Entropy-Induced Self-Assembly of Colloidal Crystals with High Reflectivity and Narrow Reflection Bandwidth
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of SPs
2.2. The Self-Assembly of Soft Matter on the Planar Interface and the Bending Interface
2.3. Effect of Temperature on the Self-Assembly Behavior of the Soft Matter System
2.4. The Preparation of CCs with High Reflectivity and Narrow Reflection Bandwidth
2.5. Determination of Optical Characteristics of CCs
2.6. Determination of Ethanol
3. Results and Discussion
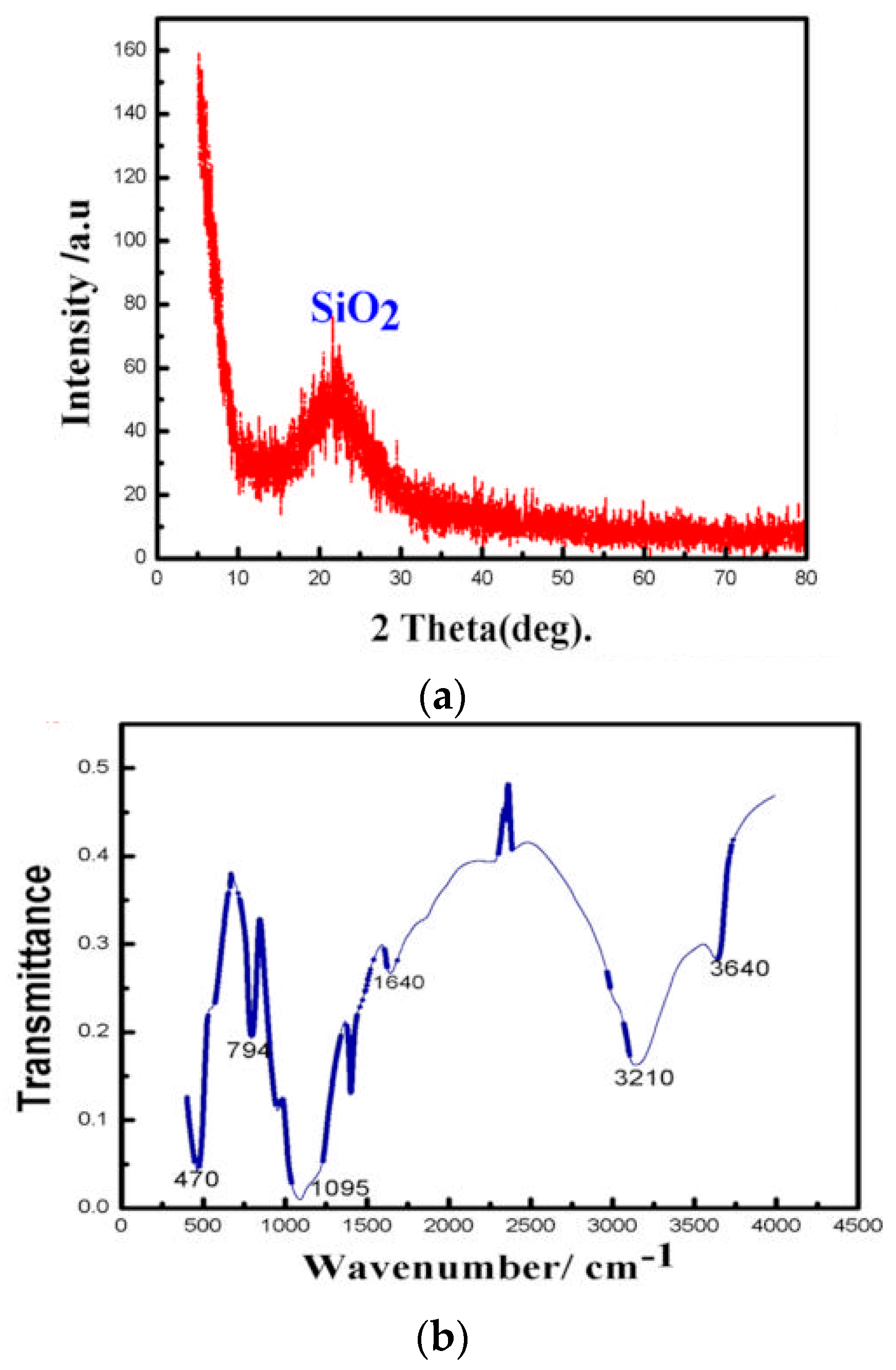
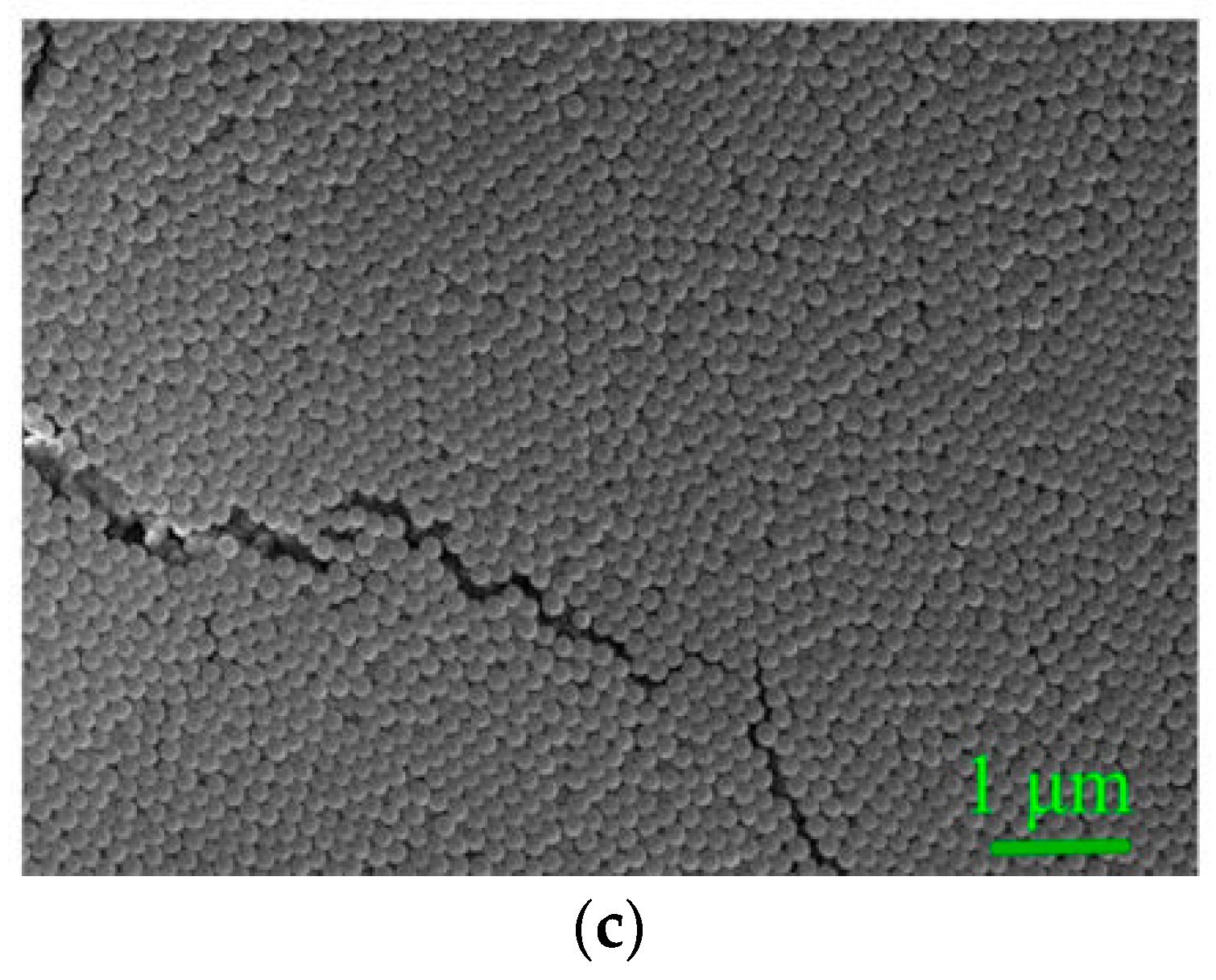
3.1. Characterization of Synthetic SPs
3.2. Determination of Composition and Proportion of the Three-Component Soft Matter
3.3. Self-Assembly of the Soft Matter System on the Planar Interface
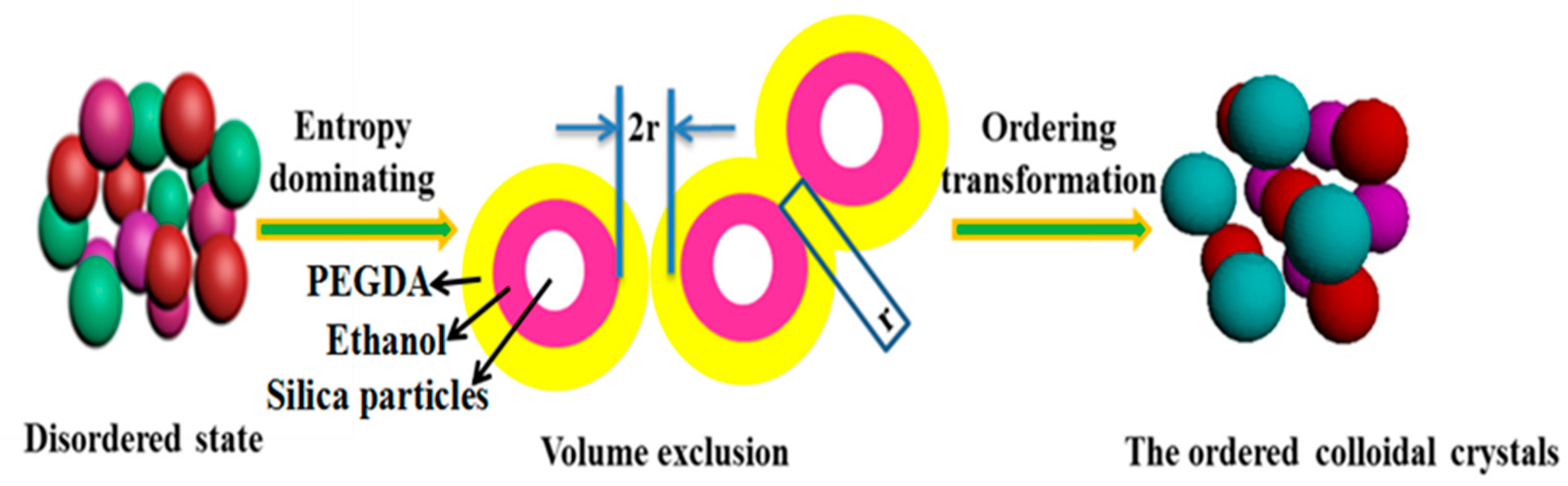
3.4. Formation Mechanism of the Ordered CC Structure Induced by Entropy on the Planar Interface
3.5. Self-Assembly of the Soft Matter System on the Bending Interface
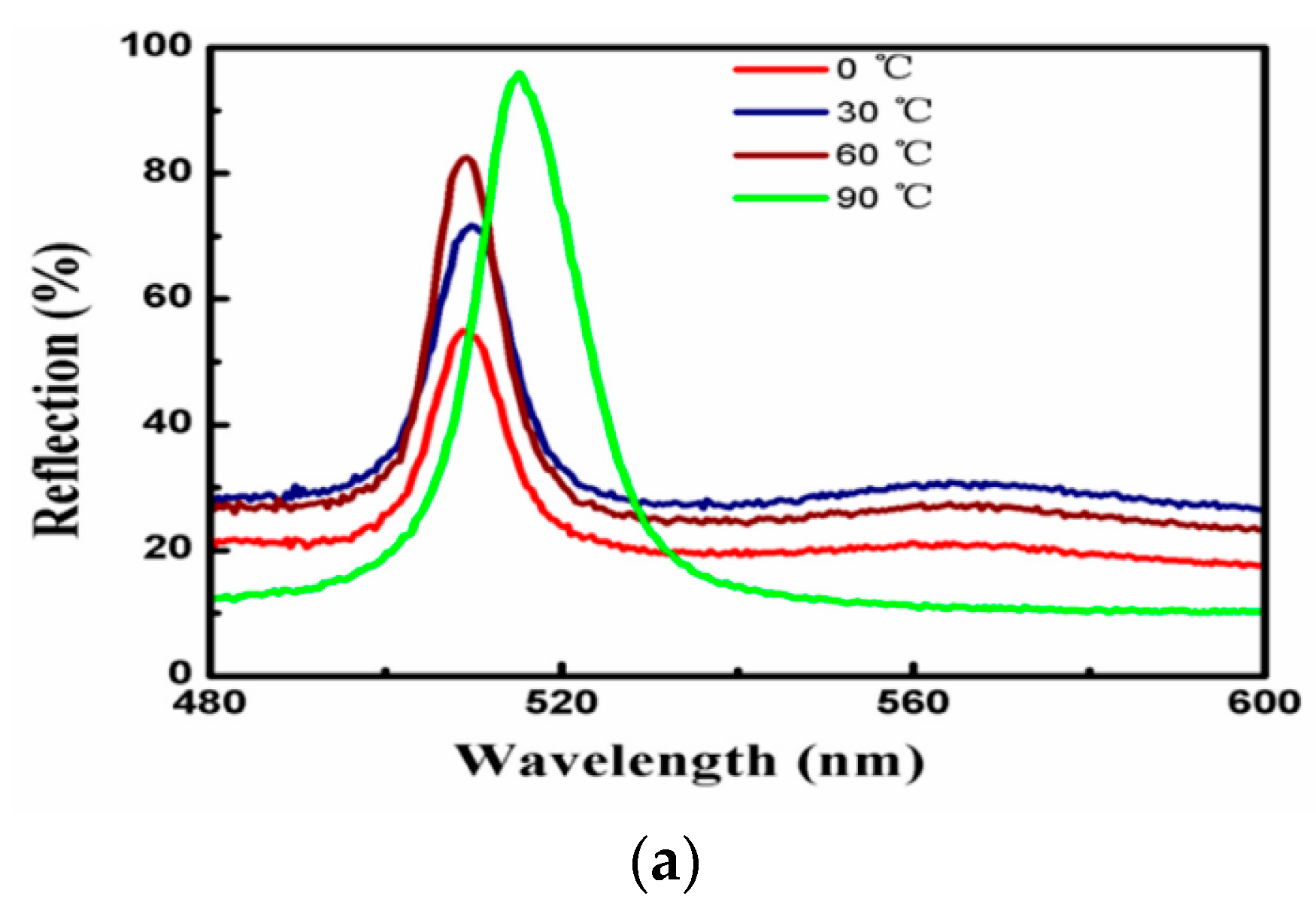
3.6. Effect of Temperature on the Self-Assembly Behavior on the Planar Interface
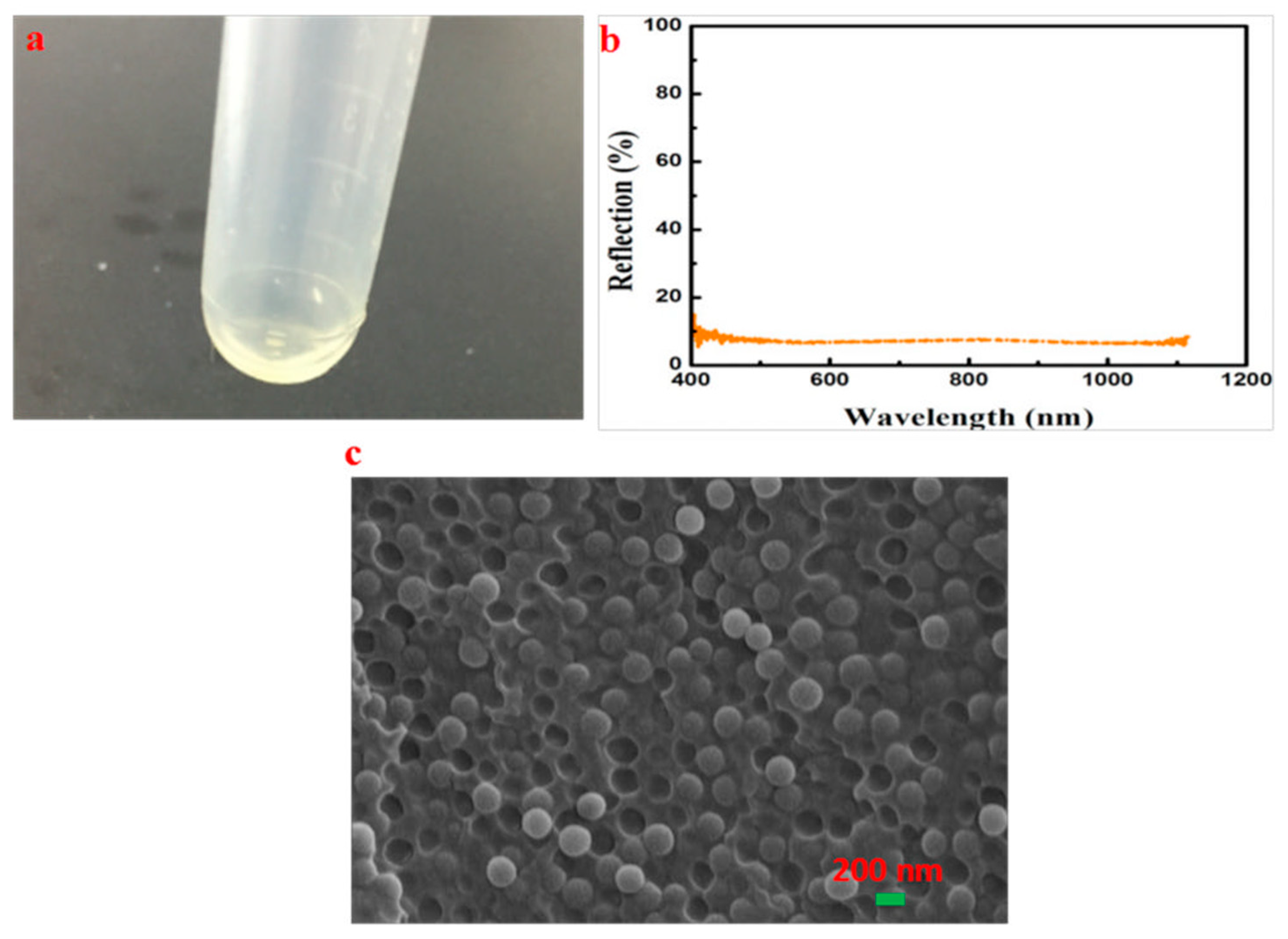
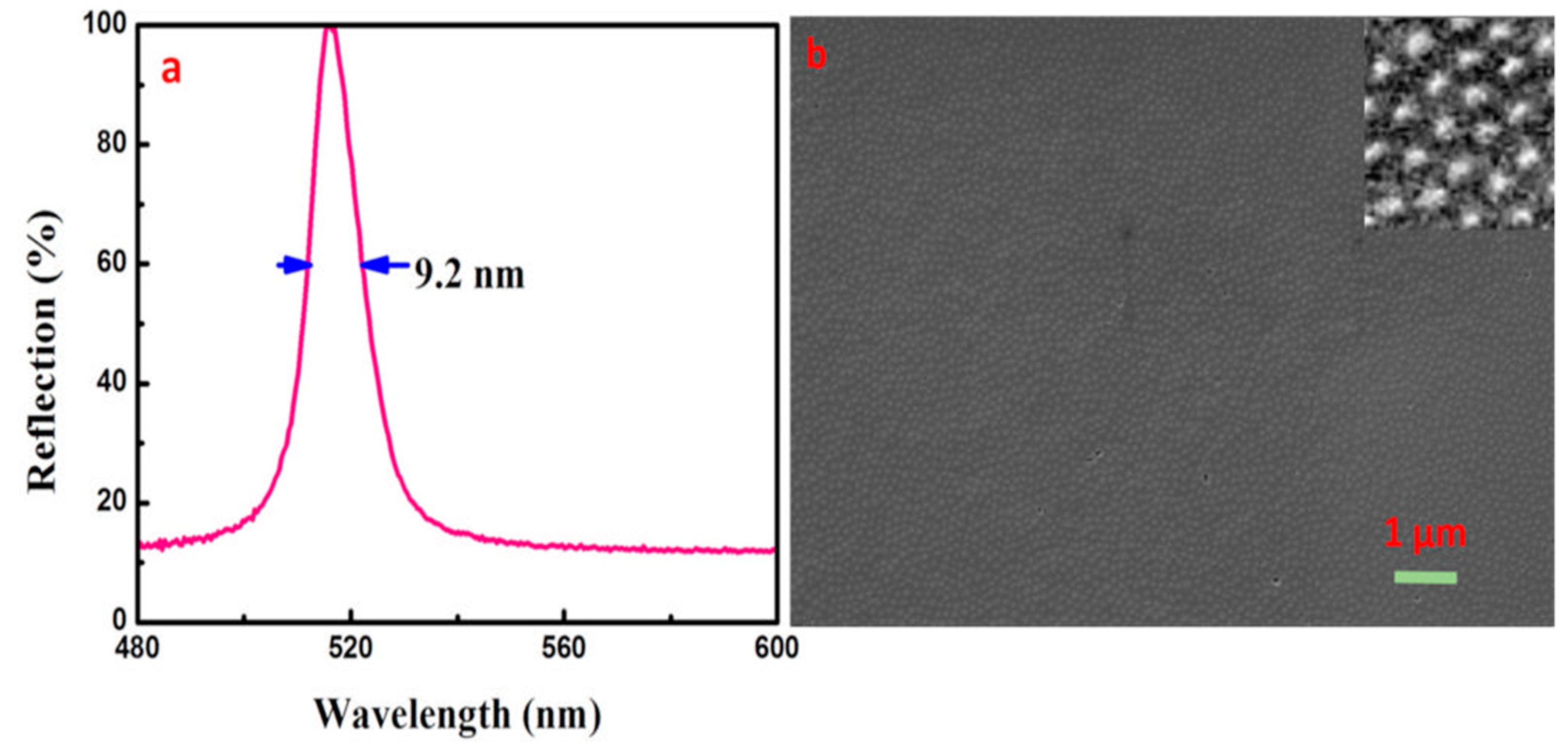
3.7. The Preparation of CCs with High Reflectivity and Narrow Reflection Bandwidth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef]
- Cho, A. High-Tech materials could render objects invisible. Science. 2006, 312, 1120. [Google Scholar] [CrossRef] [PubMed]
- Schurig, D.; Mock, J.J.; Justice, B.J.; Cummer, S.A.; Pendry, J.B.; Starr, A.F.; Smith, D.R. Metamaterial electromagnetic cloak at microwave frequencies. Science 2006, 314, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Mathias, J.P.; Seto, C.T. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Aryana, K.; Zanjani, M.B. Diamond family of colloidal supercrystals as phononic metamaterials. J. Appl. Phys. 2018, 123, 185103. [Google Scholar] [CrossRef]
- Zhou, X.; Pfeiffer, M.; Blochwitz, J.; Werner, A.; Nollau, A.; Fritz, T.; Leo, K. Very-low-operating-voltage organic light-emitting diodes using AP-doped amorphous hole injection layer. Appl. Phys. Lett. 2001, 78, 410–412. [Google Scholar] [CrossRef]
- Vos, W.L.; Sprik, R.; van Blaaderen, A.; Imhof, A.; Lagendijk, A.; Wegdam, G.H. Strong effects of photonic band structures on the diffraction of colloidal crystals. Phys. Rev. B 1996, 53, 16231. [Google Scholar] [CrossRef]
- Rogach, A.; Susha, A.; Caruso, F.; Sukhorukov, G.; Kornowski, A.; Kershaw, S.; Weller, H. Nano-and Microengineering: 3-D colloidal photonic crystals prepared from sub-μm-sized polystyrene latex spheres pre-coated with luminescent polyelectrolyte/nanocrystal shells. Adv. Mater. 2000, 12, 333–337. [Google Scholar] [CrossRef]
- Wijnhoven, J.E.; Vos, W.L. Preparation of photonic crystals made of air spheres in Titania. Science 1998, 281, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.; Xia, Y. Fabrication and characterization of chirped 3D photonic crystals. Adv. Mater. 2000, 12, 1329–1332. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V.L. Single-crystal colloidal multilayers of controlled thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]
- Gu, Z.Z.; Fujishima, A.; Sato, O. Fabrication of high-quality opal films with controllable thickness. Chem. Mater. 2002, 14, 760–765. [Google Scholar] [CrossRef]
- Hayward, R.C.; Saville, D.A.; Aksay, I.A. Electrophoretic assembly of colloidal crystals with optically tunable micropatterns. Nature 2000, 404, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Sheng, P.; Wen, W.; Wang, N.; Ma, H.; Lin, Z.; Zhang, W.Y.; Chan, C.T. Multiply coated microspheres. A platform for realizing fields-induced structural transition and photonic bandgap. Pure. Appl. Chem. 2000, 72, 309–315. [Google Scholar] [CrossRef]
- De Gennes, P.G. Soft Matter. Science 1992, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P.G. Soft matter. Rev. Mod. Phys. 1992, 64, 645. [Google Scholar] [CrossRef]
- Garbin, V.; Crocker, J.C.; Stebe, K.J. Nanoparticles at fluid interfaces: Exploiting capping ligands to control adsorption, stability and dynamics. J. Colloid. Interf. Sci. 2012, 387, 1–11. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, A.; Shi, L.; Ge, J. A polycrystalline SiO2 colloidal crystal film with ultra-narrow reflections. Chem. Commun. 2015, 51, 7382–7385. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Su, B.; Shi, L.; Wang, J.; Chen, S.; Jiang, L. Colloidal photonic crystals with narrow stopbands assembled from low-adhesive superhydrophobic substrates. J. Am. Chem. Soc. 2012, 134, 17053–17058. [Google Scholar] [CrossRef]
- Hatton, B.; Mishchenko, L.; Davis, S.; Sandhage, K.H.; Aizenberg, J. Assembly of large-area, highly ordered, crack-free inverse opal films. Proc. Natl. Acad. Sci. USA 2010, 107, 10354–10359. [Google Scholar] [CrossRef] [PubMed]
- Chabanov, A.A.; Jun, Y.; Norris, D.J. Avoiding cracks in self-assembled photonic band-gap crystals. Appl. Phys. Lett. 2004, 84, 3573–3575. [Google Scholar] [CrossRef]
- Biró, L.P.; Bálint, Z.; Kertész, K.; Vértesy, Z.; Márk, G.I.; Horváth, Z.E.; Vigneron, J.P. Role of photonic-crystal-type structures in the thermal regulation of a Lycaenid butterfly sister species pair. Phys. Rev. E 2003, 67, 021907. [Google Scholar] [CrossRef] [PubMed]
- Zi, J.; Yu, X.; Li, Y.; Hu, X.; Xu, C.; Wang, X.; Fu, R. Coloration strategies in peacock feathers. Proc. Natl. Acad. Sci. USA 2003, 100, 12576–12578. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.; Ham, A.D. Spectral reflectance and directional properties of structural coloration in bird plumage. J. Exp. Biol. 2002, 205, 2017–2027. [Google Scholar] [PubMed]
- Casey, M.T.; Scarlett, R.T.; Rogers, W.B.; Jenkins, I.; Sinno, T.; Crocker, J.C. Driving diffusionless transformations in colloidal crystals using DNA handshaking. Nat. Commun. 2012, 3, 1209. [Google Scholar] [CrossRef] [PubMed]
- Zanjani, M.B.; Jenkins, I.C.; Crocker, J.C.; Sinno, T. Colloidal cluster assembly into ordered superstructures via engineered directional binding. ACS Nano 2016, 10, 11280–11289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jenkins, I.C.; McGinley, J.T.; Sinno, T.; Crocker, J.C. Colloidal crystals with diamond symmetry at optical lengthscales. Nat. Commun. 2017, 8, 14173. [Google Scholar] [CrossRef] [PubMed]
- Ducrot, É.; Gales, J.; Yi, G.R.; Pine, D.J. Pyrochlore lattice, self-assembly and photonic band gap optimizations. Opt. Express. 2018, 26, 30052–30060. [Google Scholar] [CrossRef] [PubMed]
- Zanjani, M.B.; Crocker, J.C.; Sinno, T. Self-assembly with colloidal clusters: facile crystal design using connectivity landscape analysis. Soft matter 2017, 13, 7098–7105. [Google Scholar] [CrossRef] [PubMed]
- Ducrot, É.; He, M.; Yi, G.R.; Pine, D.J. Colloidal alloys with preassembled clusters and spheres. Nat. Mater. 2017, 16, 652. [Google Scholar] [CrossRef]
- Aryana, K.; Stahley, J.B.; Parvez, N.; Kim, K.; Zanjani, M.B. Superstructures of multielement colloidal molecules: efficient pathways to construct reconfigurable photonic and phononic crystals. Adv. Theory Simul. 2019, 1800198. [Google Scholar] [CrossRef]
- Kegel, W.K.; van Blaaderen, A. Direct observation of dynamical heterogeneities in colloidal hard-sphere suspensions. Science 2000, 287, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Phan, S.E.; Russel, W.B.; Cheng, Z.; Zhu, J.; Chaikin, P.M.; Dunsmuir, J.H.; Ottewill, R.H. Phase transition, equation of state, and limiting shear viscosities of hard sphere dispersions. Phys. Rev. E 1996, 54, 6633. [Google Scholar] [CrossRef]
- Pusey, P.N.; Zaccarelli, E.; Valeriani, C.; Sanz, E.; Poon, W.C.; Cates, M.E. Hard spheres: crystallization and glass formation. Philos. Trans. R. Soc. A 2009, 367, 4993–5011. [Google Scholar] [CrossRef] [PubMed]
- Yethiraj, A.; van Blaaderen, A. A colloidal model system with an interaction tunable from hard sphere to soft and dipolar. Nature 2003, 421, 513. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, E.; Kirsch, S.; Lindner, P.; Scherer, T.; Stölken, S. Spherical microgel colloids — hard spheres from soft matter. Ber. Bunsenges. Phys. Chem. 1998, 102, 1597–1602. [Google Scholar] [CrossRef]
- Eldridge, M.D.; Madden, P.A.; Frenkel, D. Entropy-driven formation of a superlattice in a hard-sphere binary mixture. Nature 1993, 365, 35–37. [Google Scholar] [CrossRef]
- Adams, M.; Dogic, Z.; Keller, S.L.; Fraden, S. Entropically driven microphase transitions in mixtures of colloidal rods and spheres. Nature 1998, 393, 349–352. [Google Scholar] [CrossRef]
- Lekkerkerker, H.N.; Stroobants, A. Colloids: Ordering entropy. Nature 1998, 393, 305–307. [Google Scholar] [CrossRef]
- Dinsmore, A.D.; Yodh, A.G.; Pine, D.J. Entropic control of particle motion using passive surface microstructures. Nature 1996, 383, 239–242. [Google Scholar] [CrossRef]
- Lin, K.H.; Crocker, J.C.; Prasad, V.; Schofield, A.; Weitz, D.A.; Lubensky, T.C.; Yodh, A.G. Entropically driven colloidal crystallization on patterned surfaces. Phys. Rev. Lett. 2000, 85, 1770. [Google Scholar] [CrossRef] [PubMed]
- Pusey, P.N.; Van Megen, W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature 1986, 320, 340–342. [Google Scholar] [CrossRef]
- Alder, B.J.; Wainwright, T. Phase transition for a hard sphere system. J. Chem. Phys. 1957, 27, 1208–1209. [Google Scholar] [CrossRef]
- Wood, W.W.; Jacobson, J.D. Preliminary results from a recalculation of the Monte Carlo equation of state of hard spheres. J. Chem. Phys. 1957, 27, 1207–1208. [Google Scholar] [CrossRef]
- Hoover, W.G.; Ree, F.H. Melting transition and communal entropy for hard spheres. J. Chem. Phys. 1968, 49, 3609–3617. [Google Scholar] [CrossRef]
- Woodcock, L.V. Entropy difference between the face-centred cubic and hexagonal close-packed crystal structures. Nature 1997, 385, 141. [Google Scholar] [CrossRef]
- Bolhuis, P.G.; Frenkel, D.M.S.C.; Mau, S.C.; Huse, D.A. Entropy difference between crystal phases. Nature 1997, 388, 235. [Google Scholar] [CrossRef]
- Bruce, A.D.; Wilding, N.B.; Ackland, G.J. Free energy of crystalline solids: a lattice-switch Monte Carlo method. Phys. Rev. Lett. 1997, 79, 3002. [Google Scholar] [CrossRef]
- Bolhuis, P.G.; Kofke, D.A. Monte Carlo study of freezing of polydisperse hard spheres. Phys. Rev. E 1996, 54, 634. [Google Scholar] [CrossRef]
- Fasolo, M.; Sollich, P. Equilibrium phase behavior of polydisperse hard spheres. Phys. Rev. Lett. 2003, 91, 068301. [Google Scholar] [CrossRef] [PubMed]
- Schöpe, H.J.; Bryant, G.; van Megen, W. Two-step crystallization kinetics in colloidal hard-sphere systems. Phys. Rev. Lett. 2006, 96, 175701. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, H. Effect of polydispersity on the relative stability of hard-sphere crystals. J. Chem. Phys. 2008, 128, 134510. [Google Scholar] [CrossRef] [PubMed]
- McRae, R.; Haymet, A.D.J. Freezing of polydisperse hard spheres. J. Chem. Phys. 1988, 88, 1114–1125. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Karmakar, S.; Dasgupta, C.; Krishnamurthy, H.R.; Sood, A.K. Equilibrium glassy phase in a polydisperse hard-sphere system. Phys. Rev. Lett. 2005, 95, 248301. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P. Fractionated crystallization in a polydisperse mixture of hard spheres. J. Chem. Phys. 1998, 109, 10970–10975. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, D.; Shao, G. Applied Surface Science and Technology; HIT Press: Harbin, China, 2000. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Xu, H.; Pan, M.; Zhao, J.; Li, Y.; Song, Y. Entropy-Induced Self-Assembly of Colloidal Crystals with High Reflectivity and Narrow Reflection Bandwidth. Entropy 2019, 21, 180. https://doi.org/10.3390/e21020180
Chen X, Xu H, Pan M, Zhao J, Li Y, Song Y. Entropy-Induced Self-Assembly of Colloidal Crystals with High Reflectivity and Narrow Reflection Bandwidth. Entropy. 2019; 21(2):180. https://doi.org/10.3390/e21020180
Chicago/Turabian StyleChen, Xiaoyi, Hongbo Xu, Mengyao Pan, Jiupeng Zhao, Yao Li, and Ying Song. 2019. "Entropy-Induced Self-Assembly of Colloidal Crystals with High Reflectivity and Narrow Reflection Bandwidth" Entropy 21, no. 2: 180. https://doi.org/10.3390/e21020180
APA StyleChen, X., Xu, H., Pan, M., Zhao, J., Li, Y., & Song, Y. (2019). Entropy-Induced Self-Assembly of Colloidal Crystals with High Reflectivity and Narrow Reflection Bandwidth. Entropy, 21(2), 180. https://doi.org/10.3390/e21020180





