Macrocystis pyrifera Extract Residual as Nutrient Source for the Production of Sophorolipids Compounds by Marine Yeast Rhodotorula rubra
Abstract
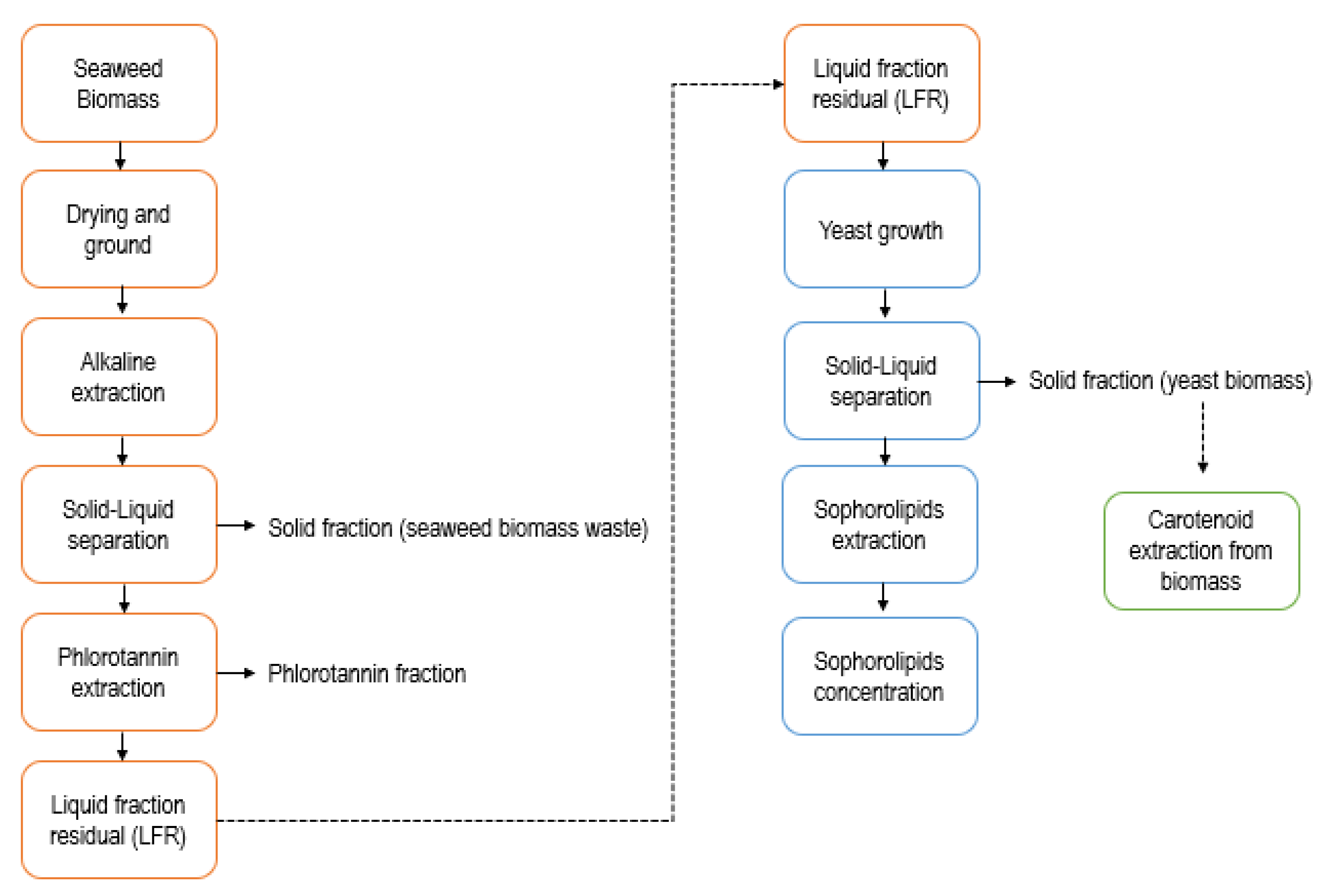
1. Introduction
2. Results
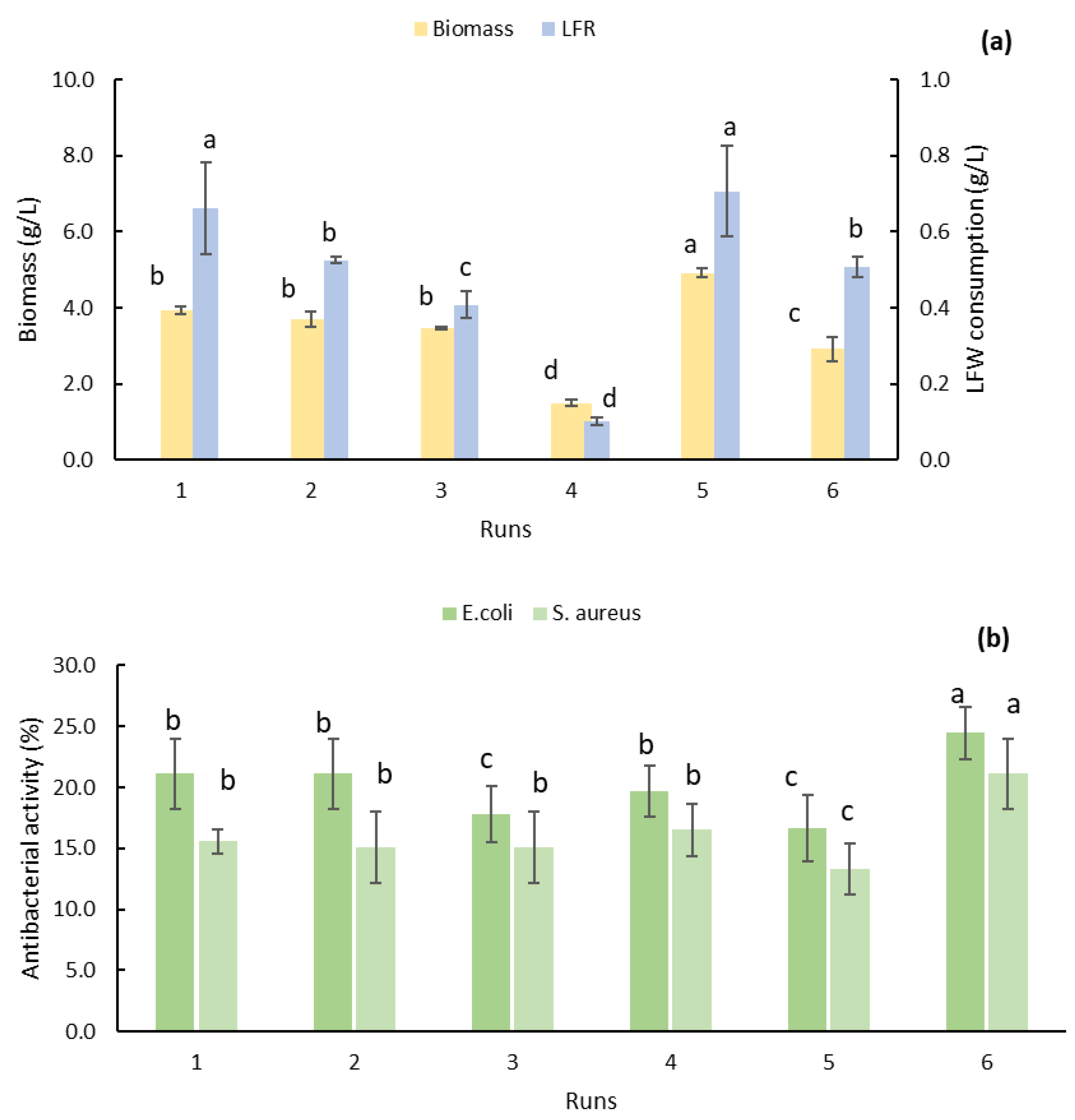
2.1. Characterization of LFR
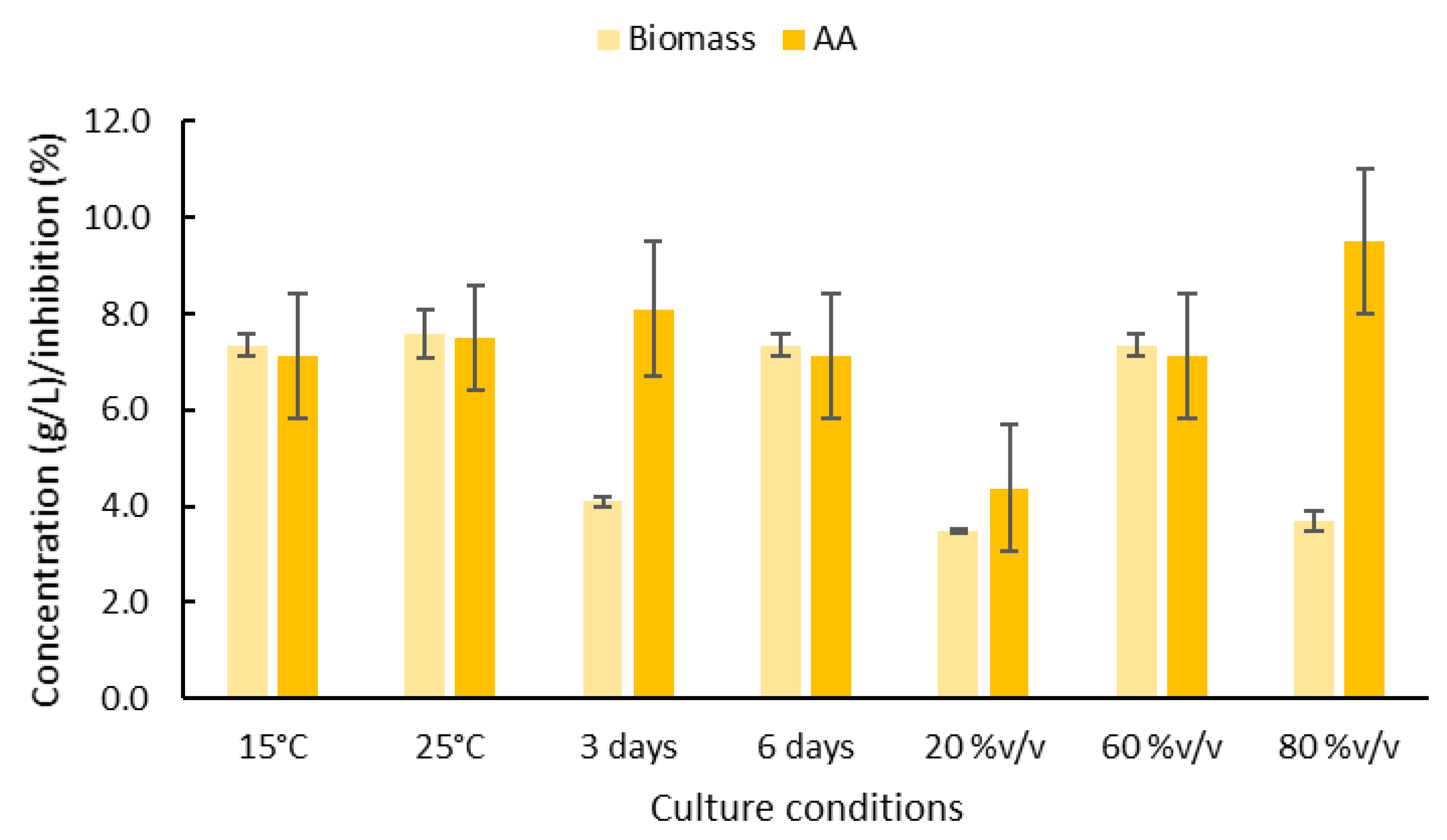
2.2. Effect of Culture Conditions on the Production of Biomass and Antibacterial Compounds by the Marine R. Rubra
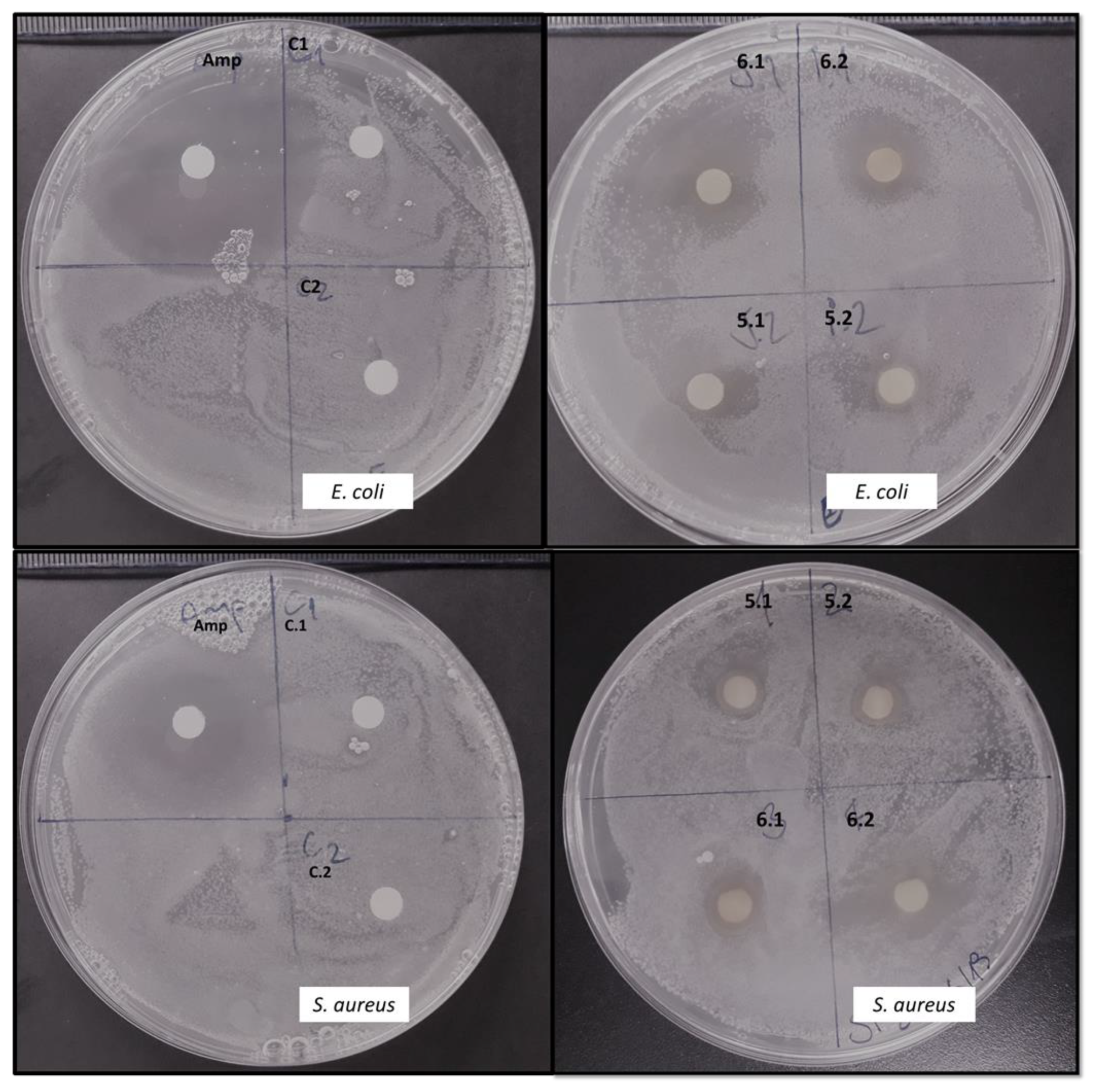
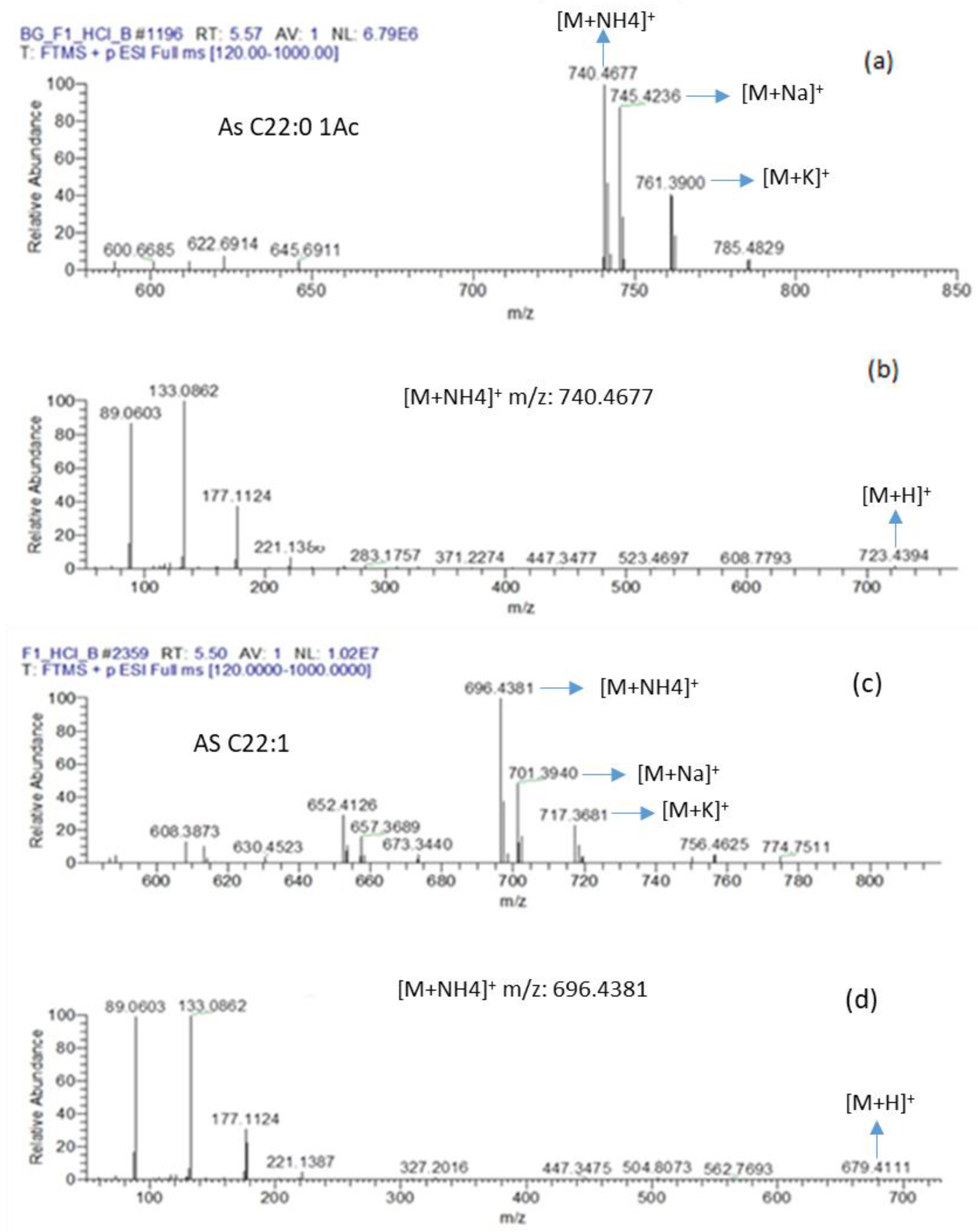
2.3. Identification of Sophorolipid with Antibacterial Activity Produced by R. Rubra
3. Discussion
4. Materials and Methods
4.1. Liquid Waste Fraction (LWR) from M. Pyrifera
4.2. Microorganism and Inoculum Preparation
4.3. Effect of Culture Conditions on the Production of Antibacterial Compounds
4.4. Separation of Antibacterial Compounds
4.5. Evaluation of the Antimicrobial Activity
4.6. Assay Methods
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nuñez, A.; Ashby, R.; Foglia, T.A.; Solaiman, D.K.Y. Analysis and characterization of sophorolipids by liquid chromatography with atmospheric pressure chemical ionization. Chromatographia 2001, 53, 673–677. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.A.; Saerens, K.; Muynck, C.; Develter, D.M.; Soetaert, W.; Vandamme, E.J. Microbial production and application of sophorolipids. Appl. Microbiol. Biotechnol. 2007, 76, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.; Shah, V.; Doncel, G.F.; Peterson, N.; Gao, W.; Gross, R. Amino Acid Conjugated Sophorolipids: A New Family of Biologically Active Functionalized Glycolipids. Bioconjugate Chem. 2006, 17, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Asmer, H.J.; Lang, S.; Wagner, F.; Wray, V. Microbial production, structure elucidation and bioconversion of sophorose lipids. J. Acad Ind. Res. 1988, 65, 1460–1466. [Google Scholar] [CrossRef]
- Hu, Y.; Ju, L.-K. Sophorolipid production from different lipid precursors observed with LC-MS. Enzym. Microb. Technol. 2001, 29, 593–601. [Google Scholar] [CrossRef]
- Joshi-Navare, K.; Khanvilkar, P.; Prabhune, A. Jatropha oil derived sophorolipids: Production and characterization as laundry detergent additive. Biochem. Res. Int. 2013, 2013, 169797. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kinta, M.; Inoue, S. Growth of yeasts on n-alkanes: Inhibition by a lactonic sophorolipid produced by Torulopsis bombicola. Agric. Biol. Chem. 1980, 44, 2221–2223. [Google Scholar] [CrossRef]
- Shao, L.; Song, X.; Ma, X.; Li, H.; Qu, Y. Bioactivities of sophorolipid with different structures against human esophageal cancer cells. J. Surg. Res. 2012, 173, 286–291. [Google Scholar] [CrossRef]
- Shah, V.; Doncel, G.F.; Seyoum, T.; Eaton, K.M.; Zalenskaya, I.; Hagver, R.; Azim, A.; Gross, R. Sophorolipids Microbial Glycolipids with Anti-Human Immunodeficiency Virus and Sperm-Immobilizing Activities. Antimicrobial Agents Chemotherapy. 2005, 49, 4093–4100. [Google Scholar] [CrossRef]
- Hirata, Y.; Ryu, M.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Kanaya, S.; Sugiura, M. Natural synergism of acid and lactone type mixed sophorolipids in interfacial activities and cytotoxicities. J. Oleo Sci. 2009, 58, 565–572. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Shao, L.-J.; Shen, J.; Song, X. Effects of nitrogen sources on production and composition of sophorolipids by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576. Appl. Microbiol. Biotechnol. 2011, 91, 1623–1632. [Google Scholar] [CrossRef]
- Cooper, D.G.; Paddock, D.A. Production of a Biosurfactant from Torulopsis bombicola. Appl. Environ. Microbiol. 1984, 47, 173–176. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Regulation of fermentation processes. Enzym. Microb. Technol. 2002, 31, 895–906. [Google Scholar] [CrossRef]
- Ribeiro, I.A.; Bronze, M.R.; Castro, M.F.; Ribeiro, M.H. Design of selective production of sophorolipids byRhodotorula bogoriensisthrough nutritional requirements. J. Mol. Recognit. 2012, 25, 630–640. [Google Scholar] [CrossRef]
- Nuñez, A.; Ashby, R.; Foglia, T.A.; Solaiman, D.K. LC/MS analysis and lipase modification of the sophorolipids produced by Rhodotorula bogoriensis**. Biotechnol. Lett. 2004, 26, 1087–1093. [Google Scholar] [CrossRef]
- Solaiman, D.K.; Ashby, R.D.; Crocker, N.V. High-titer production and strong antimicrobial activity of sophorolipids fromRhodotorula bogoriensis. Biotechnol. Prog. 2015, 31, 867–874. [Google Scholar] [CrossRef]
- Sen, S.; Borah, S.N.; Bora, A.; Deka, S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb. Cell Factories 2017, 16, 1–14. [Google Scholar] [CrossRef]
- Leyton, A.; Flores, L.; Mäki-Arvela, P.; Lienqueo, M.; Shene, C. Macrocystis pyriferasource of nutrients for the production of carotenoids by a marine yeastRhodotorula mucilaginosa. J. Appl. Microbiol. 2019, 127, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Camus, C.; Infante, J.; Buschmann, A.H. Overview of 3 year precommercial seafarming of Macrocystis pyrifera along the Chilean coast. Rev. Aquac. 2016, 10, 543–559. [Google Scholar] [CrossRef]
- Macchiavello, J.; Araya, E.; Bulboa, C. Production of Macrocystis pyrifera (Laminariales; Phaeophyceae) in northern Chile on spore-based culture. Environ. Boil. Fishes 2010, 22, 691–697. [Google Scholar] [CrossRef]
- Westermeier, R.; Patiño, D.J.; Murúa, P.; Müller, D.G. Macrocystis mariculture in Chile: Growth performance of heterosis genotype constructs under field conditions. Environ. Boil. Fishes 2010, 23, 819–825. [Google Scholar] [CrossRef]
- Gao, K.; McKinley, K.R. Use of macroalgae for marine biomass production and CO2 remediation: A review. Environ. Boil. Fishes 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.D.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Leyton, A.; Lienqueo, M.E.; Shene, C. Macrocystis pyrifera: Substrate for the production of bioactive compounds. Environ. Boil. Fishes 2020, 32, 2335–2341. [Google Scholar] [CrossRef]
- Purcell-Meyerink, D.; Packer, M.; Wheeler, T.; Hayes, M. Aquaculture Production of the Brown Seaweeds Laminaria digitata and Macrocystis pyrifera: Applications in Food and Pharmaceuticals. Mol. 2021, 26, 1306. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.-H.; Mäki-Arvela, P.; Mikkola, J.-P.; Lienqueo, M.-E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2017, 16, 201–208. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Magri, A.; Baldo, C.; Camilios-Neto, D.; Minucelli, T.; Colabone- Celligoi, M.A.P. Review: Sophorolipids A Promising Biosurfactant and it’s Applications. Int. J. Adv. Biotechnol. Res. 2015, 6, 161–174. [Google Scholar]
- Casas, J.A.; De Lara, S.G.; Garcia-Ochoa, F. Optimization of a synthetic medium for Candida bombicola growth using factorial design of experiments. Enzym. Microb. Technol. 1997, 21, 221–229. [Google Scholar] [CrossRef]
- Casas, J.A.; García-Ochoa, F. Sophorolipid production by Candida bombicola: Medium composition and culture methods. J. Biosci. Bioeng. 1999, 88, 488–494. [Google Scholar] [CrossRef]
- Daverey, A.; Pakshirajan, K. Production, Characterization, and Properties of Sophorolipids from the Yeast Candida bombicola using a Low-cost Fermentative Medium. Appl. Biochem. Biotechnol. 2009, 158, 663–674. [Google Scholar] [CrossRef]
- Leyton, A.; Vergara-Salinas, J.; Pérez-Correa, J.; Lienqueo, M. Purification of phlorotannins from Macrocystis pyrifera using macroporous resins. Food Chem. 2017, 237, 312–319. [Google Scholar] [CrossRef]
- Daniel, H.J.; Reuss, M.; Syldatk, C. Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in a two stage fed batch process using Candida bombicola ATCC 22214. Biotechnol. Lett. 1998, 20, 1153–1156. [Google Scholar] [CrossRef]
- Chandran, P.; Das, N. Characterization of sophorolipid biosurfactant produced by yeast species grown on diesel oil. Int. J. Sci. Nat. 2011, 2, 63–71. [Google Scholar]
- Polat, S.; Ozogul, Y. Seasonal proximate and fatty acid variations of some seaweeds from the northeastern Mediterranean coast. Oceanol. 2013, 55, 375–391. [Google Scholar] [CrossRef]
- Silva, G.; Pereira, R.B.; Valentao, P.; Andrade, P.B.; Sousa, C. Distinct fatty acid profile of ten Brown macroalgae. Braz. J. Pharmacogn. 2013, 23, 608–613. [Google Scholar] [CrossRef]
- Shin, J.D.; Lee, J.; Kim, Y.B.; Han, I.-S.; Kim, E.-K. Production and characterization of methyl ester sophorolipids with 22-carbon-fatty acids. Bioresour. Technol. 2010, 101, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Dengle-Pulate, V.; Chandorkar, P.; Bhagwat, S.S.; Prabhune, A.A. Antimicrobial and SEM Studies of Sophorolipids Synthesized Using Lauryl Alcohol. J. Surfactants Deterg. 2014, 17, 543–552. [Google Scholar] [CrossRef]
- Shene, C.; Leyton, A.; Rubilar, M.; Pinelo, M.; Acevedo, F.; Morales, E. Production of lipids and docosahexasaenoic acid (DHA) by a native Thraustochytrium strain. Eur. J. Lipid Sci. Technol. 2013, 115, 890–900. [Google Scholar] [CrossRef]
- AOAC, Association of Official Analytical Chemistry. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
| Chemical Composition | Unit | Seaweed Extract |
|---|---|---|
| Dry Basis | ||
| Lipids | % | 1.4 ± 0.1 |
| Ash | % | 51.5 ± 1.5 |
| Protein | % | 15 ± 0.5 |
| Carbohydrates* | % | 32 ± 1.0 |
| Runs | LFR (%) | YE (g/L) | SLs (g/L) |
|---|---|---|---|
| 1 | 20 | 6 | 0.12 |
| 2 | 20 | 0 | 0.13 |
| 3 | 30 | 6 | 0.09 |
| 4 | 30 | 0 | 0.12 |
| 5 | 40 | 6 | 0.07 |
| 6 | 40 | 0 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyton, A.; Araya, M.; Tala, F.; Flores, L.; Lienqueo, M.E.; Shene, C. Macrocystis pyrifera Extract Residual as Nutrient Source for the Production of Sophorolipids Compounds by Marine Yeast Rhodotorula rubra. Molecules 2021, 26, 2355. https://doi.org/10.3390/molecules26082355
Leyton A, Araya M, Tala F, Flores L, Lienqueo ME, Shene C. Macrocystis pyrifera Extract Residual as Nutrient Source for the Production of Sophorolipids Compounds by Marine Yeast Rhodotorula rubra. Molecules. 2021; 26(8):2355. https://doi.org/10.3390/molecules26082355
Chicago/Turabian StyleLeyton, Allison, Michael Araya, Fadia Tala, Liset Flores, María Elena Lienqueo, and Carolina Shene. 2021. "Macrocystis pyrifera Extract Residual as Nutrient Source for the Production of Sophorolipids Compounds by Marine Yeast Rhodotorula rubra" Molecules 26, no. 8: 2355. https://doi.org/10.3390/molecules26082355
APA StyleLeyton, A., Araya, M., Tala, F., Flores, L., Lienqueo, M. E., & Shene, C. (2021). Macrocystis pyrifera Extract Residual as Nutrient Source for the Production of Sophorolipids Compounds by Marine Yeast Rhodotorula rubra. Molecules, 26(8), 2355. https://doi.org/10.3390/molecules26082355








