Performance Evaluation of Enzyme Breaker for Fracturing Applications under Simulated Reservoir Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Effect of Breaker on Shear Resistance Performance and Sand-Suspending Performance of the Fracturing Fluid
2.2.2. Gel-Breaking Efficiency of Breakers
2.2.3. Reservoir Damage by the Breaker after the Fracturing Operation
2.2.4. Best-Use Concentrations of Mannanase II under Different Environmental Conditions
2.2.5. Statistical Analysis
3. Results and Discussion
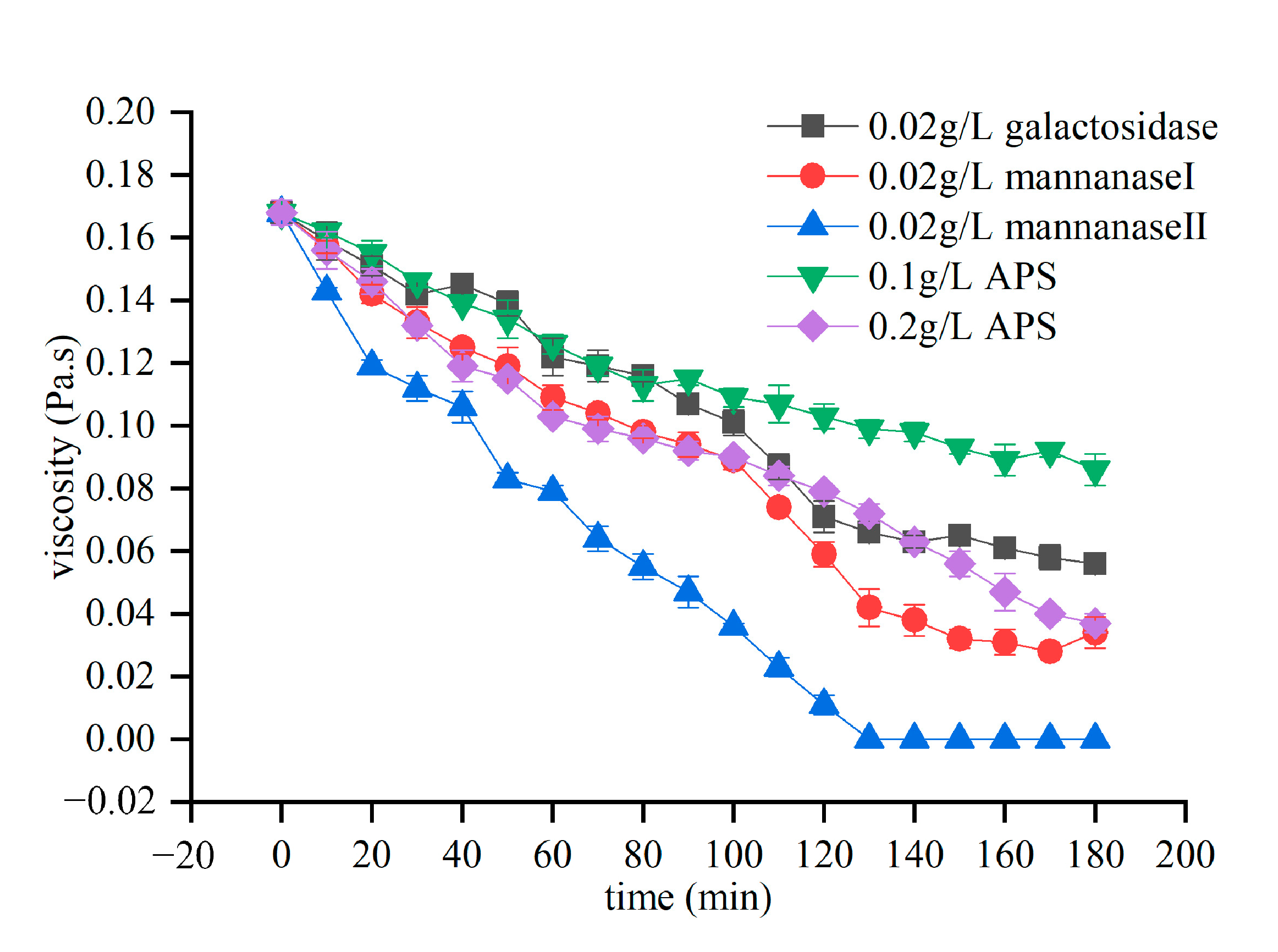
3.1. Effect of Breaker on Shear Resistance Performance and Sand-Suspending Performance of the Fracturing Fluid
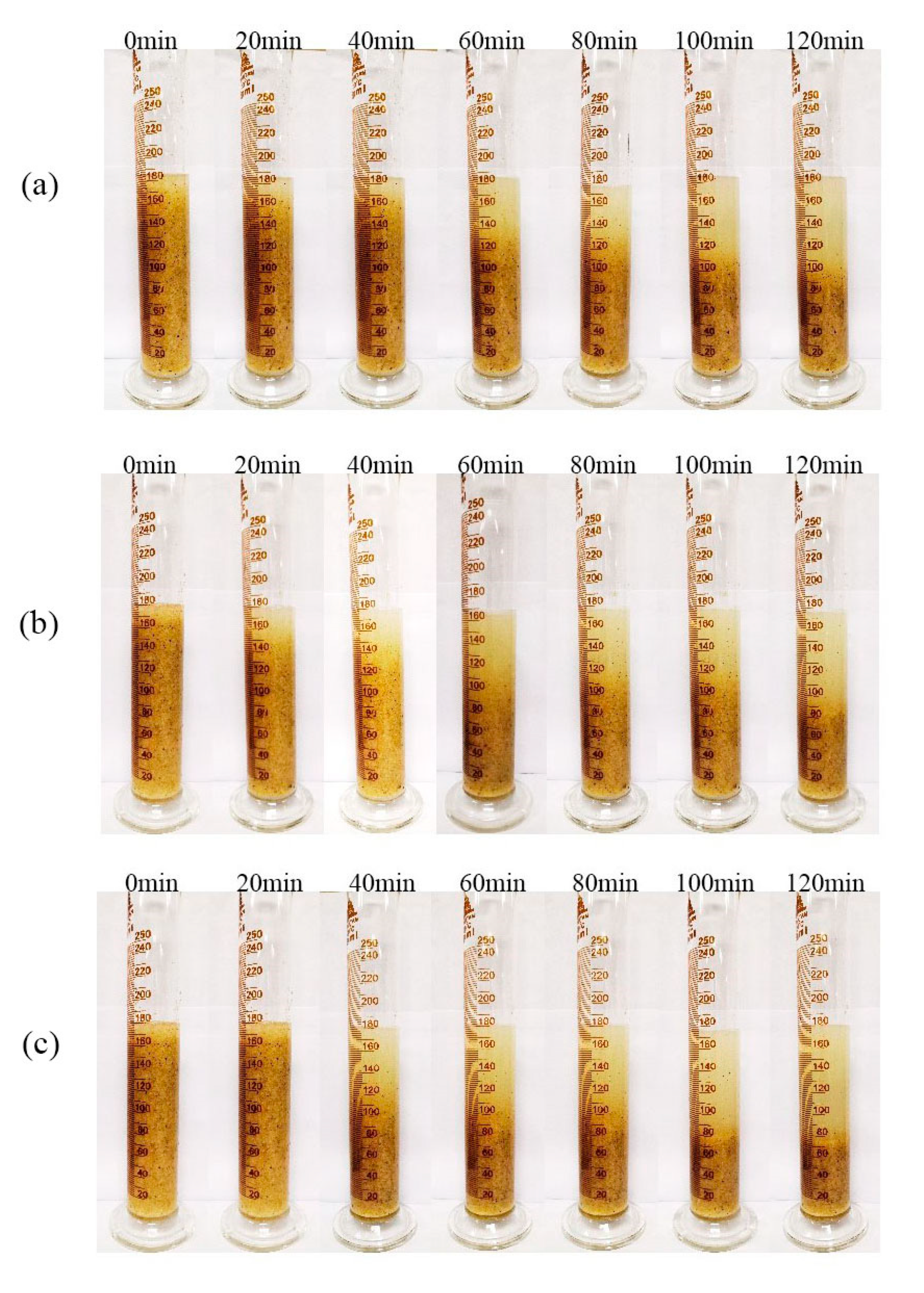
3.2. Gel-Breaking Efficiency of Breakers
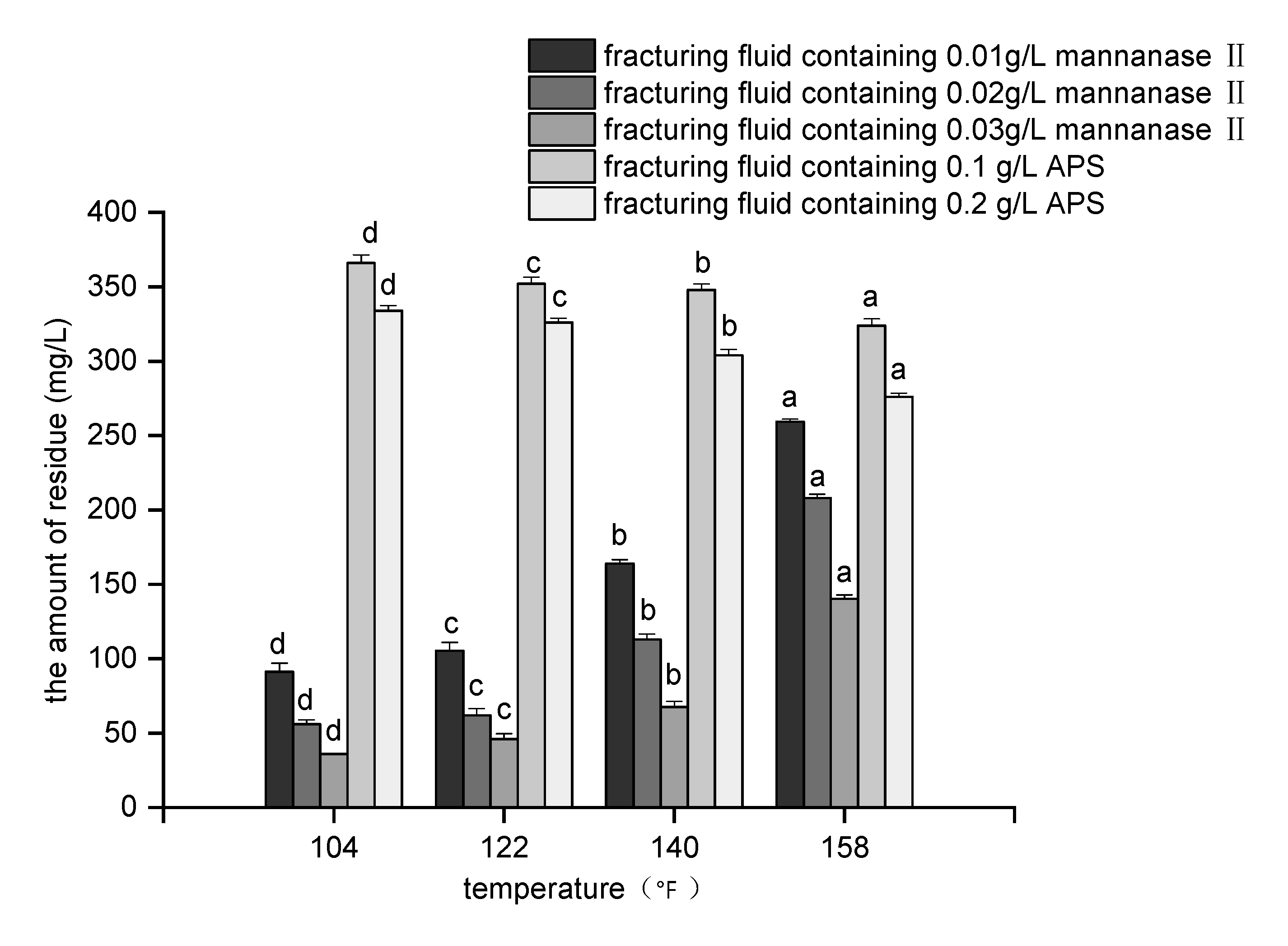
3.3. Reservoir Damage by the Breaker after the Fracturing Operation
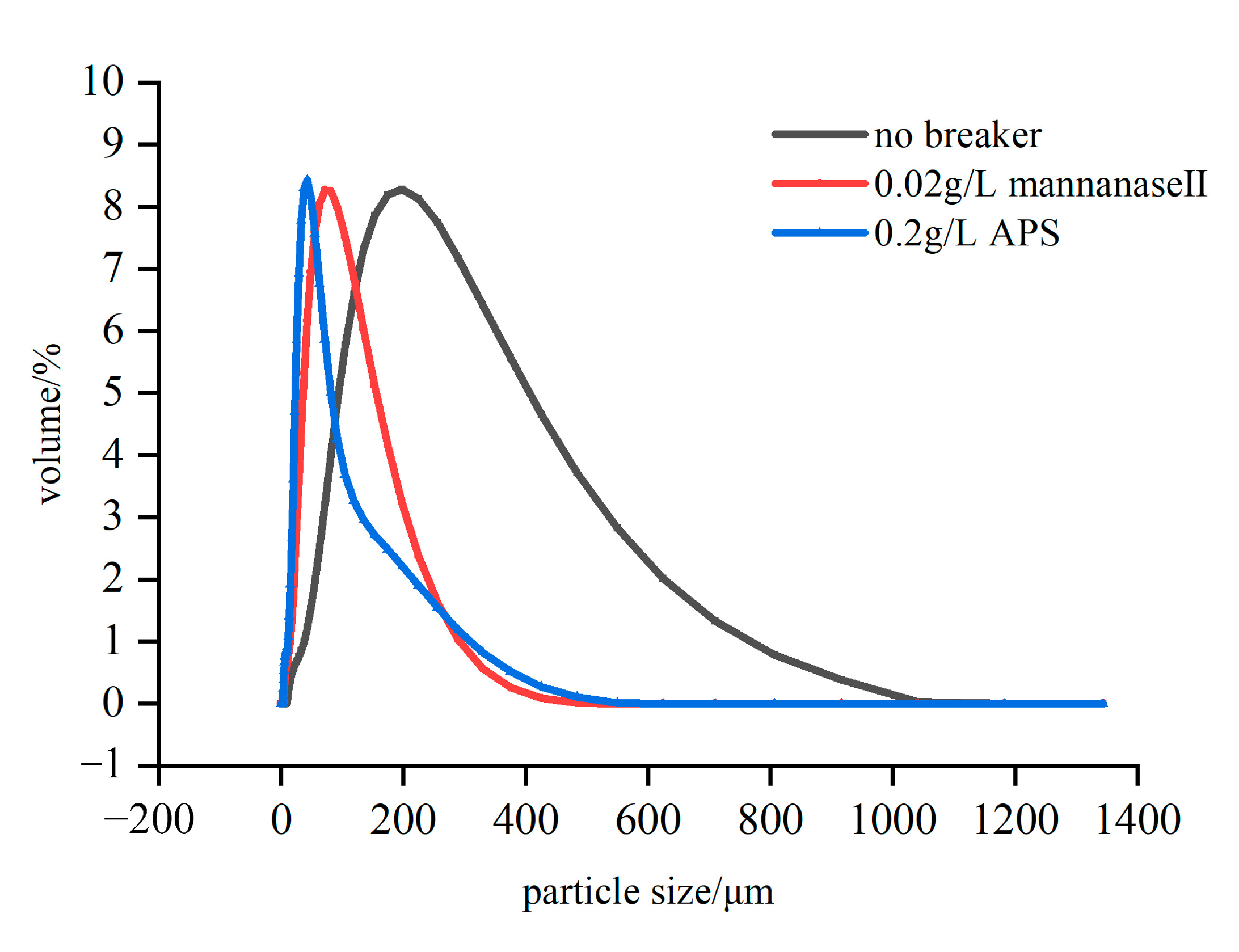
3.4. Best-Use Concentrations of Mannanase II under Different Environmental Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Stringfellow, W.T.; Domen, J.K.; Camarillo, M.K.; Sandelin, W.L.; Borglin, S. Physical, chemical, and biological characteristics of compounds used in hydraulic fracturing. J. Hazard. Mater. 2014, 275, 37–54. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.T. A Review of Fracturing Fluid Systems Used for Hydraulic Fracturing of Oil and Gas Wells. J. Appl. Polym. Sci. 2014, 131, 40735. [Google Scholar] [CrossRef]
- Jennings, A.R., Jr. Fracturing Fluids—Then and Now. J. Pet. Technol. 1996, 48, 604–610. [Google Scholar] [CrossRef]
- Bose, C.; Swartz, L.; Gupta, A.; Barati, R. Dual Application of Polyelectrolyte Complex Nanoparticles as Enzyme Breaker Carriers and Fluid Loss Additives for Fracturing Fluids. In Proceedings of the SPE/CSUR Unconventional Resources Conference, Calgary, AB, Canada, 30 September 2014; p. 171571. [Google Scholar]
- Al-Muntasheri, G.; Li, L.; Liang, F.; Gomaa, A. Concepts in Cleanup of Fracturing Fluids Used in Conventional Reservoirs: A Literature Review. SPE Prod. Oper. 2018, 33, 196–213. [Google Scholar] [CrossRef]
- Robinson, G.; Ross-Murphy, S.; Morris, E. Viscosity-Molecular Weight Relationships, Intrinsic Chain Flexibility, and Dynamic Solution Properties of Guar Galactomannan. Carbohydr. Res. 1982, 107, 17–32. [Google Scholar] [CrossRef]
- Trabelsi, S.; Kakadjian, S. Comparative Study Between Guar and Carboxymethylcellulose Used as Gelling Systems in Hydraulic Fracturing Application. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 23–26 March 2013; p. 164486. [Google Scholar]
- Wilson, A. An Innovative Approach to Gel Breakers for Hydraulic Fracturing. J. Pet. Technol. 2017, 69, 48–51. [Google Scholar] [CrossRef]
- Al-Hulail, I.; Al-Khabbaz, H.; Al-Janabi, Y.; Rahal, R.; Dahlan, M.; Al-Marshad, K. Fracturing Fluid Encapsulated Breaker: High Temperature up to 330 °F. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 28 March 2018; p. 190379. [Google Scholar]
- Prajakta, P.; Ramesh, M.; Nisha, P. Novel Controlled-Release Breakers for High-Temperature Fracturing. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 15–17 April 2013; p. 164656. [Google Scholar]
- Soe, A.; Azahar, B.; Tunio, S. Fracturing Fluid (Guar Polymer Gel) Degradation Study by using Oxidative and Enzyme Breaker. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 1667–1671. [Google Scholar]
- Watson, W.; Aften, C.; Previs, D.; Chemicals, K. Delayed-Release Coatings for Oxidative Breakers. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 10–12 September 2010; p. 127895. [Google Scholar]
- Sarwar, M.; Cawiezel, K.; Nasr-El-Din, H. Gel Degradation Studies of Oxidative and Enzyme Breakers to Optimize Breaker Type and Concentration for Effective Break Profiles at Low and Medium Temperature Ranges. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 24–26 January 2011; p. 140520. [Google Scholar]
- Caulfield, M.; Qiao, G.; Solomon, D. Some Aspects of the Properties and Degradation of Polyacrylamides. Chem. Rev. 2010, 33, 274. [Google Scholar] [CrossRef]
- Funkhouser, G.; Norman, L. Synthetic Polymer Fracturing Fluid for High-Temperature Applications. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5 February 2003; p. 80236. [Google Scholar]
- Ayoub, J.; Hutchins, R.; Van der Bas, F.; Cobianco, S.; Emiliani, C.; Glover, M.; Marino, S.; Nitters, G.; Norman, D.; Turk, G. New Results Improve Fracture-Cleanup Characterization and Damage Mitigation. SPE Prod. Oper. 2009, 24, 374–380. [Google Scholar] [CrossRef]
- Sumner, A.J.; Plata, D.L. Oxidative Breakers Can Stimulate Halogenation and Competitive Oxidation in Guar-Gelled Hydraulic Fracturing Fluids. Environ. Sci. Technol. 2019, 53, 8216–8226. [Google Scholar] [CrossRef]
- Brannon, H.; Tjon-Joe-Pin, R.; Carman, P.; Wood, W. Enzyme Breaker Technologies: A Decade of Improved Well Stimulation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5 October 2003; p. 84213. [Google Scholar]
- Vengosh, A.; Jackson, R.B.; Warner, N.; Darrah, T.H.; Kondash, A. A Critical Review of the Risks to Water Resources from Unconventional Shale Gas Development and Hydraulic Fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef] [PubMed]
- Fogang, L.; Kamal, M.; Sultan, A. Viscosity-Reducing Agents (Breakers) for Viscoelastic Surfactant Gels for Well Stimulation. Energy Fuels 2020, 34, 15686–15700. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, B.; Zhou, E.; Pu, J.; Schuman, T. Experimental Evaluation of Oxidizing Breakers for a Polyacrylamide-Based Re-Crosslinkable Preformed Particle Gel. Energy Fuels 2019, 33, 5001–5010. [Google Scholar] [CrossRef]
- Peterson, M.; Daniel, R.; Danson, M.; Eisenthal, R. The dependence of enzyme activity on temperature: Determination and validation of parameters. Biochem. J. 2007, 403, 615. [Google Scholar] [CrossRef]
- Xin, M.; Ping, S.; Licheng, L.; Qian, D.; Guang, L.; Yao, C. Low-temperature pH-regulable gel-breaking of galactomannan-based fracturing fluids by the mannanase from Bacillus aerius. Int. Biodeterior. Biodegrad. 2011, 160, 105226. [Google Scholar]
- Barati, R.; Johnson, S.J.; McCool, S.; Green, D.W.; Willhite, G.P.; Liang, J.-T. Fracturing fluid cleanup by controlled release of enzymes from polyelectrolyte complex nanoparticles. J. Appl. Polym. Sci. 2011, 121, 1292–1298. [Google Scholar] [CrossRef]
- Bhatia, S.; Singh, A.; Batra, N.; Singh, J. Microbial production and biotechnological applications of alpha-galactosidase. Int. J. Biol. Macromol. 2020, 150, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kumar, A.; Luo, X.; Shi, H.; Liu, Z.; Wu, G. Highly alkali-stable and cellulase-free xylanases from Fusarium sp. 21 and their application in clarification of orange juice. Int. J. Biol. Macromol. 2020, 155, 572–580. [Google Scholar] [CrossRef]
- Nahar, N.; Ripplinger, D.; Pryor, S.W. Process yield and economic trade-offs for enzymatic hydrolysis of alkaline pretreated corn stover. Biomass Bioenergy 2017, 99, 97–105. [Google Scholar] [CrossRef]
- Zhang, B.; Huston, A.; Whipple, L.; Urbina, H.; Barrett, K.E.; Wall, M.; Hutchins, R.D.; Mirakyan, A. A Superior, High-Performance Enzyme for Breaking Borate Crosslinked Fracturing Fluids Under Extreme Well Conditions. SPE Prod. Oper. 2013, 28, 210–216. [Google Scholar] [CrossRef]
- Angural, S.; Rana, M.; Sharma, A.; Warmoota, R.; Puri, N.; Gupta, N. Combinatorial Biobleaching of Mixedwood Pulp with Lignolytic and Hemicellulolytic Enzymes for Paper Making. Indian. J. Microbiol. 2020, 60, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Deng, J.; Zhou, K.; Liang, Y.; Chen, Y.; Wang, D.; Zhong, J.; Sun, Y.; Li, M. The effect of deacetylation degree of konjac glucomannan on microbial metabolites and gut microbiota in vitro fermentation. J. Funct. Foods. 2020, 66, 103796. [Google Scholar] [CrossRef]
- Yin, L.; Tai, H.; Jiang, S. Characterization of Mannanase from a Novel Mannanase-Producing Bacterium. J. Agric. Food. Chem. 2012, 60, 6425–6431. [Google Scholar] [CrossRef] [PubMed]
- Battistel, E.; Bianchi, D.; Fornaroli, M.; Cobianco, S. Enzymes breakers for viscosity enhancing polymers. J. Pet. Sci. Eng. 2011, 77, 10–17. [Google Scholar] [CrossRef]
- Tao, L.; Li, Z.; Bi, Y.; Zhang, J. Multi-combination exploiting technique of ultra-heavy oil reservoirs with deep and thin layers in Shengli Oilfield. Pet. Explor. Dev. 2010, 37, 732–736. [Google Scholar] [CrossRef]
- Yang, W.; Sc, A.; Kz, C.; You, H.; Hai, Y. Pressure-transient analysis of water injectors considering the multiple closures of waterflood-induced fractures in tight reservoirs: Case studies in Changqing Oilfield, China. J. Pet. Sci. Eng. 2019, 172, 643–653. [Google Scholar]
- Cao, P.F.; Mangadlao, J.D.; Advincula, R.C. Stimuli-Responsive Polymers and their Potential Applications in Oil-Gas Industry. Polym. Rev. 2015, 55, 706–733. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Zhu, Z.; Hao, L.; Deyi, Z.; Chuwen, G. Experimental Investigation on Rock Breaking Performance of Cutter Assisted with Hydraulic Fracturing. Eng. Fract. Mech. 2021, 248, 107710. [Google Scholar] [CrossRef]
- Jie, W.; Yha, B.; Yan, Z.; Fza, B.; Eya, B.; Ru, W. Study of Fracturing Fluid on Gel Breaking Performance and Damage to fracture conductivity—ScienceDirect. J. Pet. Sci. Eng. 2020, 193, 107443. [Google Scholar]
- Lin, X.; Zhang, S.; Zheng, C.; Yang, P. Optimal production of enzymatic guar gel breaker by Bacillus sp. M1-4 and its potential in hydraulic fracturing. Int. J. Oil Gas Coal Technol. 2016, 12, 231–247. [Google Scholar]
- Bankole, K.S.; Matthew, B. Applications of Oilfield Produced Formation Water for Fracturing Fluid. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 2208–2216. [Google Scholar] [CrossRef]
- Priscille, E.; Siddhamshetty, P.; Cao, K.; Mukherjee, R.; Kwon, J. Incorporation of sustainability in process control of hydraulic fracturing in unconventional reservoirs. Chem. Eng. Res. Des. 2018, 139, 62–76. [Google Scholar]
- Murthy, R.; Chavali, M. A novel hydraulic fracturing gel realization for unconventional reservoirs. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Xiong, B.; Miller, Z.; Roman-White, S.; Tasker, T.; Farina, B.; Piechowicz, B.; Burgos, W.D.; Joshi, P.; Zhu, L.; Gorski, C.A.; et al. Chemical Degradation of Polyacrylamide during Hydraulic Fracturing. Environ. Sci. Technol. 2017, 52, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Aliu, A.; Guo, J.; Wang, S.; Zhao, X. Hydraulic fracture fluid for gas reservoirs in petroleum engineering applications using sodium carboxy methyl cellulose as gelling agent. J. Nat. Gas Sci. Eng. 2016, 32, 491–500. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, S.; Wang, Q.; Feng, Y.; Shuai, Y. Improving the fracturing fluid loss control for multistage fracturing by the precise gel breaking time design. J. Nat. Gas Sci. Eng. 2015, 25, 367–370. [Google Scholar] [CrossRef]
- Khanna, A.; Luong, H.; Kotousov, A.; Nguyen, G.D.; Rose, L.F. Residual opening of hydraulic fractures created using the channel fracturing technique. Int. J. Rock Mech. Min. Sci. 2017, 100, 124–137. [Google Scholar] [CrossRef]
- Ma, X.; Lei, G.; Wang, Z.; Da, Q.; Zhang, X.; Song, P.; Yao, C. Remediation mechanism of guar degrading bacteria on hydraulic fracturing fluid damage. J. China Univ. Pet. 2018, 42, 100–110. [Google Scholar]
- Lawrence, R.A. A pocket calculator program for Duncan’s New Multiple Range test and analysis of variance. Comput. Biol. Med. 1984, 14, 357–362. [Google Scholar] [CrossRef]
- Liu, J.; Basit, A.; Miao, T.; Zheng, F.; Yu, H.; Wang, Y.; Jiang, W.; Cao, Y. Secretory expression of β-mannanase in Saccharomyces cerevisiae and its high efficiency for hydrolysis of mannans to mannooligosaccharides. Appl. Microbiol. Biotechnol. 2018, 102, 10027–10041. [Google Scholar] [CrossRef]
- Xie, J.; Pan, L.; He, Z.; Liu, W.; Zheng, D.; Zhang, Z.; Wang, B. A novel thermophilic β-mannanase with broad-range pH stability from Lichtheimia ramosa and its synergistic effect with α-galactosidase on hydrolyzing palm kernel meal. Process. Biochem. 2020, 88, 51–59. [Google Scholar] [CrossRef]
- Russell, A.; Fersht, A. Rational modification of enzyme catalysis by engineering surface charge. Nature 1987, 328, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xue, Y.; Dou, Y.; Xu, Z.; Tao, W.; Zhou, P. Characterization and gene cloning of a novel?—Mannanase from alkaliphilic Bacillus sp. N16-5. Extremophiles 2004, 8, 447–454. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Zhao, F.; Jin, X.; Feng, Y.; Sun, G.; Lin, J.; Jia, B.; Li, P. Performance Evaluation of Enzyme Breaker for Fracturing Applications under Simulated Reservoir Conditions. Molecules 2021, 26, 3133. https://doi.org/10.3390/molecules26113133
Meng Y, Zhao F, Jin X, Feng Y, Sun G, Lin J, Jia B, Li P. Performance Evaluation of Enzyme Breaker for Fracturing Applications under Simulated Reservoir Conditions. Molecules. 2021; 26(11):3133. https://doi.org/10.3390/molecules26113133
Chicago/Turabian StyleMeng, Yuling, Fei Zhao, Xianwei Jin, Yun Feng, Gangzheng Sun, Junzhang Lin, Baolei Jia, and Piwu Li. 2021. "Performance Evaluation of Enzyme Breaker for Fracturing Applications under Simulated Reservoir Conditions" Molecules 26, no. 11: 3133. https://doi.org/10.3390/molecules26113133
APA StyleMeng, Y., Zhao, F., Jin, X., Feng, Y., Sun, G., Lin, J., Jia, B., & Li, P. (2021). Performance Evaluation of Enzyme Breaker for Fracturing Applications under Simulated Reservoir Conditions. Molecules, 26(11), 3133. https://doi.org/10.3390/molecules26113133








