Bioprocessing of Marine Chitinous Wastes for the Production of Bioactive Prodigiosin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reclamation of Demineralized Shrimp Shell Powders (de-SSP) as a Potential Source for Effective Production of Prodigiosin via Fermentation
2.2. Establishment of the Process for de-SSP Bioprocessing into PG by S. marcescens on a Small Scale
- (1)
- PG production by different S. marcescens strains
- (2)
- The influence of different free protein sources on PG production
- (3)
- The influence of salt composition on PG production
- (4)
- The influence of cultivation parameters on PG production
2.3. Scale-Up of PG Production in a Bioreactor System and the Purification and Qualification of S. marcescens TNU01 PG
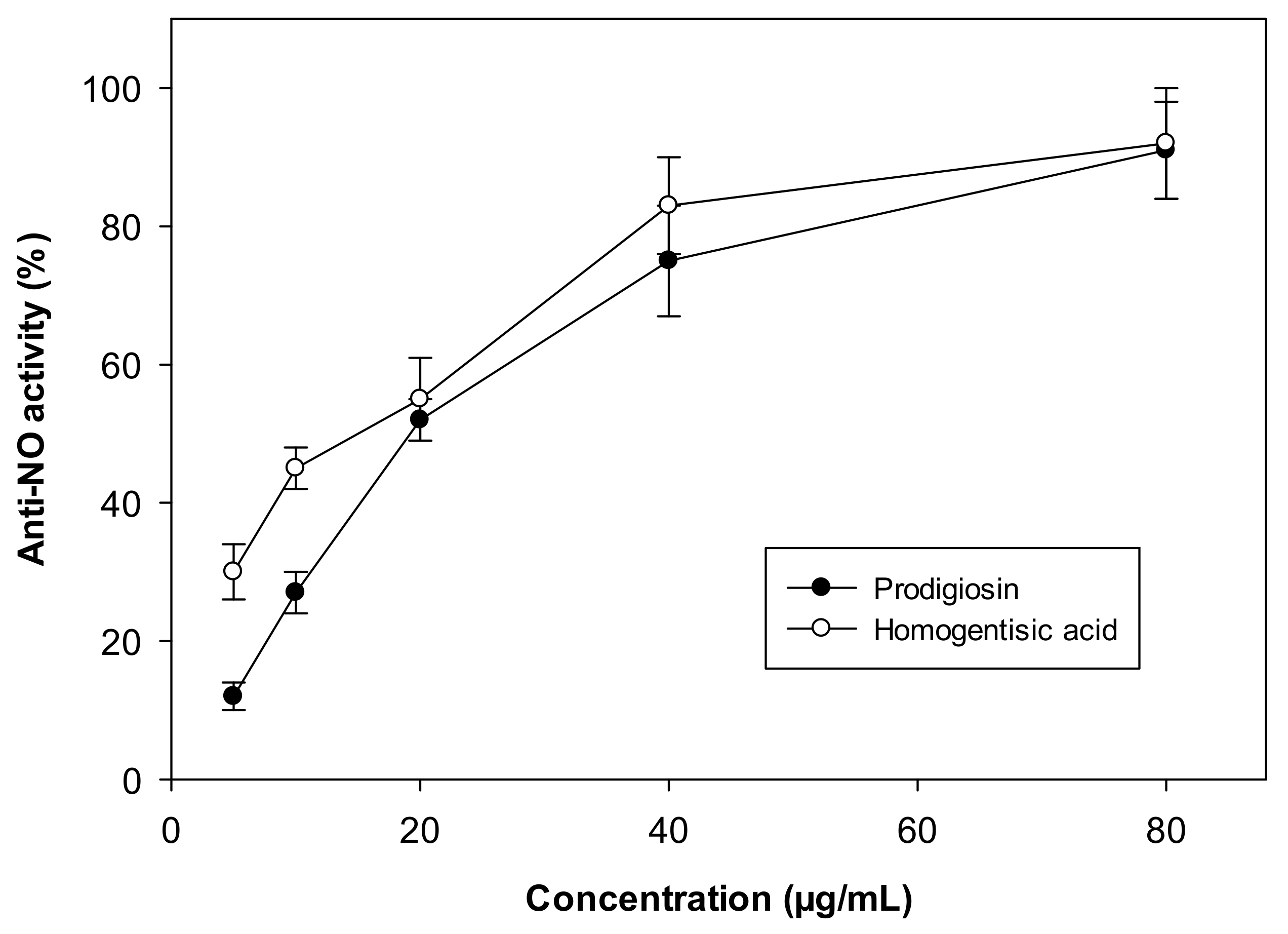
2.4. Evaluation of the Biological Effects of Prodigiosin
3. Materials and Methods
3.1. Materials
3.2. Methods
Study of Bioproduction of Prodigiosin via Bacterial Fermentation
- The Influence of Different S. marcescens Strains on PG Production
- The Effect of Different Free Protein Sources on PG Production
- The Effect of Phosphate Type and Concentration on PG Production
- The Effect of Sulfate Type and Concentration on PG Production
- The Effect of Cultivation Parameters on PG Production
- Scale-Up Production of PG in the Bioreactor System
3.3. Qualification, Extraction, and Purification of Prodigiosin
3.4. Detection of the Bioactivities of Prodigiosin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, S.L.; Nguyen, V.B.; Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, A.D. Production and potential applications of bioconversion of chitin and protein-containing fishery byproducts into prodigiosin: A review. Molecules 2020, 25, 2744. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Nguyen, D.N.; Wang, S.L. Microbial reclamation of chitin and protein-containing marine by-products for the production of prodigiosin and the evaluation of its bioactivities. Polymers 2020, 12, 1328. [Google Scholar] [CrossRef] [PubMed]
- Furstner, A. Chemistry and biology of roseophilin and the prodigiosin alkaloids: A survey of the last 2500 years. Chem. Int. Ed. Engl. 2003, 42, 3582–3603. [Google Scholar] [CrossRef]
- Cerdeno, A.M.; Bibb, M.J.; Challis, G.L. Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3(2): New mechanisms for chain initiation and termination in modular multienzymes. Chem. Biol. 2001, 8, 817–829. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.W.; Chen, S.Y.; Chen, Y.C.; Chen, Y.C.; Yen, Y.H.; Wang, S.L. Enhancement of prodigiosin production by Serratia marcescens TKU011 and its insecticidal activity relative to food colourants. J. Food Sci. 2013, 78, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- de Casullo Araújo, H.W.; Fukushima, K.; Campos Takaki, G.M. Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low-cost substrate. Molecules 2010, 15, 6931–6940. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Chandana, K.; Senthilkumar, P.; Narendra, K.G. Optimization of prodigiosin production by Serratia marcescens SU-10 and evaluation of its bioactivity. Int. Res. J. Biotechnol. 2011, 2, 128–133. [Google Scholar]
- Patricia, H.V.; Irene, M.A.; Melissa, R.D.; José Manuel, R.D.; Donato, L.M.; Francisco Guadalupe, A.A.; Juan Francisco, V.C. Photoelectric evaluation of dye-sensitized solar cells based on prodigiosin pigment derived from Serratia marcescens 11E. Dyes Pigments. 2020, 177, 108278–108287. [Google Scholar]
- Cheng, M.F.; Lin, C.S.; Chen, Y.H.; Sung, P.J.; Lin, S.R.; Tong, Y.W.; Weng, C.F. Inhibitory growth of oral squamous cell carcinoma cancer via bacterial prodigiosin. Mar. Drugs 2017, 15, 224. [Google Scholar] [CrossRef]
- Gulani, C.; Bhattacharya, S.; Das, A. Assessment of process parameters influencing the enhanced production of prodigiosin from Serratia marcescens and evaluation of its antimicrobial, antioxidant and dyeing potential. Malays. J. Microbiol. 2012, 8, 116–122. [Google Scholar]
- Wei, Y.H.; Yu, W.J.; Chen, W.C. Enhanced undecylprodigiosin production from Serratia marcescens SS-1 by medium formulation and amino-acid supplementation. J. Biosci. Bioeng. 2005, 100, 466–471. [Google Scholar] [CrossRef]
- Haddix, P.L.; Werner, T.F. Spectrophotometric assay of gene expression: Serratia marcescens pigmentation. Bioscene 2000, 26, 3–13. [Google Scholar]
- Wang, X.; Tao, J.; Wei, D.; Shen, Y.; Tong, W. Development of an adsorption procedure for the direct separation and purification of prodigiosin from culture broth. Biotechnol. Appl. Biochem. 2004, 40, 277–280. [Google Scholar]
- Montaner, B.; Navarro, S.; Piqué, M.; Vilaseca, M.; Martinell, M.; Giralt, E.; Gil, J.; Perez-Tomas, P. Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br. J. Pharmacol. 2000, 131, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Solé, M.; Rius, N.; Francia, A.; Lorén, J.G. The effect of pH on prodigiosin production by non-proliferating cells of Serratia marcescens. Lett. Appl. Microbiol. 1994, 19, 341–344. [Google Scholar] [CrossRef]
- Lin, C.; Jia, X.; Fang, Y.; Chen, L.; Zhang, H.; Lin, R.; Chen, J. Enhanced production of prodigiosin by Serratia marcescens FZSF02 in the form of pigment pellets. Electron. J. Biotechnol. 2019, 40, 58–64. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, W.C.; Ho, S.F.; Wu, H.S.; Wei, Y.H. Development of natural anti-tumor drugs by microorganisms. J. Biosci. Bioeng. 2011, 111, 501–511. [Google Scholar] [CrossRef]
- Wei, Y.H.; Chen, W.C. Enhanced production of prodigiosin-like pigment from Serratia marcescens SMdeltaR by medium improvement and oil-supplementation strategies. J. Biosci. Bioeng. 2005, 99, 616–622. [Google Scholar] [CrossRef]
- Elkenawy, N.M.; Yassin, A.S.; Elhifnawy, H.N.; Amin, M.A. Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol. Rep. 2017, 14, 47–53. [Google Scholar] [CrossRef]
- Giri, A.V.; Anandkumar, N.; Muthukumaran, G.; Pennathur, G. A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol. 2004, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Younes, I.; Hajji, S.; Rinaudo, M.; Chaabouni, M.; Jellouli, K.; Nasri, M. Optimization of proteins and minerals removal from shrimp shells to produce highly acetylated chitin. Int. J. Biol. Macromol. 2016, 84, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride–malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; De Oliveira Lima, V.A.; De Farias Rached, R.Í.; Lima, E.P.N.; Da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Ramos, P.; Mirón, J.; Valcarcel, J.; Sotelo, C.G.; Pérez-Martín, R.I. Production of chitin from Penaeus vannamei by-products to pilot plant scale using a combination of enzymatic and chemical processes and subsequent optimization of the chemical production of chitosan by response surface methodology. Mar. Drugs 2017, 15, 180. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, M.T.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. An exochitinase with N-acetyl-β-glucosaminidase-like activity from shrimp head conversion by Streptomyces speibonae and its application in hydrolyzing β-chitin powder to produce N-acetyl-D-glucosamine. Polymers 2019, 11, 1600. [Google Scholar] [CrossRef] [Green Version]
- Doan, C.T.; Tran, T.N.; Wen, I.H.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Conversion of shrimp head waste for production of a thermotolerant, detergent-Stable, alkaline protease by Paenibacillus sp. Catalysts 2019, 9, 798. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.B.; Wang, S.L. Production of potent antidiabetic compounds from shrimp head powder via Paenibacillus conversion. Process Biochem. 2019, 76, 18–24. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, T.H.; Nguyen, A.D.; Le, T.; Kuo, Y.H.; Wang, S.L. Bioprocessing shrimp shells to rat intestinal α- glucosidase inhibitor and its effect on reducing blood glucose in a mouse model. Res. Chem. Intermed. 2019, 45, 4829–4846. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Chen, S.P.; Nguyen, T.H.; Nguyen, M.T.; Tran, T.T.T.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Novel Efficient bioprocessing of marine chitins into active anticancer prodigiosin. Mar. Drugs 2020, 18, 15. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.L.; Wang, C.Y.; Yen, Y.H.; Liang, T.W.; Chen, S.Y.; Chen, C.H. Enhanced production of insecticidal prodigiosin from Serratia marcescens TKU011 in media containing squid pen. Process. Biochem. 2012, 47, 1684–1690. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, D.N.; Nguyen, A.D.; Ngo, V.A.; Ton, T.Q.; Doan, C.T.; Pham, T.P.; Tran, T.P.H.; Wang, S.L. Utilization of crab waste for cost-effective bioproduction of prodigiosin. Mar. Drugs 2020, 18, 523. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L. Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar. Drugs 2017, 15, 350. [Google Scholar] [CrossRef] [Green Version]
- Cang, S.; Sanada, M.; Johdo, O.; Ohta, S.; Nagamatsu, Y.; Yoshimoto, A. High production of prodigiosin by Serratia marcescens grown on ethanol. Biotechnol. Lett. 2000, 22, 1761–1765. [Google Scholar] [CrossRef]
- Kamble, K.D.; Hiwarale, V.D. Prodigiosin production from Serratia marcescens strains obtained from farm soil. Int. J. Environm. Sci. 2012, 3, 631–638. [Google Scholar]
- Kurbanoglu, E.B.; Ozdal, M.; Ozdal, O.G.; Algur, O.F. Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. Braz. J. Microbiol. 2015, 46, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Liu, J.; Wang, X.; Kong, D.; Du, W.; Li, H.; Hse, C.Y.; Shupe, T.; Zhou, D.; Zhao, K. Biological potentialand mechanism of prodigiosin from Serratia marcescens subsp. law sonianain human choriocarcinoma andprostate cancer cell lines. Int. J. Mol. Sci. 2018, 19, 3465. [Google Scholar] [CrossRef] [Green Version]
- Parani, K.; Saha, B.K. Optimization of prodigiosin production from a strain of Serratia marcescens SR1 and screening for antifungal activity. J. Biol. Control. 2008, 22, 73–79. [Google Scholar]
- Tao, J.; Wang, X.; Shen, Y.; Wei, D. Strategy for the improvement of prodigiosin production by a Serratia marcescens mutant through fed-batch fermentation. World. J. Microbiol. Biotechnol. 2005, 21, 969–972. [Google Scholar] [CrossRef]
- Fu, Q.; Xiao, Y.J.; Duan, X.H.; Huang, H.B.; Zhuang, Z.X.; Shen, J.H.; Pei, Y.Y.; Xu, H.J.; Gan, M.Y. Continuous fermentation of a prodigiosin-producing Serratia marcescens strain isolated from soil. Adv. Biosci. Biotechnol. 2019, 10, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Sura, J.M.; Khalid, J.K. A kinetic model for prodigiosin production by Serratia marcescens as a bio-colorant in bioreactor. AIP Conf. Proc. 2020, 2213, 020027. [Google Scholar]
- Luis R, C.; Oscar, A. An integrated process for the in-situ recovery of prodigiosin using micellar ATPS from a culture of Serratia marcescens. J. Chem. Technol. Biotechnol. 2016, 91, 2896–2903. [Google Scholar]
- Chidambaram, K.V.; Zainul, A.Z.; Wan, A.A. Optimization of culture conditions for flexirubin production by Chryseobacterium artocarpi CECT 8497 using response surface methodology. Acta Biochim. Pol. 2014, 62, 185–190. [Google Scholar]
- Song, M.J.; Bae, J.D.; Lee, D.S.; Kim, C.H.; Kim, J.S.; Kim, S.W.; Hong, S.I. Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. J. Biosci. Bioeng. 2006, 101, 157–161. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, A.; Pradeep, P.; Thigale, I.; Mohanasrinivasan, V.; Jemimah, N.S.; Devi, C.S. Exploring the bioactive potential of Serratia marcescens VITAPI (Acc: 1933637) isolated from soil. Front. Biol. 2016, 11, 476–480. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Swarnalatha, S.; Regina Mary, R.; Sekaran, G. Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate. J. Food Sci. Technol. 2018, 55, 2661. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L.; Nguyen, A.D.; Lin, Z.H.; Doan, C.T.; Tran, T.N.; Huang, H.T.; Kuo, Y.H. Bioactivity-guided purification of novel herbal antioxidant and anti-NO compounds from Euonymus laxiflorus Champ. Molecules 2019, 24, 120. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.B.; Nguyen, T.H.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Production and bioactivity-guided isolation of antioxidants with α-glucosidase inhibitory and anti-NO properties from marine chitinous material. Molecules 2018, 23, 1124. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.B.; Ton, T.Q.; Nguyen, D.N.; Nguyen, T.T.; Ngu, T.N.; Nguyen, T.H.; Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Ho, N.D. Reclamation of beneficial bioactivities of herbal antioxidant condensed tannin extracted from Euonymus laxiflorus. Res. Chem. Intermed. 2020, 46, 4751–4766. [Google Scholar] [CrossRef]
- Krishna, P.S.; Vani, K.; Prasad, M.R.; Samatha, B.; Hima Bindu, N.S.V.S.S.S.L.; Charya, M.A.S.; Shetty, P.R. In-silico molecular docking analysis of prodigiosin and cycloprodigiosin as COX-2 inhibitors. Springerplus 2013, 2, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.B.; Wang, S.L.; Nguyen, T.H.; Nguyen, T.H.; Trinh, T.H.T.; Nong, T.T.; Nguyen, T.U.; Nguyen, V.N.; Nguyen, A.D. Reclamation of rhizobacteria newly isolated from black pepper plant roots as potential biocontrol agents of root-knot nematodes. Res. Chem. Intermed. 2019, 45, 5293–5307. [Google Scholar] [CrossRef]
- Wang, S.L.; Yeh, P.Y. Production of a surfactant- and solvent-stable alkaliphilic protease by bioconversion of shrimp shell wastes fermented by Bacillus subtilis TKU007. Process. Biochem. 2006, 41, 1545–1552. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Liang, T.W.; Liu, K.C.; Hsu, Y.W.; Hsu, H.C.; Wang, S.L. Isolation and identification of a novel antioxidant with antitumor activity from Serratia ureilytica using squid pen as fermentation substrate. Mar. Biotechnol. 2011, 13, 451–461. [Google Scholar] [CrossRef]
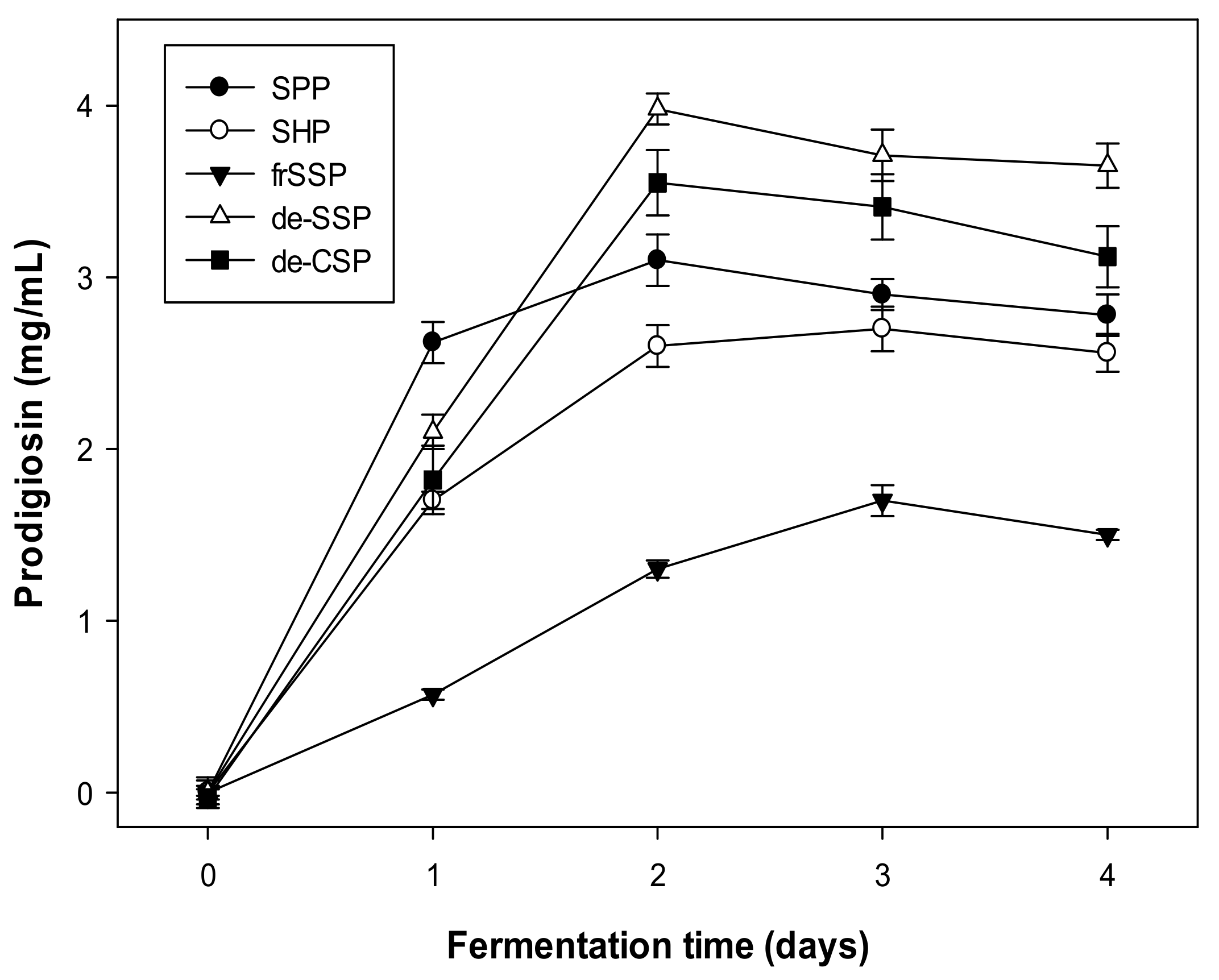
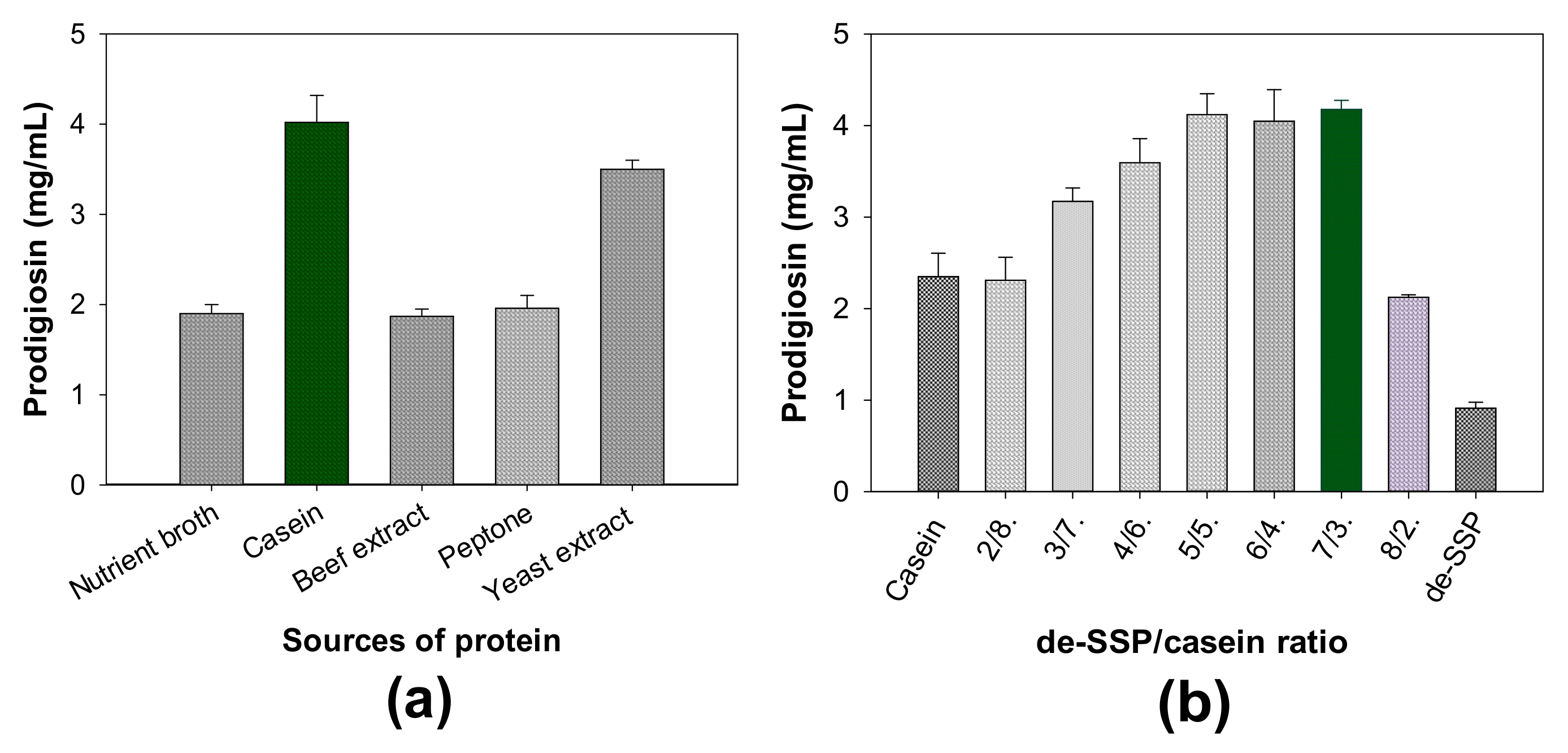
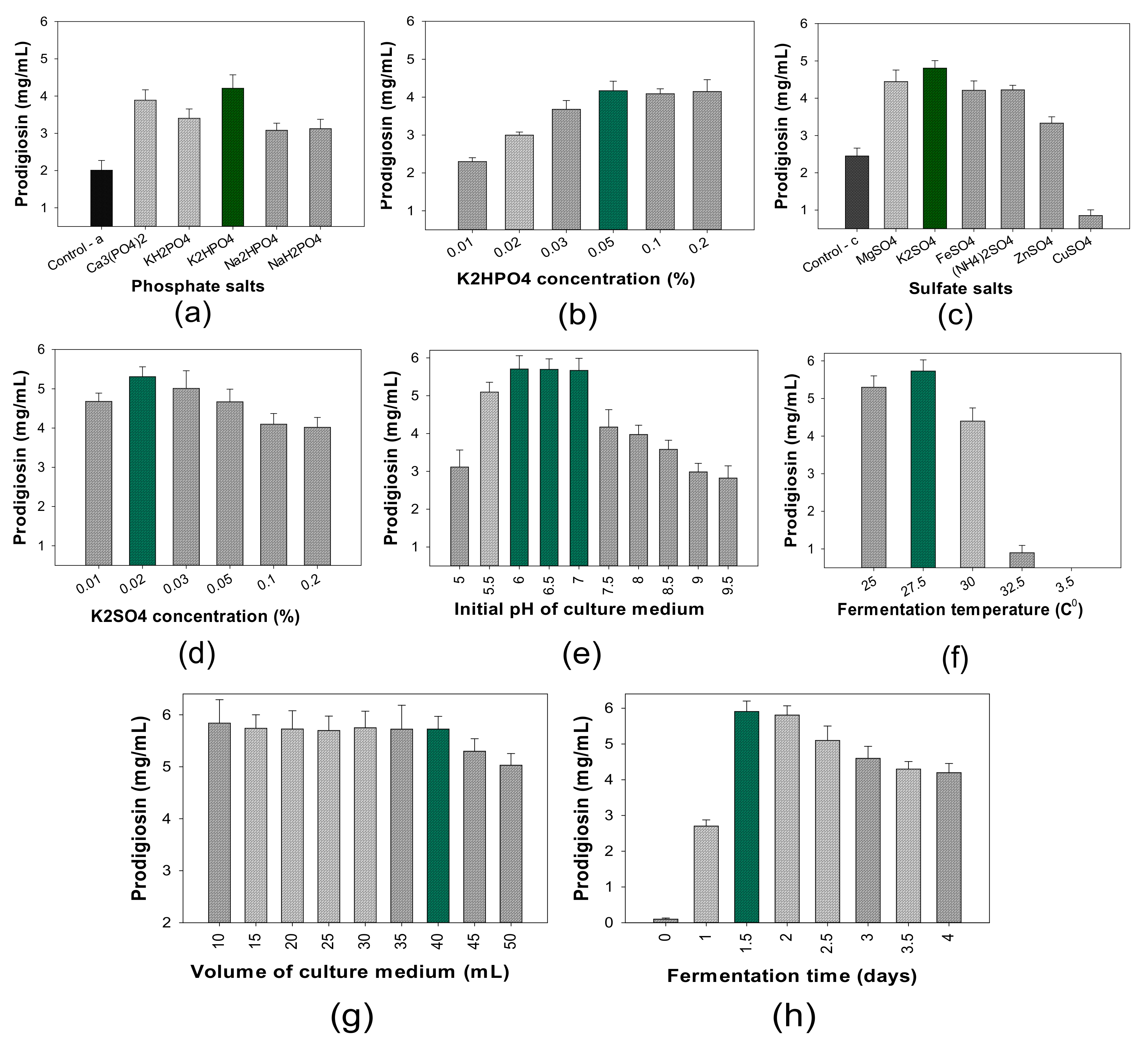
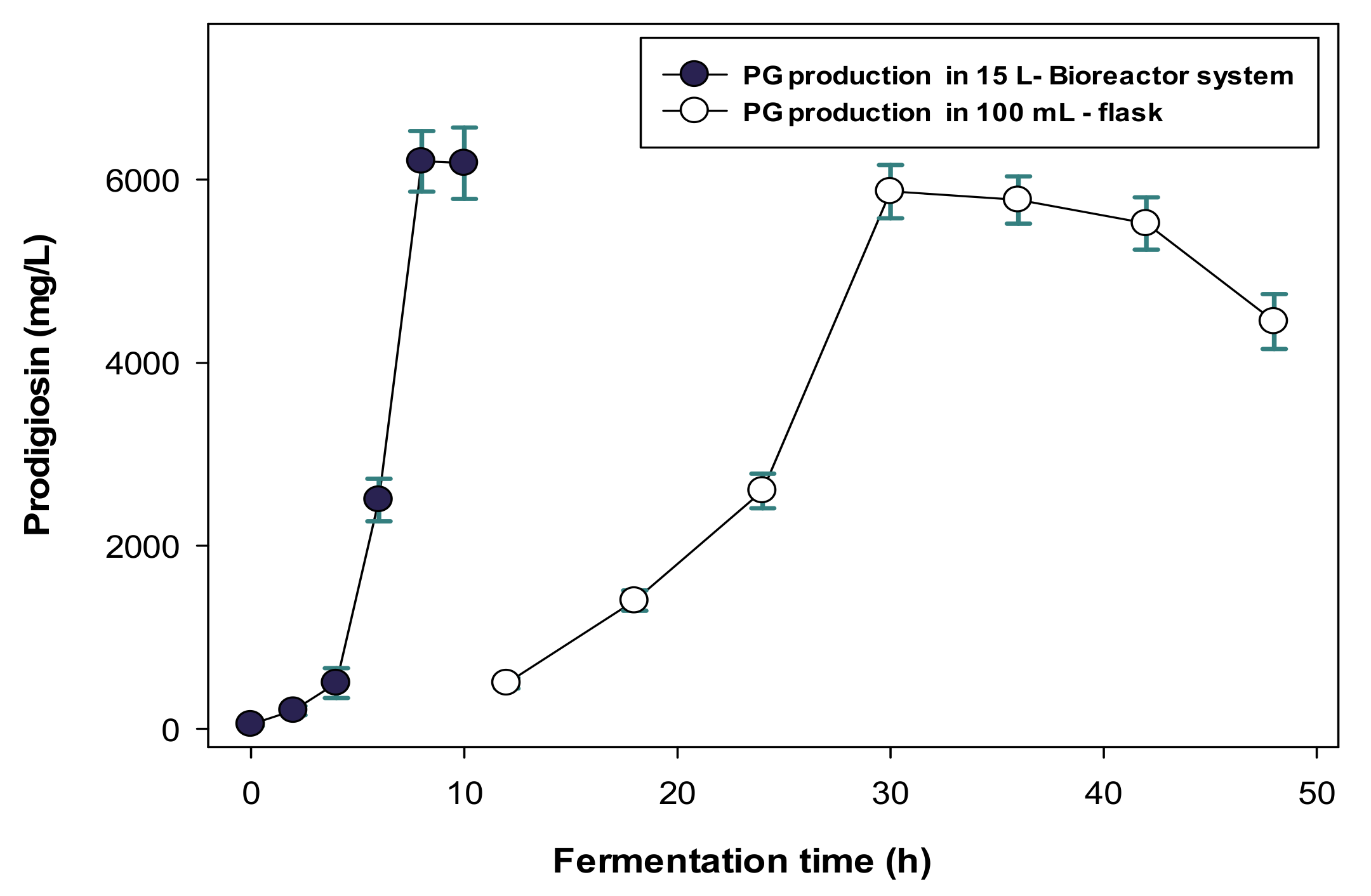
| No. | S. marcescens Strains | PG Concentration in Culture Broths (mg/mL) |
|---|---|---|
| 1 | TKU011 | 3.832 ± 0.142 |
| 2 | TNU01 | 4.015 ± 0.161 |
| 3 | TNU02 | 3.721 ± 0.177 |
| 4 | CC17 | 3.562 ± 0.145 |
| Control (no bacterium) | - |
| Factors | Initial Experiment | Optimal Conditions |
|---|---|---|
| S. marcescens strain | TNU02 | TNU01 |
| C/N source | de-SSP/casein = 7/3 | de-SSP/casein = 7/3 |
| Salts compositions | 0.03% K2HPO4 and 0.05% CaSO4 | 0.02% K2SO4 and 0.05% K2HPO4 |
| Initial pH of medium | 6.15 | 7.0 |
| Fermentation temperature (°C) | 27 | 27 |
| Ratio volume of medium/flask | 3/10 | 4/10 |
| Time course of fermentation (day) | 2 | 1.5 |
| PG yield (mg/mL) | 3.98 | 5.61 |
| Strain | C/N Sources | Scale Production | PG Yield | Culture Time (h) | Ref. |
|---|---|---|---|---|---|
| S. marcescens TNU01 | 1.12% de-SSP/0.48% casein | 5/15 L | 6200 mg/L | 8 | This study |
| S. marcescens TNU01 | 1.75% SPP | 3/10 L | 3450 mg/L | 12 | [2] |
| S. marcescens TNU02 | 1.12% de-CSP /0.48% casein | 4.5/15 L | 5100 mg/L | 8 | [33] |
| S. marcescens 02 | 1.0% glycerol, 1.0% tryptone, 1.0% extract of yeast | 2.75/5 L | 583 mg/L | 20 | [40] |
| S. marcescens | 0.865% sucrose/0.662% peptone | 6.5/7 L | 594.88mg /L | 52 | [42] |
| S. marcescens BS 303 (ATCC® 13880™) | 3.0% glycerol/1.05% casein peptone | 0.935/1.5 L | 872mg/L | 65 | [43] |
| Chryseobacterium artocarpi CECT 849 | 1.125% Lactose and 0.6% l-tryptophan. | 50/100 L | 521.64 mg/L | 24 | [44] |
| Inhibitory Activity against Cancer Cell Lines (IC50 Value, µg/mL) | ||||
|---|---|---|---|---|
| A549 | MCF-7 | WiDr | Hep G2 | |
| PG | 0.07 ± 0.015 | 0.05 ± 0.009 | 0.22 ± 0.02 | 0.06 ± 0.015 |
| Mitomycin C | 0.15 ± 0.011 | 0.11 ± 0.009 | 0.13 ± 0.012 | 0.14 ± 0.021 |
| DPPH Assay | ABTS Assay | |||
|---|---|---|---|---|
| Max Inhibition Value (%) | IC50 Value (µg/mL) | Max Inhibition Value (%) | IC50 Value (µg/mL) | |
| PG | 99 ± 1.67 | 235 ± 19.32 | 100 ± 1.98 | 115 ± 10.12 |
| α-tocopherol | 100 ± 2.21 | 24.3 ± 1.62 | 97 ± 1.81 | 12.7 ± 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-H.; Wang, S.-L.; Nguyen, D.-N.; Nguyen, A.-D.; Nguyen, T.-H.; Doan, M.-D.; Ngo, V.-A.; Doan, C.-T.; Kuo, Y.-H.; Nguyen, V.-B. Bioprocessing of Marine Chitinous Wastes for the Production of Bioactive Prodigiosin. Molecules 2021, 26, 3138. https://doi.org/10.3390/molecules26113138
Nguyen T-H, Wang S-L, Nguyen D-N, Nguyen A-D, Nguyen T-H, Doan M-D, Ngo V-A, Doan C-T, Kuo Y-H, Nguyen V-B. Bioprocessing of Marine Chitinous Wastes for the Production of Bioactive Prodigiosin. Molecules. 2021; 26(11):3138. https://doi.org/10.3390/molecules26113138
Chicago/Turabian StyleNguyen, Thi-Hanh, San-Lang Wang, Dai-Nam Nguyen, Anh-Dzung Nguyen, Thi-Huyen Nguyen, Manh-Dung Doan, Van-Anh Ngo, Chien-Thang Doan, Yao-Haur Kuo, and Van-Bon Nguyen. 2021. "Bioprocessing of Marine Chitinous Wastes for the Production of Bioactive Prodigiosin" Molecules 26, no. 11: 3138. https://doi.org/10.3390/molecules26113138
APA StyleNguyen, T.-H., Wang, S.-L., Nguyen, D.-N., Nguyen, A.-D., Nguyen, T.-H., Doan, M.-D., Ngo, V.-A., Doan, C.-T., Kuo, Y.-H., & Nguyen, V.-B. (2021). Bioprocessing of Marine Chitinous Wastes for the Production of Bioactive Prodigiosin. Molecules, 26(11), 3138. https://doi.org/10.3390/molecules26113138










