Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano
Abstract
1. Introduction
2. Environmentally Friendly Techniques in the Extraction of Polyphenols
2.1. Microwave-Assisted Extraction (MAE)
2.2. Ultrasound-Assisted Extraction (UAE)
2.3. Pulsed Electric Field Extraction (PEF) and High-Voltage Electrical Discharges (HVED)
2.4. Enzyme-Assisted Extraction (EAE)
2.5. High Hydrostatic Pressure Extration (HHPE)
2.6. Deep Eutectic Solvents (DESs)
3. Application of Environmentally Friendly Techniques in Some Food Species
3.1. Clove (Syzygium aromaticum)
3.1.1. MAE
3.1.2. UAE
3.1.3. DESs
3.2. Green Tea (Camellia sinensis)
3.2.1. MAE
3.2.2. UAE
3.2.3. PEF
3.2.4. HHPEE
3.2.5. DESs
3.3. Oregano (Origanum vulgare L.)
MAE
3.4. Rosemary (Rosmarinus officinalis L.)
3.4.1. MAE
3.4.2. UAE
3.4.3. EAE
3.4.4. DESs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahidi, F. Antioxidants: Principles and Applications. In Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–14. [Google Scholar]
- Dasgupta, A.; Klein, K. Fruits, Vegetables, and Nuts. In Antioxidants in Food, Vitamins and Supplements; Elsevier: San Diego, CA, USA, 2014; pp. 209–235. [Google Scholar]
- Arias, M.; Penichet, I.; Ysambertt, F.; Bauza, R.; Zougagh, M.; Ríos, Á. Fast supercritical fluid extraction of low- and high-density polyethylene additives: Comparison with conventional reflux and automatic Soxhlet extraction. J. Supercrit. Fluids 2009, 50, 22–28. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Molina, G.A.; González-Fuentes, F.; Loske, A.M.; Fernández, F.; Estevez, M. Shock wave-assisted extraction of phenolic acids and flavonoids from Eysenhardtia polystachya heartwood: A novel method and its comparison with conventional methodologies. Ultrason. Sonochem. 2020, 61, 104809. [Google Scholar] [CrossRef]
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M.J. Assisted extraction of rosemary antioxidants with green solvents. J. Food Eng. 2012, 109, 98–103. [Google Scholar] [CrossRef]
- Silva, A.S.; Reboredo-Rodríguez, P.; Sanchez-Machado, D.I.; López-Cervantes, J.; Barreca, D.; Pittala, V.; Samec, D.; Orhan, I.E.; Gulcan, H.O.; Forbes-Hernandez, T.Y.; et al. Evaluation of the status quo of polyphenols analysis: Part II—Analysis methods and food processing effects. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3219–3240. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Extraction of antioxidant compounds from Jabuticaba (Myrciaria cauliflora) skins: Yield, composition and economical evaluation. J. Food Eng. 2010, 101, 23–31. [Google Scholar] [CrossRef]
- Poompavai, S.; Sree, V.G. Anti-proliferative Efficiency of Pulsed Electric Field Treated Curcuma Longa (Turmeric) Extracts on Breast Cancer Cell Lines. IETE J. Res. 2020, 1–15. [Google Scholar] [CrossRef]
- Moreira, S.A.; Silva, S.; Costa, E.; Pinto, S.; Sarmento, B.; Saraiva, J.A.; Pintado, M. Effect of High Hydrostatic Pressure Extraction on Biological Activities and Phenolics Composition of Winter Savory Leaf Extracts. Antioxidants 2020, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Lovrić, V.; Putnik, P.; Kovačević, D.B.; Jukić, M.; Dragović-Uzelac, V. Effect of Microwave-Assisted Extraction on the Phenolic Compounds and Antioxidant Capacity of Blackthorn Flowers. Food Technol. Biotechnol. 2017, 55, 243–250. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and nonconventional extraction techniques for optimal extraction processes of rosmarinic acid from six Lamiaceae plants as determined by HPLC-DAD measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef]
- Bimakr, M.; Ganjloo, A.; Zarringhalami, S.; Ansarian, E. Ultrasound-assisted extraction of bioactive compounds from Malva sylvestris leaves and its comparison with agitated bed extraction technique. Food Sci. Biotechnol. 2017, 26, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.-U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Alcántara, C.; Žugčić, T.; Abdelkebir, R.; García-Pérez, J.V.; Jambrak, A.R.; Lorenzo, J.M.; Collado, M.C.; Granato, D.; Barba, F.J. Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules 2020, 25, 1718. [Google Scholar] [CrossRef]
- Miljanović, A.; Bielen, A.; Grbin, D.; Marijanović, Z.; Andlar, M.; Rezić, T.; Roca, S.; Jerković, I.; Vikić-Topić, D.; Dent, M. Effect of Enzymatic, Ultrasound, and Reflux Extraction Pretreatments on the Yield and Chemical Composition of Essential Oils. Molecules 2020, 25, 4818. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, A.; Celeiro, M.; Jozinović, A.; Jelinić, J.; Kovač, T.; Jokić, S.; Babić, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef]
- Moghaddam, T.N.; Elhamirad, A.H.; Asl, M.R.S.; Noghabi, M.S. Pulsed electric field-assisted extraction of phenolic antioxidants from tropical almond red leaves. Chem. Pap. 2020, 74, 3957–3961. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass-Valorization 2019, 10, 889–897. [Google Scholar] [CrossRef]
- Liu, Z.; Esveld, E.; Vincken, J.-P.; Bruins, M.E. Pulsed Electric Field as an Alternative Pre-treatment for Drying to Enhance Polyphenol Extraction from Fresh Tea Leaves. Food Bioprocess Technol. 2019, 12, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-W.; Zeng, X.-A.; Ngadi, M. Enhanced extraction of phenolic compounds from onion by pulsed electric field (PEF). J. Food Process. Preserv. 2018, 42, e13755. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-Assisted Extraction of Flavorings and Colorants from Plant Materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef]
- Macedo, G.A.; Santana, Á.L.; Crawford, L.M.; Wang, S.C.; Dias, F.F.G.; de Moura Bell, J.M.L.N. Integrated microwave- and enzyme-assisted extraction of phenolic compounds from olive pomace. LWT 2021, 138, 110621. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Amarowicz, R.; Kaur, C. Evaluation of Cellulolytic Enzyme-Assisted Microwave Extraction of Punica granatum Peel Phenolics and Antioxidant Activity. Plant Foods Hum. Nutr. 2020, 75, 614–620. [Google Scholar] [CrossRef]
- Scepankova, H.; Martins, M.; Estevinho, L.; Delgadillo, I.; Saraiva, J.A. Enhancement of Bioactivity of Natural Extracts by Non-Thermal High Hydrostatic Pressure Extraction. Plant Foods Hum. Nutr. 2018, 73, 253–267. [Google Scholar] [CrossRef]
- Duarte, K.; Justino, C.I.L.; Gomes, A.M.; Rocha-Santos, T.; Duarte, A.C. Green Analytical Methodologies for Preparation of Extracts and Analysis of Bioactive Compounds. In Analysis of Marine Samples in Search of Bioactive Compounds; Elsevier: Kidlington, UK, 2014; Volume 65, pp. 59–78. [Google Scholar]
- De La Guardia, M.; Armenta, S. Greening Sample Treatments. In Advances in Ion Mobility-Mass Spectrometry: Fundamentals, Instrumentation and Applications; Elsevier: Amsterdam, The Netherland, 2011; Volume 57, pp. 87–120. [Google Scholar]
- Jun, X. High-pressure processing as emergent technology for the extraction of bioactive ingredients from plant materials. Crit. Rev. Food Sci. Nutr. 2013, 53, 837–852. [Google Scholar] [CrossRef]
- Rivalain, N.; Roquain, J.; Demazeau, G. Development of high hydrostatic pressure in biosciences: Pressure effect on biological structures and potential applications in Biotechnologies. Biotechnol. Adv. 2010, 28, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Briones-Labarca, V.; Giovagnoli-Vicuña, C.; Cañas-Sarazúa, R. Optimization of extraction yield, flavonoids and lycopene from tomato pulp by high hydrostatic pressure-assisted extraction. Food Chem. 2019, 278, 751–759. [Google Scholar] [CrossRef]
- Grassino, A.N.; Pedisić, S.; Dragović-Uzelac, V.; Karlović, S.; Ježek, D.; Bosiljkov, T. Insight into High-Hydrostatic Pressure Extraction of Polyphenols from Tomato Peel Waste. Plant Foods Hum. Nutr. 2020, 75, 427–433. [Google Scholar] [CrossRef]
- Jamaludin, R.; Kim, D.-S.; Salleh, L.M.; Lim, S.-B. Optimization of high hydrostatic pressure extraction of bioactive compounds from noni fruits. J. Food Meas. Charact. 2020, 14, 2810–2818. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Coelho, M.A.Z.; Rosenthal, A.; Gottschalk, L.M.F.; Tonon, R.V. Combination of enzyme-assisted extraction and high hydrostatic pressure for phenolic compounds recovery from grape pomace. J. Food Eng. 2021, 288, 110128. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Giovagnoli-Vicuña, C.; Chacana-Ojeda, M. High pressure extraction increases the antioxidant potential and in vitro bio-accessibility of bioactive compounds from discarded blueberries. CyTA-J. Food 2019, 17, 622–631. [Google Scholar] [CrossRef]
- Ratti, R. Ionic Liquids: Synthesis and Applications in Catalysis. Adv. Chem. 2014, 2014, 729842. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; de la Guardia, M. Are deep eutectic solvents useful in chromatography? A short review. J. Chromatogr. A 2021, 1639, 461918. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Plastiras, O.-E.; Andreasidou, E.; Samanidou, V. Microextraction Techniques with Deep Eutectic Solvents. Molecules 2020, 25, 6026. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.T.; Rodrigues, R.F.; Machado, N.M.; Maurer, L.H.; Ferreira, L.F.; Somacal, S.; da Veiga, M.L.; Rocha, M.I.d.U.M.d.; Vizzotto, M.; Rodrigues, E.; et al. Natural deep eutectic solvent (NADES)-based blueberry extracts protect against ethanol-induced gastric ulcer in rats. Food Res. Int. 2020, 138, 109718. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; An, Q.; Sun, H.; Mao, M. Application of ultrasound-assisted deep eutectic solvent extraction combined with liquid-liquid extraction method to the extraction of three pesticide residues from fruit and vegetable samples. Acta Chromatogr. 2020, 1–7. [Google Scholar] [CrossRef]
- Barbieri, J.B.; Goltz, C.; Cavalheiro, F.B.; Toci, A.T.; Igarashi-Mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crop. Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.-R.; Li, H.-B.; Wu, D.-T.; Geng, F.; Corke, H.; Wei, X.-L.; Gan, R.-Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef]
- Alexandru, L.; Cravotto, G.; Giordana, L.; Binello, A.; Chemat, F. Ultrasound-assisted extraction of clove buds using batch- and flow-reactors: A comparative study on a pilot scale. Innov. Food Sci. Emerg. Technol. 2013, 20, 167–172. [Google Scholar] [CrossRef]
- Taşkın, B.; Özbek, Z.A. Optimisation of microwave effect on bioactives contents and colour attributes of aqueous green tea extracts by central composite design. J. Food Meas. Charact. 2020, 14, 2240–2252. [Google Scholar] [CrossRef]
- Sanz, V.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Clean technologies applied to the recovery of bioactive extracts from Camellia sinensis leaves agricultural wastes. Food Bioprod. Process. 2020, 122, 214–221. [Google Scholar] [CrossRef]
- Vural, N.; Cavuldak, Ö.A.; Akay, M.A.; Anlı, R.E. Determination of the various extraction solvent effects on polyphenolic profile and antioxidant activities of selected tea samples by chemometric approach. J. Food Meas. Charact. 2020, 14, 1286–1305. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.S.; Keum, Y.-S. Total phenolics, antioxidant, antitumor, and enzyme inhibitory activity of Indian medicinal and aromatic plants extracted with different extraction methods. 3 Biotech 2017, 7, 76. [Google Scholar] [CrossRef]
- Psarrou, I.; Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction Kinetics of Phenolic Antioxidants from the Hydro Distillation Residues of Rosemary and Effect of Pretreatment and Extraction Parameters. Molecules 2020, 25, 4520. [Google Scholar] [CrossRef]
- Calinescu, I.; Asofiei, I.; Gavrila, A.I.; Trifan, A.; Ighigeanu, D.; Martin, D.; Matei, C.; Buleandra, M. Integrating Microwave-Assisted Extraction of Essential Oils and Polyphenols from Rosemary and Thyme Leaves. Chem. Eng. Commun. 2017, 204, 965–973. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Alfikri, F.N.; Pujiarti, R.; Wibisono, M.G.; Hardiyanto, E.B. Yield, Quality, and Antioxidant Activity of Clove (Syzygium aromaticum L.) Bud Oil at the Different Phenological Stages in Young and Mature Trees. Scientifica 2020, 2020, 9701701. [Google Scholar] [CrossRef]
- Han, F.; Ma, G.-Q.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.-C.; Zhao, Z.-D.; Xu, H.-L. Chemical composition and antioxidant activities of essential oils from different parts of the oregano. J. Zhejiang Univ. Sci. B 2017, 18, 79–84. [Google Scholar] [CrossRef]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Kuete, V. Syzygium aromaticum. In Medicinal Spices and Vegetables from Africa; Academic Press: London, UK, 2017; pp. 611–625. [Google Scholar]
- El Ghallab, Y.; Al Jahid, A.; Eddine, J.J.; Said, A.A.H.; Zarayby, L.; Derfoufi, S. Syzygium aromaticum L.: Phytochemical investigation and comparison of the scavenging activity of essential oil, extracts and eugenol. Adv. Tradit. Med. 2020, 20, 153–158. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Zare, M.; Razzaghi, S. Eugenol Enrichment of Clove Bud Essential Oil Using Different Microwave-assisted Distillation Methods. Food Sci. Technol. Res. 2017, 23, 385–394. [Google Scholar] [CrossRef]
- Kapadiya, S.M.; Parikh, J.; Desai, M.A. A greener approach towards isolating clove oil from buds of Syzygium aromaticum using microwave radiation. Ind. Crop. Prod. 2018, 112, 626–632. [Google Scholar] [CrossRef]
- González-Rivera, J.; Duce, C.; Falconieri, D.; Ferrari, C.; Ghezzi, L.; Piras, A.; Tine, M.R. Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): Chemical composition and thermal analysis. Innov. Food Sci. Emerg. Technol. 2016, 33, 308–318. [Google Scholar] [CrossRef]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of Essential Oils of Rosmarinus officinalis L. by Two Different Methods: Hydrodistillation and Microwave Assisted Hydrodistillation. Sci. World J. 2019, 2019, 3659432. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, F.; Mafi, M.; Farhadi, F.; Tabar-Heidar, K.; Aghapoor, K.; Mohsenzadeh, F.; Darabi, H.R. Supercritical CO2 Extraction of Essential Oil from Clove Bud: Effect of Operation Conditions on the Selective Isolation of Eugenol and Eugenyl Acetate. Zeitschrift für Naturforschung B 2005, 60, 1197–1201. [Google Scholar] [CrossRef]
- Wei, M.-C.; Xiao, J.; Yang, Y.-C. Extraction of α-humulene-enriched oil from clove using ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. 2016, 210, 172–181. [Google Scholar] [CrossRef]
- Triaux, Z.; Petitjean, H.; Marchioni, E.; Boltoeva, M.; Marcic, C. Deep eutectic solvent–based headspace single-drop microextraction for the quantification of terpenes in spices. Anal. Bioanal. Chem. 2020, 412, 933–948. [Google Scholar] [CrossRef]
- Cooper, R.; Morré, D.J.; Morré, D.M. Medicinal Benefits of Green Tea: Part II. Review of Anticancer Properties. J. Altern. Complement. Med. 2005, 11, 639–652. [Google Scholar] [CrossRef]
- Johnson, R.; Bryant, S.; Huntley, A.L. Green tea and green tea catechin extracts: An overview of the clinical evidence. Maturitas 2012, 73, 280–287. [Google Scholar] [CrossRef]
- Basu, A.; Lucas, E.A. Mechanisms and Effects of Green Tea on Cardiovascular Health. Nutr. Rev. 2007, 65, 361–375. [Google Scholar] [CrossRef]
- Vishnoi, H.; Bodla, R.B.; Kant, R. Green Tea (Camellia sinensis) and Its Antioxidant Property: A Review. Int. J. Pharm. Sci. Res. 2018, 9, 1723–1736. [Google Scholar] [CrossRef]
- Ghasemzadeh-Mohammadi, V.; Zamani, B.; Afsharpour, M.; Mohammadi, A. Extraction of caffeine and catechins using microwave-assisted and ultrasonic extraction from green tea leaves: An optimization study by the IV-optimal design. Food Sci. Biotechnol. 2017, 26, 1281–1290. [Google Scholar] [CrossRef]
- Bindes, M.M.M.; Reis, M.H.M.; Cardoso, V.L.; Boffito, D.C. Ultrasound-assisted extraction of bioactive compounds from green tea leaves and clarification with natural coagulants (chitosan and Moringa oleífera seeds). Ultrason. Sonochem. 2019, 51, 111–119. [Google Scholar] [CrossRef]
- Ayyildiz, S.S.; Karadeniz, B.; Sagcan, N.; Bahar, B.; Us, A.A.; Alasalvar, C. Optimizing the extraction parameters of epigallocatechin gallate using conventional hot water and ultrasound assisted methods from green tea. Food Bioprod. Process. 2018, 111, 37–44. [Google Scholar] [CrossRef]
- Xi, J.; Shen, D.; Zhao, S.; Lu, B.; Li, Y.; Zhang, R. Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction. Int. J. Pharm. 2009, 382, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, B.; Row, K. Extraction of catechin compounds from green tea with a new green solvent. Chem. Res. Chin. Univ. 2014, 30, 37–41. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J.; Sobska, A. Application of Deep Eutectic Solvents and Ionic Liquids in the Extraction of Catechins from Tea. Molecules 2020, 25, 3216. [Google Scholar] [CrossRef]
- Ma, W.; Row, K.H. Solid-Phase Extraction of Catechins from Green Tea with Deep Eutectic Solvent Immobilized Magnetic Molybdenum Disulfide Molecularly Imprinted Polymer. Molecules 2020, 25, 280. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Row, K.H. Ternary deep eutectic solvent magnetic molecularly imprinted polymers for the dispersive magnetic solid-phase microextraction of green tea. J. Sep. Sci. 2018, 41. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K. Oregano: Overview of the Literature on Health Benefits. Nutr. Today 2010, 45, 129–138. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 2017, 9, 968. [Google Scholar] [CrossRef]
- Hassani, F.V.; Shirani, K.; Hosseinzadeh, H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: A review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 931–949. [Google Scholar] [CrossRef]
- Himed-Idir, H.; Mouhoubi, K.; Siar, E.; Boudries, H.; Mansouri, H.; Adjeroud, N.; Madani, K.; Boulekbache-Makhlouf, L. Effect of rosemary (Rosmarinus officinalis L.) supplementation on fresh cheese: Physicochemical properties, antioxidant potential, and sensory attributes. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Munekata, P.E.; Alcántara, C.; Žugčić, T.; Abdelkebir, R.; Collado, M.C.; García-Pérez, J.V.; Jambrak, A.R.; Gavahian, M.; Barba, F.J.; Lorenzo, J.M. Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary. Food Res. Int. 2020, 134, 109242. [Google Scholar] [CrossRef]
- Hosseini, H.; Bolourian, S.; Hamgini, E.Y.; Mahababadi, E.G. Optimization of heat- and ultrasound-assisted extraction of polyphenols from dried rosemary leaves using response surface methodology. J. Food Process. Preserv. 2018, 42, e13778. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Binello, A.; Boffa, L.; Mulinacci, N.; Cravotto, G. Selective recovery of rosmarinic and carnosic acids from rosemary leaves under ultrasound- and microwave-assisted extraction procedures. C. R. Chim. 2016, 19, 699–706. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.-S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.T.; Fagundes, M.B.; Cichoski, A.J.; de Menezes, C.R.; Barin, J.S.; Lorenzo, J.M.; Wagner, R.; Campagnol, P.C.B. Volatile compounds and sensory profile of burgers with 50% fat replacement by microparticles of chia oil enriched with rosemary. Meat Sci. 2019, 148, 164–170. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, H.N.T.; Huang, M.-Y.; Lin, K.-H.; Pham, D.-C.; Tran, Y.B.; Su, C.-H. Optimization of aqueous enzyme-assisted extraction of rosmarinic acid from rosemary (Rosmarinus officinalis L.) leaves and the antioxidant activity of the extract. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Marques, C.; Igarashi-Mafra, L.; Coutinho, J.A.; Mafra, M.R. Extraction of phenolic compounds from rosemary using choline chloride-based Deep Eutectic Solvents. Sep. Purif. Technol. 2021, 258, 117975. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a Method Based on Natural Deep Eutectic Solvents for Extraction of Flavonoids from Food Samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
| Method | Advantage | Disadvantage |
|---|---|---|
| Microwave-assisted extraction (MAE) | Short time (15–30 min). May or may not use a solvent other than water Low solvent usage Easy industrial escalation Low power consumption Low levels of CO2 released into the atmosphere Non-contact heat source Accelerates mass and energy transfer | Needs a solvent separation method. It can affect thermolabile metabolites and in some cases causes oxidation Non-selective extraction |
| Ultrasound-assisted extraction (UAE) | Uses room temperatures. Less energy Lower solvent volume | Difficulty scaling Decrease in its efficiency in systems with high viscosity Temperature stability Solvent contamination Non-selective extraction |
| Pulsed electric field extraction (PEF) and high voltage electrical discharges (HVED) | Low energy Continuous operability Short times | Difficulty scaling Possible oxidation of compounds (HVED) |
| Enzyme-assisted extraction (EAE) | Easy operation and high specificity if the choice of enzymes is right High efficiency Environmentally friendly Low energy requirements and low operating temperature | Scaling and influencing factors such as enzyme concentration, oxygen, pH, temperature, and agitation Establishment of operating conditions if two or more enzymes are used in the process |
| High-hydrostatic pressure extraction (HHPE) | Can be operated at room temperature or in refrigeration temperatures Short operation time Less solvent use compared to heat techniques Better quality, efficiency, and biological activity for extracts Reduced extraction times | May promote oxidation reactions At an industrial level it is a semi-continuous or batch process |
| Deep eutectic solvents (DESs) | Biodegradable solutions Non-toxic Easy to prepare | Expensive to scale The final solution possesses high viscosities and densities |
| Material | Method | Experimental Conditions | Total Phenolic Content | Other Relevant Data | Reference |
|---|---|---|---|---|---|
| Clove | UEA (batch reactor) | 1 kg in 20 L ethanol/water (1:1) in 45 min at 25 kHz effective power 360 W at 28–30 °C | Around 195 ± 1 mg GAE/L extract | Around 45% in inhibition of DDPH | [52] |
| UEA (multi-horn flow reactor) | 1 kg in 20 L ethanol/water (1:1) in 45 min at effective power 350 W, four horns of 21.0 kHz, flow 1350 mL/min | 215 ± 3 mg GAE/L of extract | Around 52% in inhibition of DDPH | [52] | |
| Conventional method | 95% ethanol at room temperature with agitation for 24 h | 54.3 ± 7.3 mg GAE/g | Total flavonoids: 6.9 ± 0.36 mg catechin equivalents/g DPPH (IC50) 0.45 ± 0.08 FRAP: 1216 ± 45.3 mg Trolox/g extract | Unpublished data | |
| Green tea | MAE | 350.65 W and 5 min irradiation | 116.58 mg GAE/g | Total flavonoid content: 49.33 mg catechin equivalent/g, condensed tannins content: 9.89 mg catechin equivalents/g, DPPH IC50: 294.46 µg/mL | [53] |
| Microwave hydro-diffusion and gravity + UEA | Leave wastes in water (15:1). Extraction conditions: 300 W irradiation + UAE at 30 min at 80 °C and 80 kHz | 130 mg GAE/g extract | Antioxidant activity of 0.4 g Trolox equivalents/g | [54] | |
| UEA | 50% methanol in an ultrasonic bath at 28 kHz for 15 min at 55°C | 90.87 ± 1.52 mg GAE/g dw | Total flavonoids content: 26.18 ± 0.86 mg CAT/g dw Total antioxidant capacity: 94.18 ± 0.49% inhibition DPPH | [55] | |
| UEA + DES | DES solvent: choline chloride + glycerol. Ratio of liquid:solid of 36:1 (mL/g), ultrasonic power of 461.5 W with 21 min | 243 ± 7 mg GAE/g dw | Important presence of 4 catechins: epicatechin, epigallocatechin, epicatechin gallate and epigallocatechin gallate. DPPH: 215 ± 6 mmol Trolox/100 g dw FRAP: 332 ± 9 mmol Fe (II)/100 g dw | [51] | |
| Conventional method | 95% ethanol at room temperature with agitation for 24 h | 108.4 ± 6.9 mg GAE/g | Total flavonoids: 32.10 ± 1.91 mg catechin equivalents/g DPPH (IC50) 0.65 ± 0.05 FRAP: 382.3 ± 58.3 mg Trolox/g extract | Unpublished data | |
| Oregano | MAE | 5 g with 100 mL of absolute ethanol for 20 min at 150 W microwave power at 60 °C | 65.40 ± 1.58 mg GAE/g dw | ---- | [56] |
| UAE | 5 g with 500 mL ethanol in an ultrasonic bath for 20 min and then extraction with water at 60 °C for 1 h | 56.22 ± 2.11 mg GAE/g dw | ----- | [56] | |
| SE (Soxhlet extraction) | 5 g with 200 mL of absolute ethanol for 8 h | 50.88 ± 1.32 mg GAE/g dw | ----- | [56] | |
| Conventional method | 95% ethanol at room temperature with agitation for 24 h | 22.8 ± 0.63 mg GAE/g | Total flavonoids: 1.60 ± 0.25 mg catechin equivalents/g DPPH (IC50) 1.08 ± 0.07 FRAP: 178.1 ± 14.8 mg Trolox/g extract | Unpublished data | |
| Rosemary | Maceration + PEF | Wet and ground plant material exposed to 1000 pulses of 15 µsec in a field strength of 5.2 kV/cm. Extraction with 60% ethanol | 64.0 ± 0.3 mg GAE/g dw | ------ | [57] |
| MAE + microwave hydro-diffusion and gravity (pretreatment MHG) | Pretreatment MHG for 579 s (100 g fresh plant with 50% ethanol in residual water of other MHG). MAE AT 60 °C | 55.5 mg GAE/ dw | ------ | [58] | |
| UAE | Extraction with 60% ethanol for 70 min with an ultrasonic bath with a frequency of 37 kHz at 22 °C | 77.5 ± 1.2 mg GAE/g dw | Flavonoids: 16.1 ± 0.3 mg QUE/g dw, carnosic acid: 29.1 ± 0.9 mg/g dw; carnosol: 16.1 ± 0.7 mg/g dw; Rosmarinic acid: 10.1 ± 0.6 mg/g dw | [57] | |
| UAE + DES | 150 mg rosemary leaves + 2.85 mL DES (choline chloride: 1,2-propaneidol) for 120 min with an ultrasound frequency of 50–60 Hz | 62.21 ± 3.85 mg GAE/g plant | 150.63 ± 0.3 mM Trolox equivalent/g plant for DPPH antioxidant activity and 148.24 ± 8.75 mM Trolox equivalent/g plant for FRAP activity | [50] | |
| Conventional method | 50% ethanol at 60 °C | 35.29 mg GAE/g dw | ------- | [58] | |
| Conventional method | 95% ethanol at room temperature with agitation for 24 h | 14.59 ± 1.31 mg GAE/g | Total flavonoids: 2.9 ± 0.31 mg catechin equivalents/g DPPH (IC50) 4.7 ± 0.37 FRAP: 51.36 ± 1.2 mg Trolox/g extract | Unpublished data |
| Material | Name and Empirical Formula | Structure |
|---|---|---|
| Rosemary | Rosmarinic acid (C18H16O8) | 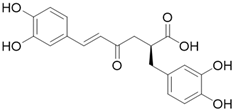 |
| Carnosol C20H26O4 | 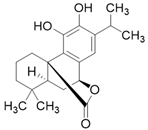 | |
| Carnosic acid C20H28O4 | 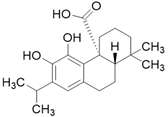 | |
| Clove | Eugenol C10H12O2 | 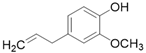 |
| β-caryophyllene C15H24 | 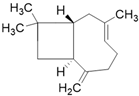 | |
| Eugenyl acetate C12H14O3 | 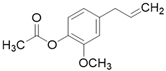 | |
| α-humulene C15H24 | 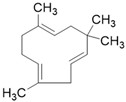 | |
| Green tea | Catechin C15H14O6 |  |
| Epicatechin C15H14O6 | 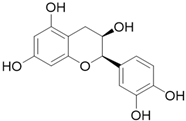 | |
| Epicatechin gallate C22H18O10 | 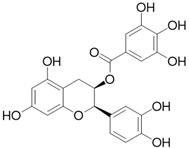 | |
| Epigallocatechin C15H14O7 | 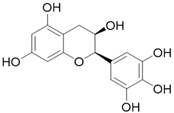 | |
| Epigallocatechin gallate C22H18O11 | 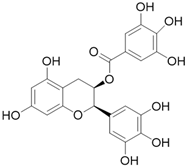 | |
| Quercetin C15H10O7 | 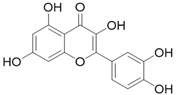 | |
| Oregano | Carvacrol C10H14O |  |
| Thymol C10H14O | 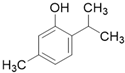 | |
| p-cymene C10H14 |  | |
| β-caryophyllene C15H24 | 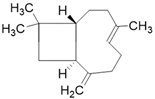 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869. https://doi.org/10.3390/molecules26071869
Calderón-Oliver M, Ponce-Alquicira E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules. 2021; 26(7):1869. https://doi.org/10.3390/molecules26071869
Chicago/Turabian StyleCalderón-Oliver, Mariel, and Edith Ponce-Alquicira. 2021. "Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano" Molecules 26, no. 7: 1869. https://doi.org/10.3390/molecules26071869
APA StyleCalderón-Oliver, M., & Ponce-Alquicira, E. (2021). Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules, 26(7), 1869. https://doi.org/10.3390/molecules26071869






