Hense, H.W.; Pauleikhoff, D. Macular pigment optical density measurement in autofluorescence imaging: Comparison of one- and two-wavelength methods. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Wooten, B.R.; Hammond, B.R.; Land, R.I.; Snodderly, D.M. A practical method for measuring macular pigment optical density. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2481–2489. [Google Scholar]
- Bone, R.A.; Landrum, J.T.; Friedes, L.M.; Gomez, C.A.; Kilburn, M.D.; Menendez, E.; Vidal, I.; Wang, W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997, 64, 211–218. [Google Scholar] [CrossRef]
- Rapp, L.M.; Maple, S.S.; Choi, J.H. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1200–1209. [Google Scholar]
- Bernstein, P.S.; Delori, F.C.; Richer, S.; van Kuijk, F.J.M.; Wenzel, A.J. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vis. Res. 2010, 50, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, M.; Zhao, D.-Y.; Bernstein, P.S.; Gellermann, W. Resonance Raman imaging of macular pigment distributions in the human retina. J. Opt. Soc. Am. A 2008, 25, 947–957. [Google Scholar] [CrossRef]
- Theelen, T.; Berendschot, T.T.J.M.; Klevering, B.J.; Fuijkschot, J.; Hoyng, C.B.; Willemsen, M.A.A.P. Multimodal imaging of the macula in hereditary and acquired lack of macular pigment. Acta Ophthalmol. 2014, 92, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; van der Veen, R.L.P.; Stijfs, A.; Holz, F.G.; Scholl, H.P.N.; Berendschot, T.T.J.M. Quantification of reduced macular pigment optical density in the central retina in macular telangiectasia type 2. Exp. Eye Res. 2009, 89, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Brignole-Baudouin, F.; Desbenoit, N.; Hamm, G.; Liang, H.; Both, J.-P.; Brunelle, A.; Fournier, I.; Guerineau, V.; Legouffe, R.; Stauber, J.; et al. A new safety concern for glaucoma treatment demonstrated by mass spectrometry imaging of benzalkonium chloride distribution in the eye, an experimental study in rabbits. PLoS ONE 2012, 7, e50180. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.C.; Nakanishi, H.; Takahashi, K.; Nakanishi, S.; Kajihara, S.; Hayasaka, T.; Setou, M.; Ogawa, K.; Taguchi, R.; Naito, T. Salamander retina phospholipids and their localization by MALDI imaging mass spectrometry at cellular size resolution. J. Lipid Res. 2011, 52, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zemski Berry, K.A.; Gordon, W.C.; Murphy, R.C.; Bazan, N.G. Spatial organization of lipids in the human retina and optic nerve by MALDI imaging mass spectrometry. J. Lipid Res. 2014, 55, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Ly, A.; Meding, S.; Witting, M.; Hauck, S.M.; Ueffing, M.; Schmitt-Kopplin, P.; Aichler, M.; Walch, A. High-resolution metabolite imaging of light and dark treated retina using MALDI-FTICR mass spectrometry. Proteomics 2014, 14, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, T.; Goto-Inoue, N.; Sugiura, Y.; Zaima, N.; Nakanishi, H.; Ohishi, K.; Nakanishi, S.; Naito, T.; Taguchi, R.; Setou, M. Matrix-assisted laser desorption/ionization quadrupole ion trap time-of-flight (MALDI-QIT-TOF)-based imaging mass spectrometry reveals a layered distribution of phospholipid molecular species in the mouse retina. Rapid Commun. Mass Spectrom. 2008, 22, 3415–3426. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.D.; Griffiths, R.; Styles, I.; Claridge, E.; Calcagni, A.; Bunch, J. Sucrose cryo-protection facilitates imaging of whole eye sections by MALDI mass spectrometry. J. Mass Spectrom. 2012, 47, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.C.; Chaurand, P.; Caprioli, R.M.; Schey, K.L. MALDI imaging mass spectrometry of integral membrane proteins from ocular lens and retinal tissue. J. Proteome Res. 2009, 8, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.G.; Ablonczy, Z.; Koutalos, Y.; Spraggins, J.; Crouch, R.K.; Caprioli, R.M.; Schey, K.L. High resolution MALDI imaging mass spectrometry of retinal tissue lipids. J. Am. Soc. Mass Spectrom. 2014, 25, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Lanni, E.J.; Rubakhin, S.S.; Sweedler, J.V. Mass spectrometry imaging and profiling of single cells. J. Proteomics 2012, 75, 5036–5051. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, T.A.; Rubakhin, S.S.; Sweedler, J.V. MALDI mass spectrometry imaging of neuronal cell cultures. J. Am. Soc. Mass Spectrom. 2011, 22, 828–836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rubakhin, S.S.; Sweedler, J.V. Mass Spectrometry Imaging; Humana Press: New York, NY, USA, 2010. [Google Scholar]
- Snodderly, D.M.; Handelman, G.J.; Adler, A.J. Distribution of individual macular pigment carotenoids in central retina of macaque and squirrel monkeys. Investig. Ophthalmol. Vis. Sci. 1991, 32, 268–279. [Google Scholar]
- Van Lieshout, M.; West, C.E.; van Breemen, R.B. Isotopic tracer techniques for studying the bioavailability and bioefficacy of dietary carotenoids, particularly beta-carotene, in humans: A review. Am. J. Clin. Nutr. 2003, 77, 12–28. [Google Scholar] [PubMed]
- Burri, B.J.; Clifford, A.J. Carotenoid and retinoid metabolism: Insights from isotope studies. Arch. Biochem. Biophys. 2004, 430, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Chang, A.-N. Total synthesis of (3R,3′R,6′R)-lutein and its stereoisomers. J. Org. Chem. 2009, 74, 3875–3885. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Lu, C.-H.; Choi, J.-H.; Engelmann Moran, N.; Jin, Y.-S.; Erdman, J.W. Laboratory-scale production of 13C-labeled lycopene and phytoene by bioengineered Escherichia coli. J. Agric. Food Chem. 2011, 59, 9996–10005. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.K.; Rogers, R.B.; Lila, M.A.; Erdman, J.W. Biosynthesis of 14C-phytoene from tomato cell suspension cultures (Lycopersicon esculentum) for utilization in prostate cancer cell culture studies. J. Agric. Food Chem. 2006, 54, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Rogers, R.B.; Lu, C.-H.; Conlon, L.E.; Lila, M.A.; Clinton, S.K.; Erdman, J.W. Biosynthesis of highly enriched 13C-lycopene for human metabolic studies using repeated batch tomato cell culturing with 13C-glucose. Food Chem. 2013, 139, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Gowik, U.; Westhoff, P. The path from C3 to C4 photosynthesis. Plant Physiol. 2011, 155, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; White, W.S.; Yao, L.; Serfass, R.E. Use of high-precision gas isotope ratio mass spectrometry to determine natural abundance 13C in lutein isolated from C3 and C4 plant sources. J. Chromatogr. A 1998, 800, 51–58. [Google Scholar] [CrossRef]
- Lu, C.-H.; Engelmann, N.J.; Lila, M.A.; Erdman, J.W. Optimization of lycopene extraction from tomato cell suspension culture by response surface methodology. J. Agric. Food Chem. 2008, 56, 7710–7714. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, N.J.; Campbell, J.K.; Rogers, R.B.; Rupassara, S.I.; Garlick, P.J.; Lila, M.A.; Erdman, J.W. Screening and selection of high carotenoid producing in vitro tomato cell culture lines for [13C]-carotenoid production. J. Agric. Food Chem. 2010, 58, 9979–9987. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdman, J.W., Jr.; Smith, J.W.; Kuchan, M.J.; Mohn, E.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.V.; Neuringer, M. Lutein and Brain Function. Foods 2015, 4, 547-564. https://doi.org/10.3390/foods4040547
Erdman JW Jr., Smith JW, Kuchan MJ, Mohn ES, Johnson EJ, Rubakhin SS, Wang L, Sweedler JV, Neuringer M. Lutein and Brain Function. Foods. 2015; 4(4):547-564. https://doi.org/10.3390/foods4040547
Chicago/Turabian StyleErdman, John W., Jr., Joshua W. Smith, Matthew J. Kuchan, Emily S. Mohn, Elizabeth J. Johnson, Stanislav S. Rubakhin, Lin Wang, Jonathan V. Sweedler, and Martha Neuringer. 2015. "Lutein and Brain Function" Foods 4, no. 4: 547-564. https://doi.org/10.3390/foods4040547
APA StyleErdman, J. W., Jr., Smith, J. W., Kuchan, M. J., Mohn, E. S., Johnson, E. J., Rubakhin, S. S., Wang, L., Sweedler, J. V., & Neuringer, M. (2015). Lutein and Brain Function. Foods, 4(4), 547-564. https://doi.org/10.3390/foods4040547




