Laboratory and Field Evaluation of the Phytotoxic Activity of Sapindus mukorossi Gaertn Pulp Extract and Identification of a Phytotoxic Substance
Abstract
1. Introduction
2. Results
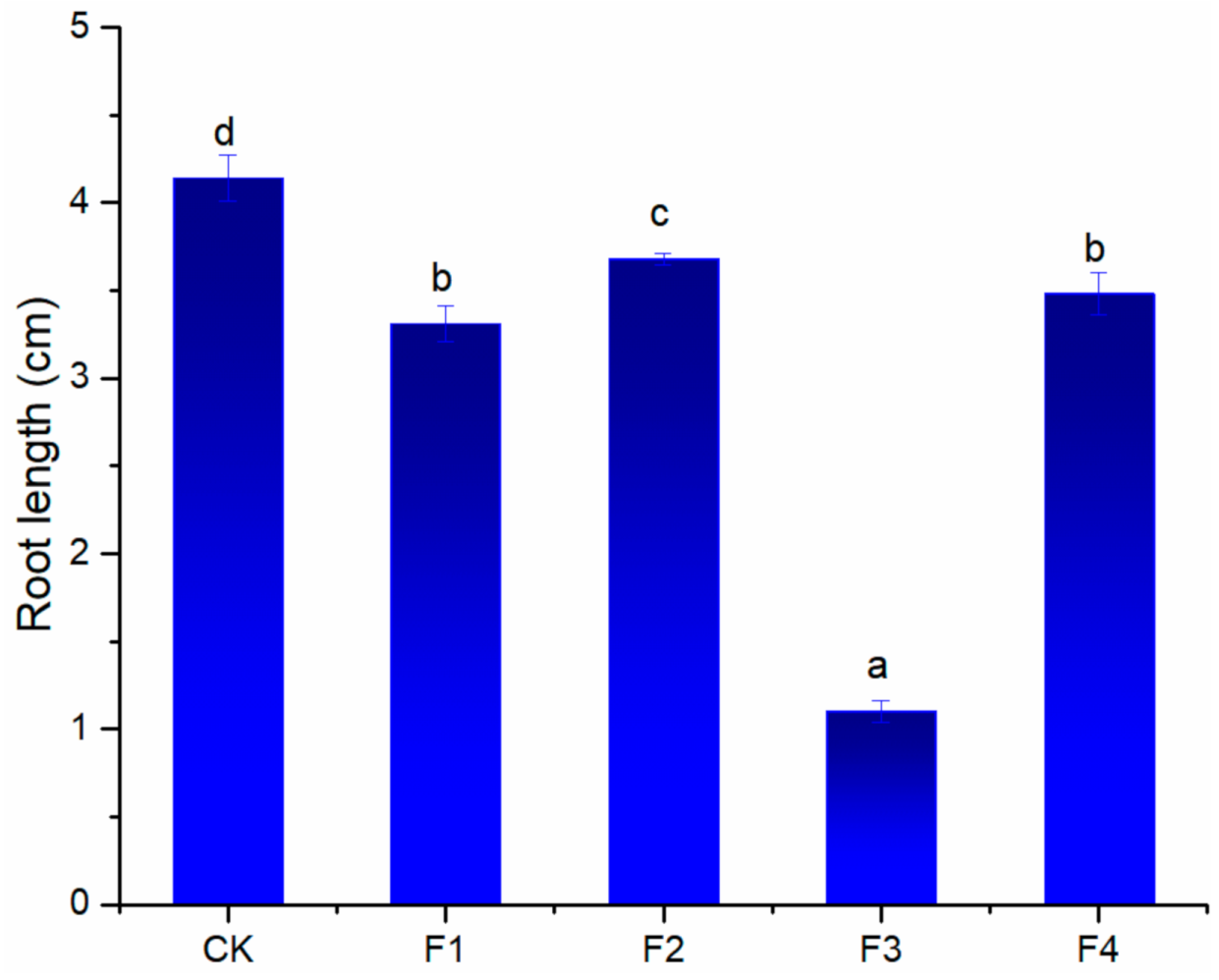
2.1. Phytotoxicity Screening of the Extract and Fractions from S. mukorossi Pulp
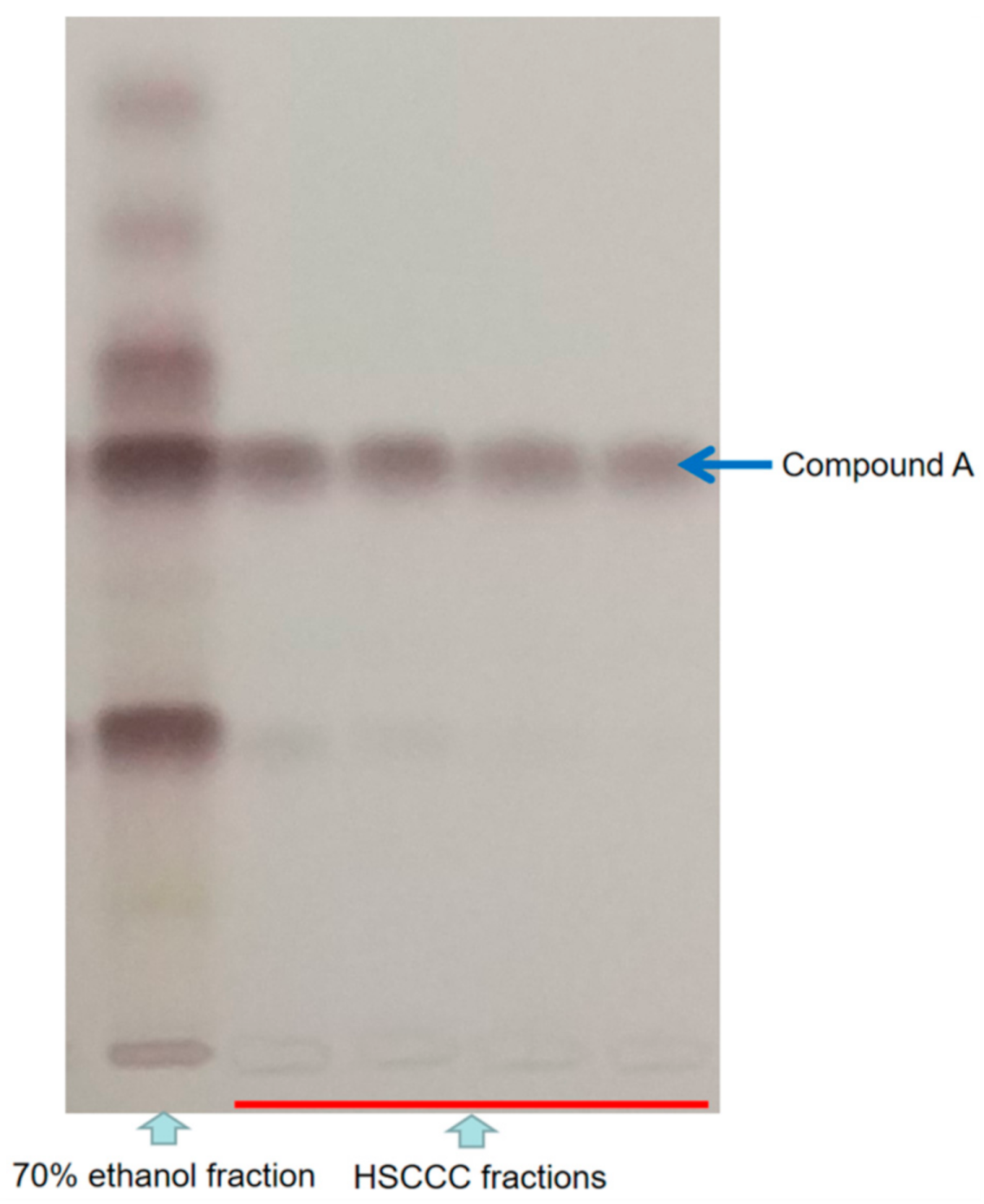
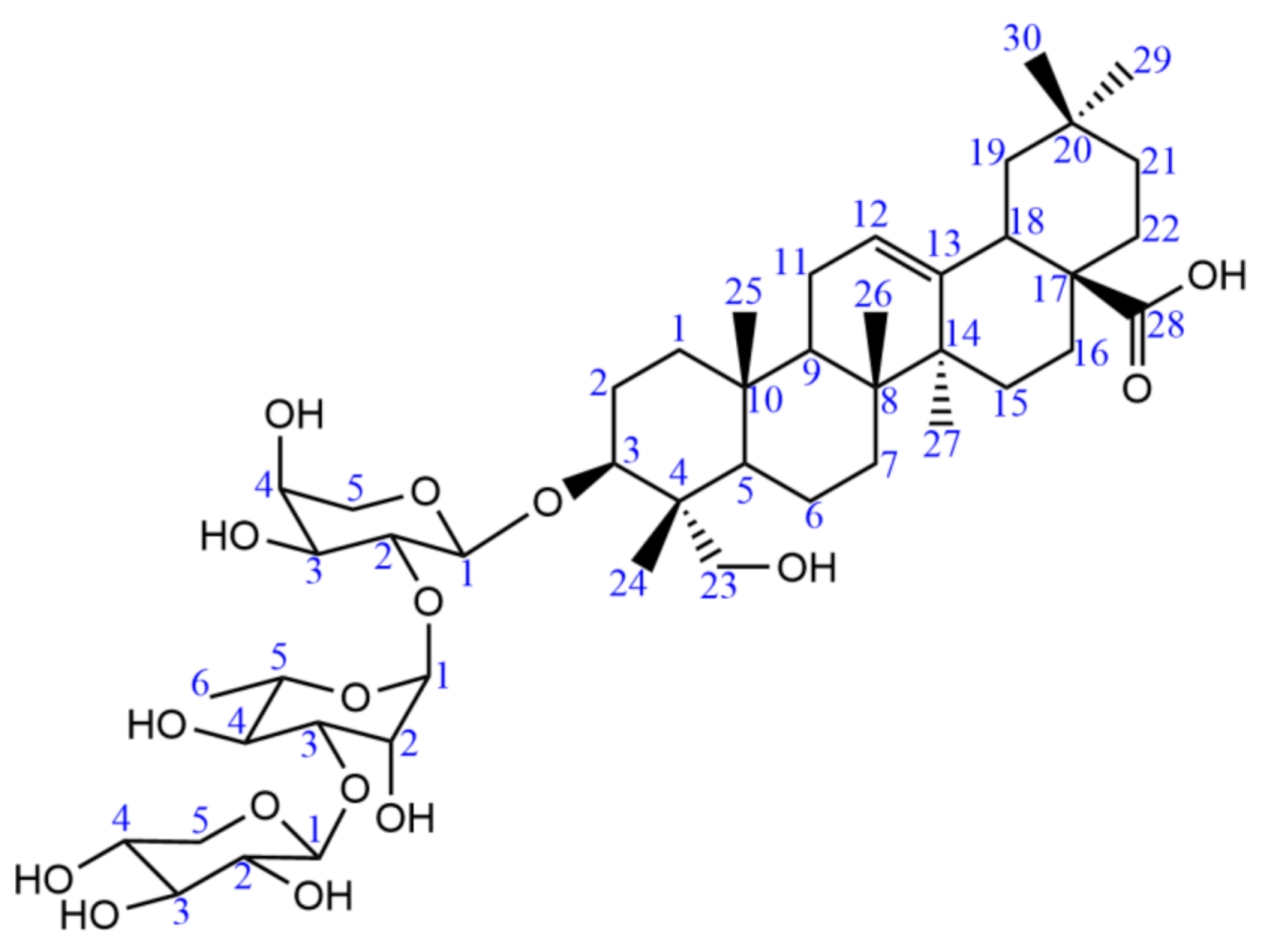
2.2. HSCCC Separation and Phytotoxic Activity of Isolated Compounds
2.3. Field Efficacy of 20% Pulp Extract AS against Weeds in Carrot
3. Discussion
4. Materials and Methods
4.1. Material and Reagents
4.2. Apparatus
4.3. Preparation of Pulp Extract of S. mukorossi
4.4. HSCCC Separation and TLC Analysis
4.5. Phytotoxic Activity in Laboratory
4.6. Formulation Preparation and Its Field Test
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kaur, S.; Kaur, R.; Chauhan, B.S. Understanding crop-weed-fertilizer-water interactions and their implications for weed management in agricultural systems. Crop. Prot. 2018, 103, 65–72. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Akhter, M.J.; Iqbal, N.; Peerzada, A.M.; Hanif, Z.; Manalil, S.; Hashim, S.; Ali, H.H.; Kebaso, L.; Frimpong, D.; et al. Biology and management of Avena fatua and Avena ludoviciana: Two noxious weed species of agro-ecosystems. Environ. Sci. Pollut. Res. 2017, 24, 19465–19479. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, M.; Wolgemuth, D.; Pittman, C.U.; Mohan, D.; Mlsna, T. Phenoxy herbicide removal from aqueous solutions using fast pyrolysis switchgrass biochar. Chemosphere 2017, 174, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Benvenuti, S.; Cioni, P.L.; Flamini, G.; Pardossi, A. Weeds for weed control: Asteraceae essential oils as natural herbicides. Weed Res. 2017, 57, 342–353. [Google Scholar] [CrossRef]
- Ni, W.; Hua, Y.; Liu, H.Y.; Teng, R.W.; Kong, Y.C.; Hu, X.Y.; Chen, C.X. Tirucallane-type triterpenoid saponins from the roots of Sapindus mukorossi. Chem. Pharm. Bull. 2006, 54, 1443–1446. [Google Scholar] [CrossRef]
- Dutta, S.K.; Akoijam, R.S.; Boopathi, T.; Saha, S. Bioactivity and traditional uses of 26 underutilized ethno-medicinal fruit species of North-East Himalaya, India. J. Food Meas. Charact. 2018, 12, 2503–2514. [Google Scholar] [CrossRef]
- Wei, M.; Qiu, J.; Li, L.; Xie, Y.; Guo, Y.; Yu, H.; Cheng, Y.; Qian, H.; Yao, W. The chemical profile and biological activity of different extracts of Sapindus mukorossi Gaertn. against Cutibacterium acnes. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef]
- Li, L.; Qiu, J.; Wei, M.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Yao, W. Inhibition of Candida albicans and induced vaginitis by sapindus water extract. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Huong, V.T.L.; Nguyen, N.T. Green synthesis, characterization and antibacterial activity of silver nanoparticles using Sapindus mukorossi fruit pericarp extract. Mate. Today Proc. 2020. [Google Scholar] [CrossRef]
- Dinda, G.; Halder, D.; Mitra, A.; Pal, N.; Vazquez-Vazquez, C.; Lopez-Quintela, M.A. Study of the antibacterial and catalytic activity of silver colloids synthesized using the fruit of Sapindus mukorossi. New J. Chem. 2017, 41, 10703–10711. [Google Scholar] [CrossRef]
- Wei, M.; Zhu, X.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Isolation of two sesquiterpene glycosides from Sapindus mukorossi Gaertn. with cytotoxic properties and analysis of their mechanism based on network pharmacology. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Fu, L.L.; He, S.Q.; Lu, X.P.; Wu, Y.Y.; Ma, Z.Q.; Zhang, X. Potent herbicidal activity of Sapindus mukorossi Gaertn. against Avena fatua L. and Amaranthus retroflexus L. Ind. Crop. Prod. 2018, 122, 1–6. [Google Scholar] [CrossRef]
- Singh, N. Efficacy of saponin glycosides against the weed, Echinochloa colanum. Nat. Environ. Pol. Technol. 2010, 9, 379–381. [Google Scholar]
- Huang, H.C.; Liao, S.C.; Chang, F.R.; Kuo, Y.H.; Wu, Y.C. Molluscicidal saponins from Sapindus mukorossi, inhibitory agents of golden apple snails, Pomacea canaliculata. J. Agric. Food Chem. 2003, 51, 4916–4919. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kumar, J.; Dhingra, S.; Parmar, B.S. Screening for feeding deterrent and insect growth regulatory activity of triterpenic saponins from Diploknema butyracea and Sapindus mukorossi. J. Agric. Food Chem. 2010, 58, 434–440. [Google Scholar] [CrossRef]
- Hu, Q.W.; Chen, Y.Y.; Jiao, Q.Y.; Khan, A.; Li, F.; Han, D.F.; Cao, G.D.; Lou, H.X. Triterpenoid saponins from the pulp of Sapindus mukorossi and their antifungal activities. Phytochemistry 2018, 147, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Gui, M.L.; Xu, T.T.; Zhang, L.; Kong, L.T.; Qin, L.; Zou, Z.R. Efficient aqueous enzymatic-ultrasonication extraction of oil from Sapindus mukorossi seed kernels. Ind. Crop. Prod. 2019, 134, 124–133. [Google Scholar] [CrossRef]
- Singh, R.; Kumari, N. Comparative determination of phytochemicals and antioxidant activity from leaf and fruit of Sapindus mukorrossi Gaertn.—A valuable medicinal tree. Ind. Crop. Prod. 2015, 73, 1–8. [Google Scholar] [CrossRef]
- Macías, F.A.; Molinillo, J.M.G.; Oliveros-Bastidas, A.; Marín, D.; Chinchilla, D. Allelopathy. A natural strategy for weed control. Commun. Agric. Appl. Biol. Sci. 2004, 69, 13–23. [Google Scholar]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Thapaliyal, S.; Bali, R.S.; Singh, B.; Todaria, N.P. Allelopathic effects of tree of economic importance on germination and growth of food crops. J. Herbs Spices Med. Plants 2008, 13, 11–23. [Google Scholar] [CrossRef]
- Siddiqui, S.; Alamri, S.; Al-Rumman, S.; Moustafa, M. Allelopathic and Cytotoxic Effects of Medicinal Plants on Vegetable Crop Pea (Pisum sativum). Cytologia 2018, 83, 276–281. [Google Scholar] [CrossRef]
- Murgu, M.; Santos, L.F.A.; de Souza, G.D.; Daolio, C.; Schneider, B.; Ferreira, A.G.; Rodrigues-Filho, E. Hydroxylation of a hederagenin derived saponin by a Xylareaceous fungus found in fruits of Sapindus saponaria. J. Braz. Chem. Soc. 2008, 19, 831–835. [Google Scholar] [CrossRef]
- Nakayama, K.; Fujino, H.; Kasai, R.; Tanaka, O.; Zhou, J. Saponins of pericarps of Chinese Sapindus delavayi (Pyi-shiau-tzu), a source of natural surfactants. Chem. Pharm. Bull. 1986, 34, 2209–2213. [Google Scholar] [CrossRef]
- Sodaeizadeh, H.; Rafieiolhossaini, M.; Havlik, J.; Van Damme, P. Allelopathic activity of different plant parts of Peganum harmala L. and identification of their growth inhibitors substances. Plant Growth Regul. 2009, 59, 227–236. [Google Scholar] [CrossRef]
- Barkatullah, A.I.; Ibrar, M.; Jelani, G. Allelopathic potential of Sapindus mukorossi Gaertn tested against Pennisetum americanum (L.) Leeke, Setaria italica (L.) Beauv. and Lactuca sativa L. Pak. J. Bot. 2015, 47, 1879–1882. [Google Scholar]
- Begum, K.; Shammi, M.; Hasan, N.; Appiah, K.S.; Fujii, Y. Evaluation of potential volatile allelopathic plants from Bangladesh, with Sapindus mukorossi as a Candidate Species. Agronomy 2020, 10, 49. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.S.; Doohan, D. Safety of bicyclopyrone on several vegetable crops and efficacy of weed control. Weed Technol. 2018, 32, 498–505. [Google Scholar] [CrossRef]
- Rao, M.S.; Asad, B.S.; Fazil, M.; Sudharshan, R.; Rasheed, S.; Pradeep, H.; Aboobacker, S.; Thayyil, A.; Riyaz, A.; Mansoor, M.; et al. Evaluation of protective effect of Sapindus mukorossi saponin fraction on CCl(4)-induced acute hepatotoxicity in rats. Clin. Exp. Gastroenter. 2012, 5, 129–137. [Google Scholar]
- Du, M.; Huang, S.; Zhang, J.; Wang, J.; Hu, L.; Jiang, J. Toxicolological test of saponins from Sapindus mukorossi Gaerth. Open J. For. 2015, 5, 749–753. [Google Scholar]
- Wei, M.-P.; Qiu, J.-D.; Li, L.; Xie, Y.-F.; Yu, H.; Guo, Y.-H.; Yao, W.-R. Saponin fraction from Sapindus mukorossi Gaertn as a novel cosmetic additive: Extraction, biological evaluation, analysis of anti-acne mechanism and toxicity prediction. J. Ethnopharmacol. 2021, 268, 113552. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Kumar, N.; Singh, B.; Bhat, T.K. An improved method for thin layer chromatographic analysis of saponins. Food Chem. 2012, 132, 671–674. [Google Scholar] [CrossRef]
- Kerem, Z.; German-Shashoua, H.; Yarden, O. Microwave-assisted extraction of bioactive saponins from chickpea (Cicer arietinum L). J. Sci. Food Agric. 2005, 85, 406–412. [Google Scholar] [CrossRef]

| Sample | IC50 (95% CI) (mg/L) | Slope ± SE | χ2 |
|---|---|---|---|
| Compound A | 16.64 (12.34–21.47) | 0.97 ± 0.11 | 2.39 |
| 70% ethanol fraction | 35.13 (25.29–46.40) | 0.87 ± 0.11 | 1.37 |
| C | DEPT | Compound A | Reference | C | DEPT | Compound A | Reference |
|---|---|---|---|---|---|---|---|
| 1 | CH2 | 39.4 | 39.3 | 26 | CH3 | 17.8 | 17.9 |
| 2 | CH2 | 26.7 | 26.5 | 27 | CH3 | 26.5 | 26.4 |
| 3 | CH | 81.4 | 81.3 | 28 | - | 180.9 | 182.0 |
| 4 | - | 43.9 | 43.8 | 29 | CH3 | 33.7 | 33.1 |
| 5 | CH | 47.9 | 47.9 | 30 | CH3 | 24.1 | 23.9 |
| 6 | CH2 | 18.5 | 18.4 | ||||
| 7 | CH2 | 33.2 | 33.2 | Arabinose | |||
| 8 | - | 40.0 | 40.0 | 1 | CH | 105.0 | 104.3 |
| 9 | CH | 48.5 | 48.5 | 2 | CH | 75.9 | 75.4 |
| 10 | - | 37.2 | 37.2 | 3 | CH | 75.4 | 74.8 |
| 11 | CH2 | 24.2 | 24.0 | 4 | CH | 69.8 | 69.4 |
| 12 | CH | 122.8 | 122.6 | 5 | CH2 | 66.6 | 65.9 |
| 13 | - | 145.2 | 145.5 | Rhamnose | |||
| 14 | - | 42.4 | 42.4 | 1 | CH | 101.6 | 101.1 |
| 15 | CH2 | 28.6 | 28.7 | 2 | CH | 72.3 | 71.7 |
| 16 | CH2 | 24.0 | 24.0 | 3 | CH | 82.8 | 82.6 |
| 17 | - | 46.7 | 46.9 | 4 | CH | 72.7 | 72.7 |
| 18 | CH | 42.5 | 42.4 | 5 | CH | 69.9 | 69.4 |
| 19 | CH2 | 46.7 | 47.0 | 6 | CH3 | 18.7 | 18.3 |
| 20 | - | 31.3 | 31.2 | Xylose | |||
| 21 | CH2 | 34.5 | 34.6 | 1 | CH | 107.8 | 107.1 |
| 22 | CH2 | 33.6 | 33.6 | 2 | CH | 75.5 | 75.2 |
| 23 | CH2 | 64.3 | 64.4 | 3 | CH | 78.7 | 78.1 |
| 24 | CH3 | 14.5 | 14.4 | 4 | CH | 71.4 | 70.8 |
| 25 | CH3 | 16.4 | 16.4 | 5 | CH2 | 67.7 | 67.1 |
| Treatment | Dose (g a.i./ha) | Fresh Weight Control Efficacy (%) | ||||
|---|---|---|---|---|---|---|
| Eleusine indica (L.) Gaertn. | Cyperus rotundus L. | Acalypha australis L. | Physalis minima L. | Total | ||
| 20% pulp extract AS | 1500.0 | 69.6 ± 2.5 | 83.9 ± 3.9 | 88.5 ± 5.2 | 76.7 ± 3.7 | 78.7 ± 1.0 b |
| 1200.0 | 50.0 ± 4.7 | 63.4 ± 3.7 | 67.7 ± 4.3 | 60.5 ± 5.1 | 61.5 ± 2.8 c | |
| 900.0 | 25.6 ± 3.4 | 37.7 ± 4.5 | 41.1 ± 4.7 | 49.0 ± 3.6 | 28.2 ± 1.7 d | |
| 33% pendimethalin EC | 742.5 | 100.0 ± 0.0 | 42.5 ± 4.5 | 65.4 ± 4.0 | 99.7 ± 0.5 | 93.4 ± 0.8 a |
| Blank control (water) | 0 | 0 | 0 | 0 | 0 | 0 e |
| Treatment | Dose (g a.i./ha) | Weed Control Efficacy (%) | |
|---|---|---|---|
| 30 DAT | 45 DAT | ||
| 20% pulp extract AS | 1500.0 | 70.1 ± 2.8 b | 72.2 ± 2.4 b |
| 1200.0 | 53.1 ± 2.7 c | 59.7 ± 2.9 c | |
| 900.0 | 39.0 ± 3.4 d | 45.0 ± 2.1 d | |
| 33% pendimethalin EC | 742.5 | 84.8 ± 0.98 a | 88.3 ± 1.4 a |
| Blank control (water) | 0 | 0 e | 0 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Wang, J.; Ma, X.; Sun, J.; Tang, F. Laboratory and Field Evaluation of the Phytotoxic Activity of Sapindus mukorossi Gaertn Pulp Extract and Identification of a Phytotoxic Substance. Molecules 2021, 26, 1318. https://doi.org/10.3390/molecules26051318
Dai Z, Wang J, Ma X, Sun J, Tang F. Laboratory and Field Evaluation of the Phytotoxic Activity of Sapindus mukorossi Gaertn Pulp Extract and Identification of a Phytotoxic Substance. Molecules. 2021; 26(5):1318. https://doi.org/10.3390/molecules26051318
Chicago/Turabian StyleDai, Ziyang, Jin Wang, Xiaojiang Ma, Jia Sun, and Feng Tang. 2021. "Laboratory and Field Evaluation of the Phytotoxic Activity of Sapindus mukorossi Gaertn Pulp Extract and Identification of a Phytotoxic Substance" Molecules 26, no. 5: 1318. https://doi.org/10.3390/molecules26051318
APA StyleDai, Z., Wang, J., Ma, X., Sun, J., & Tang, F. (2021). Laboratory and Field Evaluation of the Phytotoxic Activity of Sapindus mukorossi Gaertn Pulp Extract and Identification of a Phytotoxic Substance. Molecules, 26(5), 1318. https://doi.org/10.3390/molecules26051318





