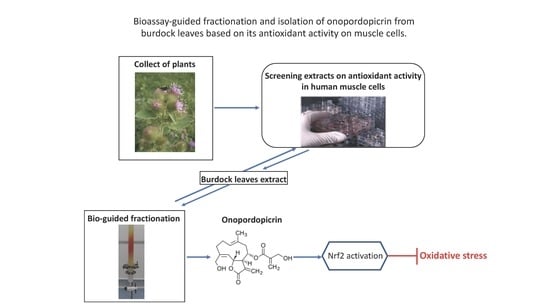
Identification of a Sesquiterpene Lactone from Arctium lappa Leaves with Antioxidant Activity in Primary Human Muscle Cells
Abstract
1. Introduction
2. Results
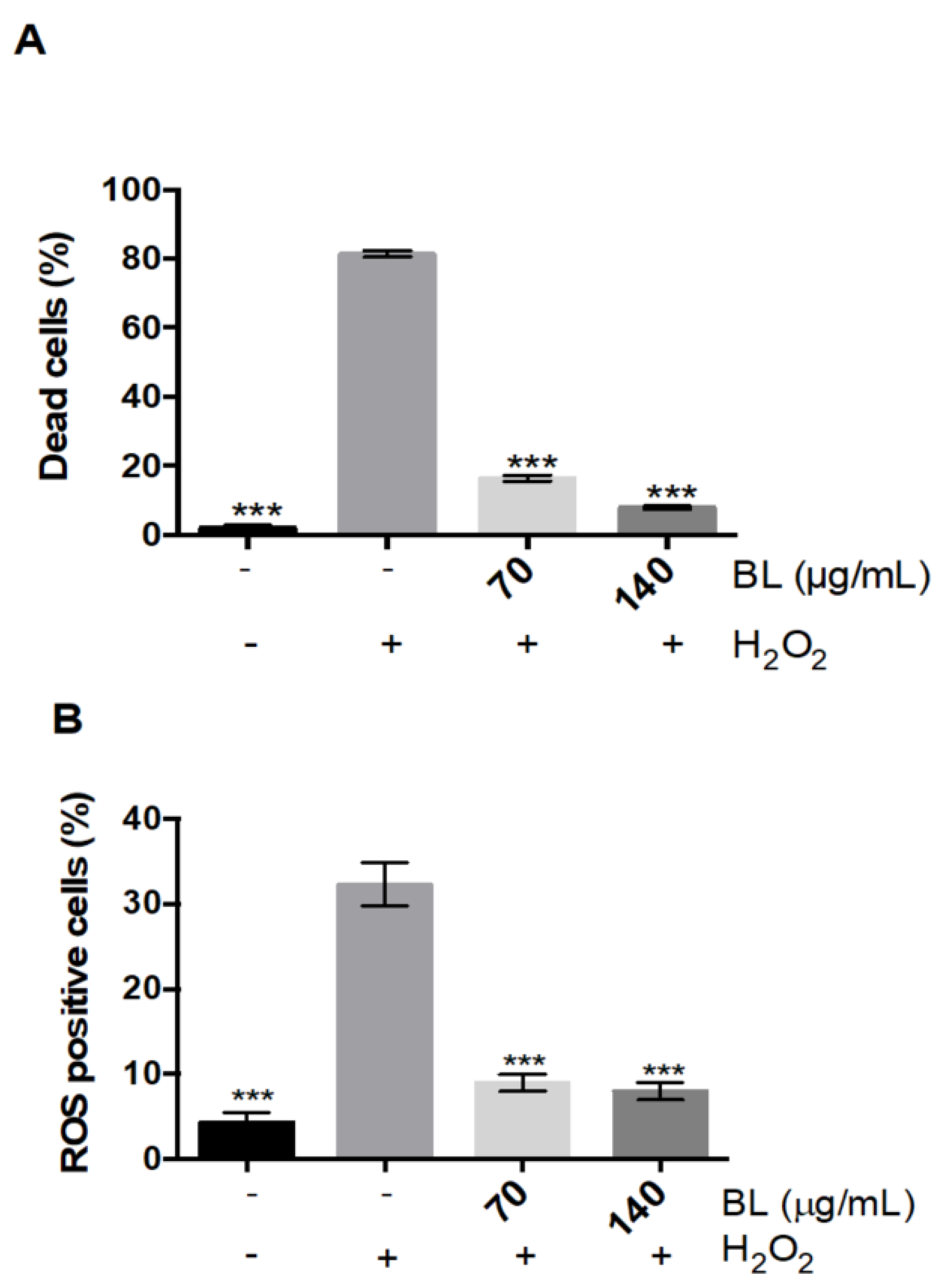
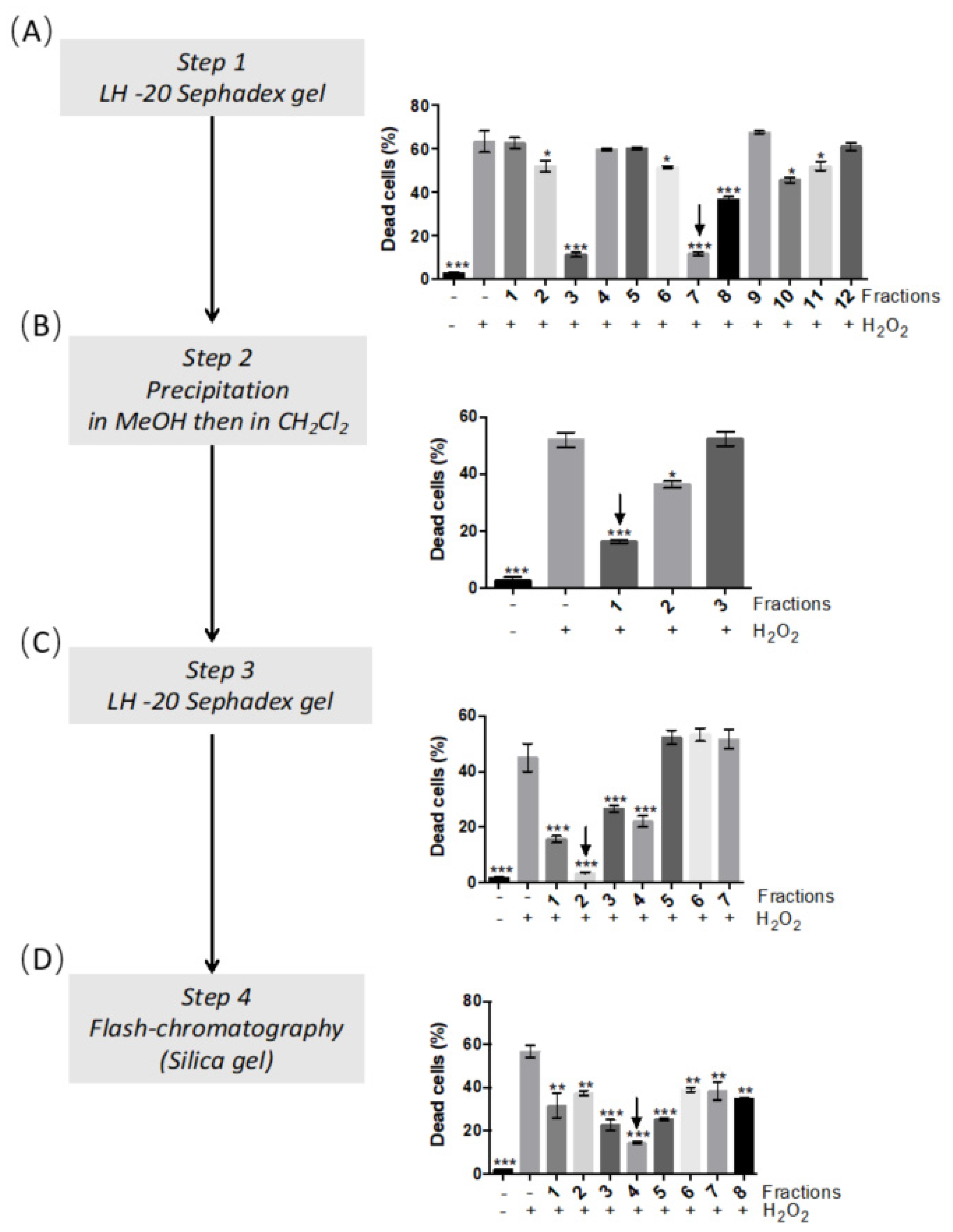
2.1. Antioxidant Activity of Arctium lappa Leaf Extract and Bioactivity-Guided Separation of Antioxidant Compounds
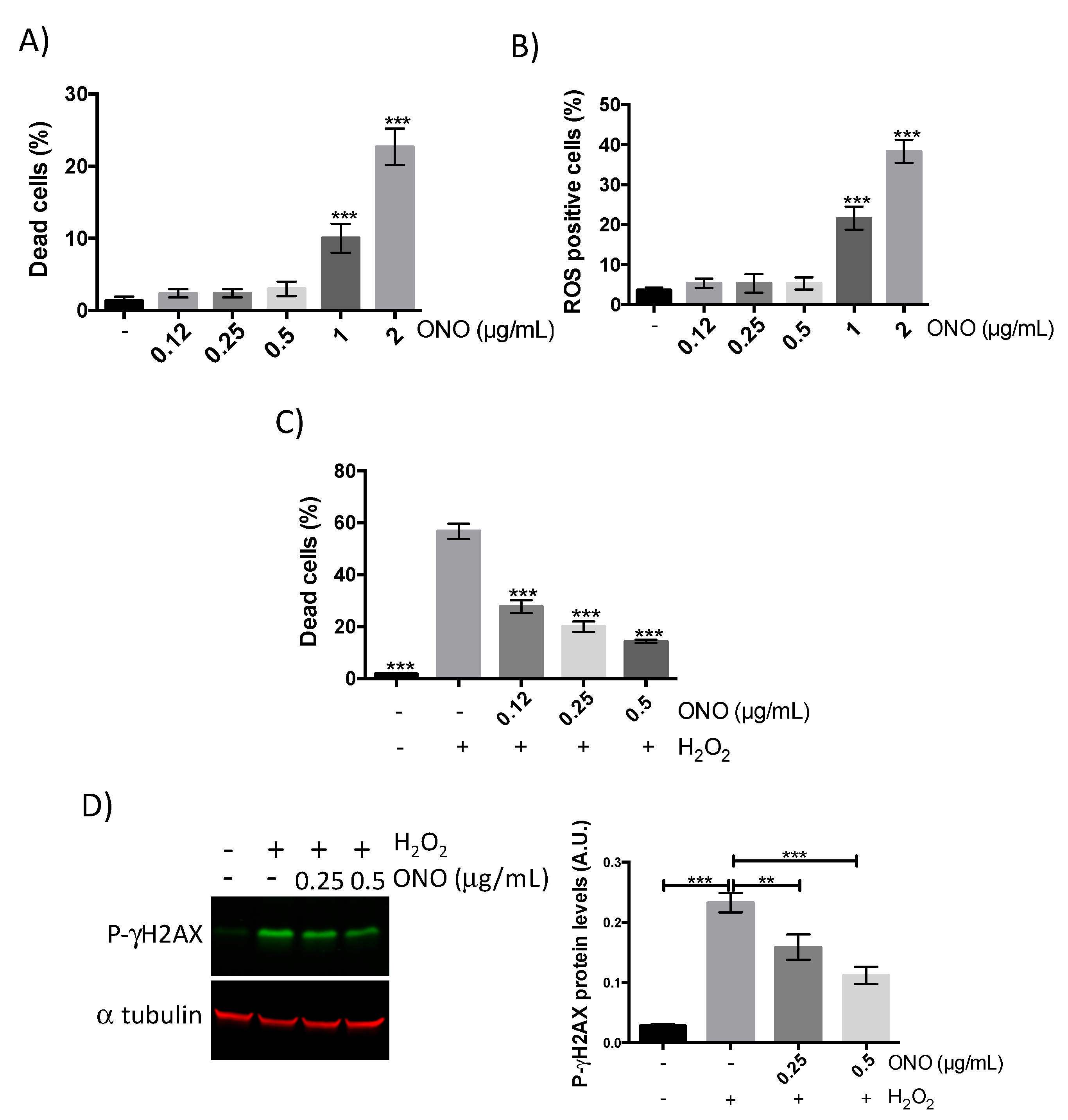
2.2. Antioxidant Capacity of the Sesquiterpene Lactone Onopordopicrin
2.2.1. Onopordopicrin Protects Human Myoblasts against H2O2-Induced Stress
2.2.2. Onopordopicrin Activates the Nrf2/HO-1 Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedure
4.2. Reagent and Standards
4.3. Plant Material
4.4. Extraction
4.5. Bioassay-Guided Isolation of Onopordopicrin from the Burdock Leaves Extract
4.6. High-Performance Liquid Chromatography (HPLC) Analysis
4.7. Primary Cultures of Human Myoblasts
4.8. Cell Death and ROS Quantification
4.9. siRNA Transfections
4.10. Western Blotting
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cacciapuoti, F. Oxidative stress as “mother” of many human diseases at strong clinical impact. J. Cardiovasc. Med. Cardiol. 2016, 3, 001–006. [Google Scholar] [CrossRef]
- Doria, E.; Buonocore, D.; Focarelli, A.; Marzatico, F. Relationship between human aging muscle and oxidative system pathway. Oxid. Med. Cell. Longev. 2012, 2012, 830257. [Google Scholar] [CrossRef]
- Canton, M.; Menazza, S.; Di Lisa, F. Oxidative stress in muscular dystrophy: From generic evidence to specific sources and targets. J. Muscle Res. Cell Motil. 2014, 35, 23–36. [Google Scholar] [CrossRef]
- Renjini, R.; Gayathri, N.; Nalini, A.; Srinivas Bharath, M.M. Oxidative damage in muscular dystrophy correlates with the severity of the pathology: Role of glutathione metabolism. Neurochem. Res. 2012, 37, 885–898. [Google Scholar] [CrossRef]
- Powers, S.K.; Morton, A.B.; Ahn, B.; Smuder, A.J. Redox Control of Skeletal Muscle Atrophy. Free Radic. Biol. Med. 2016, 98, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Ow, J.R.; Yang, N.D.; Taneja, R. Oxidative Stress-Mediated Skeletal Muscle Degeneration: Molecules, Mechanisms, and Therapies. Oxid. Med. Cell. Longev. 2016, 2016, 6842568. [Google Scholar] [CrossRef]
- Renault, V.; Thornell, L.E.; Butler-Browne, G.; Mouly, V. Human skeletal muscle satellite cells: Aging, oxidative stress and the mitotic clock. Exp. Gerontol. 2002, 37, 1229–1236. [Google Scholar] [CrossRef]
- Sacco, A.; Mourkioti, F.; Tran, R.; Choi, J.; Llewellyn, M.; Kraft, P.; Shkreli, M.; Delp, S.; Pomerantz, J.H.; Artandi, S.E.; et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 2010, 143, 1059–1071. [Google Scholar] [CrossRef]
- Aguennouz, M.; Vita, G.L.; Messina, S.; Cama, A.; Lanzano, N.; Ciranni, A.; Rodolico, C.; Di Giorgio, R.M.; Vita, G. Telomere shortening is associated to TRF1 and PARP1 overexpression in Duchenne muscular dystrophy. Neurobiol. Aging 2011, 32, 2190–2197. [Google Scholar] [CrossRef]
- Di Foggia, V.; Zhang, X.; Licastro, D.; Gerli, M.F.; Phadke, R.; Muntoni, F.; Mourikis, P.; Tajbakhsh, S.; Ellis, M.; Greaves, L.C.; et al. Bmi1 enhances skeletal muscle regeneration through MT1-mediated oxidative stress protection in a mouse model of dystrophinopathy. J. Exp. Med. 2014, 211, 2617–2633. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 2008, 101, 14D–19D. [Google Scholar] [CrossRef] [PubMed]
- Crespo, F.L.; Sobrado, V.R.; Gomez, L.; Cervera, A.M.; McCreath, K.J. Mitochondrial reactive oxygen species mediate cardiomyocyte formation from embryonic stem cells in high glucose. Stem Cells 2010, 28, 1132–1142. [Google Scholar] [CrossRef]
- Ding, Y.; Choi, K.J.; Kim, J.H.; Han, X.; Piao, Y.; Jeong, J.H.; Choe, W.; Kang, I.; Ha, J.; Forman, H.J.; et al. Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phosphatidylinositol 3-kinase during muscle differentiation. Am. J. Pathol. 2008, 172, 1529–1541. [Google Scholar] [CrossRef]
- Krieger-Brauer, H.I.; Kather, H. Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochem. J. 1995, 307, 549–556. [Google Scholar] [CrossRef]
- Li, J.; Stouffs, M.; Serrander, L.; Banfi, B.; Bettiol, E.; Charnay, Y.; Steger, K.; Krause, K.H.; Jaconi, M.E. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol. Biol. Cell 2006, 17, 3978–3988. [Google Scholar] [CrossRef]
- Won, H.; Lim, S.; Jang, M.; Kim, Y.; Rashid, M.A.; Jyothi, K.R.; Dashdorj, A.; Kang, I.; Ha, J.; Kim, S.S. Peroxiredoxin-2 upregulated by NF-kappaB attenuates oxidative stress during the differentiation of muscle-derived C2C12 cells. Antioxid. Redox Signal. 2012, 16, 245–261. [Google Scholar] [CrossRef]
- Xiao, R.; Ferry, A.L.; Dupont-Versteegden, E.E. Cell death-resistance of differentiated myotubes is associated with enhanced anti-apoptotic mechanisms compared to myoblasts. Apoptosis 2011, 16, 221–234. [Google Scholar] [CrossRef]
- Miazga-Karska, M.; Michalak, K.; Ginalska, G. Anti-Acne Action of Peptides Isolated from Burdock Root-Preliminary Studies and Pilot Testing. Molecules 2020, 25, 2027. [Google Scholar] [CrossRef]
- Maghsoumi-Norouzabad, L.; Alipoor, B.; Abed, R.; Eftekhar Sadat, B.; Mesgari-Abbasi, M.; Asghari Jafarabadi, M. Effects of Arctium lappa L. (Burdock) root tea on inflammatory status and oxidative stress in patients with knee osteoarthritis. Int. J. Rheum Dis. 2016, 19, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.; Leung, G.P.; Yu, P.H.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2010, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Barrea, L.; Ciampaglia, R.; Cicala, C.; Arnone, A.; Savastano, S.; Nabavi, S.M.; Tenore, G.C.; Novellino, E. Arctium lappa contributes to the management of type 2 diabetes mellitus by regulating glucose homeostasis and improving oxidative stress: A critical review of in vitro and in vivo animal-based studies. Phytother. Res. 2019, 33, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Badarau, A.S.; Swamy, M.K.; Shaw, S.; Maggi, F.; da Silva, L.E.; Lopez, V.; Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Arctium Species Secondary Metabolites Chemodiversity and Bioactivities. Front. Plant. Sci. 2019, 10, 834. [Google Scholar] [CrossRef]
- He, Y.; Fan, Q.; Cai, T.; Huang, W.; Xie, X.; Wen, Y.; Shi, Z. Molecular mechanisms of the action of Arctigenin in cancer. Biomed. Pharm. 2018, 108, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects †A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Franco, R.R.; da Silva Carvalho, D.B.; de Moura, F.B.R.; Justino, A.B.; Silva, H.C.G.; Peixoto, L.G.; Espindola, F.S. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. J. Ethnopharmacol. 2018, 215, 140–146. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Li, J.; Chen, S.; Zhu, S.; Ma, C.; Wang, Z. Antioxidant activity and chemical composition of the fractions from burdock leaves. J. Food Sci. 2010, 75, C413–C419. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, Y.R.; Shim, J.; Choi, Y.S.; Kim, Y.T.; Kim, M.K.; Kim, M.J. Suppressive Effect of Arctium Lappa L. Leaves on Retinal Damage Against A2E-Induced ARPE-19 Cells and Mice. Molecules 2020, 25, 1737. [Google Scholar] [CrossRef]
- Jean, E.; Laoudj-Chenivesse, D.; Notarnicola, C.; Rouger, K.; Serratrice, N.; Bonnieu, A.; Gay, S.; Bacou, F.; Duret, C.; Carnac, G. Aldehyde dehydrogenase activity promotes survival of human muscle precursor cells. J. Cell Mol. Med. 2011, 15, 119–133. [Google Scholar] [CrossRef]
- de Almeida, A.B.; Luiz-Ferreira, A.; Cola, M.; Di Pietro Magri, L.; Batista, L.M.; de Paiva, J.A.; Trigo, J.R.; Souza-Brito, A.R. Anti-ulcerogenic mechanisms of the sesquiterpene lactone onopordopicrin-enriched fraction from Arctium lappa L. (Asteraceae): Role of somatostatin, gastrin, and endogenous sulfhydryls and nitric oxide. J. Med. Food 2012, 15, 378–383. [Google Scholar] [CrossRef]
- Machado, F.B.; Yamamoto, R.E.; Zanoli, K.; Nocchi, S.R.; Novello, C.R.; Schuquel, I.T.; Sakuragui, C.M.; Luftmann, H.; Ueda-Nakamura, T.; Nakamura, C.V.; et al. Evaluation of the antiproliferative activity of the leaves from Arctium lappa by a bioassay-guided fractionation. Molecules 2012, 17, 1852. [Google Scholar] [CrossRef]
- Barbosa Filho, J.M.; Costa, M. Isolation of onopordopicrin, the toxic constituent of Arctium lappa L. J. Brazyl. Chem. Soc. 1993, 4, 186–187. [Google Scholar] [CrossRef]
- Drozdz, B.; Holub, M.; Samek, V.; Sorm, F. On terpenes. CXCII. The constitution and absolute configuration of onopordopircine. A sesquiterpenic lactone from Onopordon acanthium L. Collect. Czech. Chem. Commun. 1968, 33, 1730–1737. [Google Scholar] [CrossRef]
- Zimmermann, S.; Thomi, S.; Kaiser, M.; Hamburger, M.; Adams, M. Screening and HPLC-Based Activity Profiling for New Antiprotozoal Leads from European Plants. Sci. Pharm. 2012, 80, 205–213. [Google Scholar] [CrossRef]
- Formisano, C.; Sanna, C.; Ballero, M.; Chianese, G.; Sirignano, C.; Rigano, D.; Millan, E.; Munoz, E.; Taglialatela-Scafati, O. Anti-inflammatory sesquiterpene lactones from Onopordum illyricum L. (Asteraceae), an Italian medicinal plant. Fitoterapia 2017, 116, 61–65. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev. Pharm. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Kourakis, S.; Timpani, C.A.; de Haan, J.B.; Gueven, N.; Fischer, D.; Rybalka, E. Dimethyl Fumarate and Its Esters: A Drug with Broad Clinical Utility? Pharmaceuticals 2020, 13, 306. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef]
- Jurasek, M.; Rimpelova, S.; Kmonickova, E.; Drasar, P.; Ruml, T. Tailor-made fluorescent trilobolide to study its biological relevance. J. Med. Chem. 2014, 57, 7947–7954. [Google Scholar] [CrossRef]
- Rimpelova, S.; Jurasek, M.; Peterkova, L.; Bejcek, J.; Spiwok, V.; Majdl, M.; Jirasko, M.; Budesinsky, M.; Harmatha, J.; Kmonickova, E.; et al. Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica. Beilstein J. Org. Chem. 2019, 15, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity of crop residues from Burdock and an active substance. J. Environ. Sci. Health B 2019, 54, 877–882. [Google Scholar] [CrossRef]
- Moricz, A.M.; Kruzselyi, D.; Alberti, A.; Darcsi, A.; Horvath, G.; Csontos, P.; Beni, S.; Ott, P.G. Layer chromatography-bioassays directed screening and identification of antibacterial compounds from Scotch thistle. J. Chromatogr. A 2017, 1524, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, A.; Frederich, M.; Ledoux, A.; Campos, P.E.; Clerc, P.; Hermann, T.; Quetin-Leclercq, J.; Cieckiewicz, E. In vitro antiplasmodial and cytotoxic activities of sesquiterpene lactones from Vernonia fimbrillifera Less. (Asteraceae). Nat. Prod. Res. 2017, 32, 1463–1466. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Noh, J.S.; Jin, C.Y.; Kim, G.Y.; Hyun, J.W.; Leem, S.H.; Choi, Y.H. Hemistepsin A protects human keratinocytes against hydrogen peroxide-induced oxidative stress through activation of the Nrf2/HO-1 signaling pathway. Arch. Biochem. Biophys. 2020, 691, 108512. [Google Scholar] [CrossRef]
- Peterkova, L.; Kmonickova, E.; Ruml, T.; Rimpelova, S. Sarco/Endoplasmic Reticulum Calcium ATPase Inhibitors: Beyond Anticancer Perspective. J. Med. Chem. 2020, 63, 1937–1963. [Google Scholar] [CrossRef]
- Morel, S.; Saint, N.; Vitou, M.; Cicero, A.L.; Nissan, X.; Vernus, B.; Chabi, B.; Bonnieu, A.; Hugon, G.; Fons, F.o.; et al. The abietane diterpene taxodione contributes to the antioxidant activity of rosemary by-product in muscle tissue. J. Funct. Foods 2019, 62, 103565. [Google Scholar] [CrossRef]
- Beauchamp, J.R.; Morgan, J.E.; Pagel, C.N.; Partridge, T.A. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J. Cell Biol. 1999, 144, 1113–1122. [Google Scholar] [CrossRef]
- Drowley, L.; Okada, M.; Beckman, S.; Vella, J.; Keller, B.; Tobita, K.; Huard, J. Cellular antioxidant levels influence muscle stem cell therapy. Molcules 2010, 18, 1865–1873. [Google Scholar] [CrossRef]
- Rodriguez-Porcel, M.; Gheysens, O.; Paulmurugan, R.; Chen, I.Y.; Peterson, K.M.; Willmann, J.K.; Wu, J.C.; Zhu, X.; Lerman, L.O.; Gambhir, S.S. Antioxidants improve early survival of cardiomyoblasts after transplantation to the myocardium. Mol. Imaging Biol. 2010, 12, 325–334. [Google Scholar] [CrossRef][Green Version]
- Suzuki, K.; Murtuza, B.; Beauchamp, J.R.; Smolenski, R.T.; Varela-Carver, A.; Fukushima, S.; Coppen, S.R.; Partridge, T.A.; Yacoub, M.H. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 2004, 18, 1153–1155. [Google Scholar] [CrossRef]
- El Haddad, M.; Jean, E.; Turki, A.; Hugon, G.; Vernus, B.; Bonnieu, A.; Passerieux, E.; Hamade, A.; Mercier, J.; Laoudj-Chenivesse, D.; et al. Glutathione peroxidase 3, a new retinoid target gene, is crucial for human skeletal muscle precursor cell survival. J. Cell Sci. 2012, 125, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Gussoni, E.; Blau, H.M.; Kunkel, L.M. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat. Med. 1997, 3, 970–977. [Google Scholar] [CrossRef]
- Mendell, J.R.; Kissel, J.T.; Amato, A.A.; King, W.; Signore, L.; Prior, T.W.; Sahenk, Z.; Benson, S.; McAndrew, P.E.; Rice, R.; et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N. Engl. J. Med. 1995, 333, 832–838. [Google Scholar] [CrossRef]
- Partridge, T.A.; Morgan, J.E.; Coulton, G.R.; Hoffman, E.P.; Kunkel, L.M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 1989, 337, 176–179. [Google Scholar] [CrossRef]
- Tremblay, J.P.; Malouin, F.; Roy, R.; Huard, J.; Bouchard, J.P.; Satoh, A.; Richards, C.L. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transpl. 1993, 2, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Montarras, D.; L’Honore, A.; Buckingham, M. Lying low but ready for action: The quiescent muscle satellite cell. FEBS J. 2013, 280, 4036–4050. [Google Scholar] [CrossRef]
- Dang, X.; He, B.; Ning, Q.; Liu, Y.; Guo, J.; Niu, G.; Chen, M. Alantolactone suppresses inflammation, apoptosis and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-kappaB pathways. Respir. Res. 2020, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Zhu, Z. Parthenolide inhibits hydrogen peroxideinduced osteoblast apoptosis. Mol. Med. Rep. 2018, 17, 8369–8376.63. [Google Scholar]
- Kim, C.Y.; Kang, B.; Suh, H.J.; Choi, H.S. Parthenolide, a feverfew-derived phytochemical, ameliorates obesity and obesity-induced inflammatory responses via the Nrf2/Keap1 pathway. Pharmacol. Res. 2019, 145, 104259. [Google Scholar] [CrossRef]
- Siedle, B.; Garcia-Pineres, A.J.; Murillo, R.; Schulte-Monting, J.; Castro, V.; Rungeler, P.; Klaas, C.A.; Da Costa, F.B.; Kisiel, W.; Merfort, I. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-kappaB. J. Med. Chem. 2004, 47, 6042–6054. [Google Scholar] [CrossRef]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2 by costunolide provides neuroprotective effect in PC12 cells. Food Funct. 2019, 10, 4143–4152. [Google Scholar] [CrossRef]
- Gach, K.; Dlugosz, A.; Janecka, A. The role of oxidative stress in anticancer activity of sesquiterpene lactones. Naunyn Schmiedebergs Arch. Pharm. 2015, 388, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Won, Y.K.; Ong, C.N.; Shen, H.M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer Agents 2005, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Kitzmann, M.; Bonnieu, A.; Duret, C.; Vernus, B.; Barro, M.; Laoudj-Chenivesse, D.; Verdi, J.M.; Carnac, G. Inhibition of Notch signaling induces myotube hypertrophy by recruiting a subpopulation of reserve cells. J. Cell Physiol. 2006, 208, 538–548. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khatib, N.; Morel, S.; Hugon, G.; Rapior, S.; Carnac, G.; Saint, N. Identification of a Sesquiterpene Lactone from Arctium lappa Leaves with Antioxidant Activity in Primary Human Muscle Cells. Molecules 2021, 26, 1328. https://doi.org/10.3390/molecules26051328
El Khatib N, Morel S, Hugon G, Rapior S, Carnac G, Saint N. Identification of a Sesquiterpene Lactone from Arctium lappa Leaves with Antioxidant Activity in Primary Human Muscle Cells. Molecules. 2021; 26(5):1328. https://doi.org/10.3390/molecules26051328
Chicago/Turabian StyleEl Khatib, Nour, Sylvie Morel, Gérald Hugon, Sylvie Rapior, Gilles Carnac, and Nathalie Saint. 2021. "Identification of a Sesquiterpene Lactone from Arctium lappa Leaves with Antioxidant Activity in Primary Human Muscle Cells" Molecules 26, no. 5: 1328. https://doi.org/10.3390/molecules26051328
APA StyleEl Khatib, N., Morel, S., Hugon, G., Rapior, S., Carnac, G., & Saint, N. (2021). Identification of a Sesquiterpene Lactone from Arctium lappa Leaves with Antioxidant Activity in Primary Human Muscle Cells. Molecules, 26(5), 1328. https://doi.org/10.3390/molecules26051328