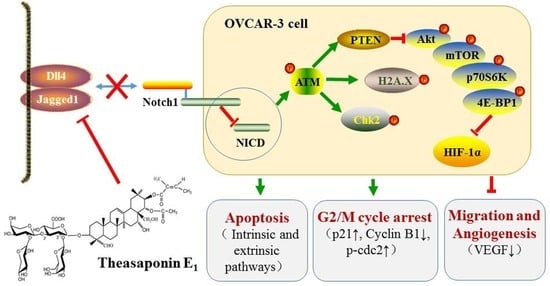
Theasaponin E1 Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis
Abstract
1. Introduction
2. Results
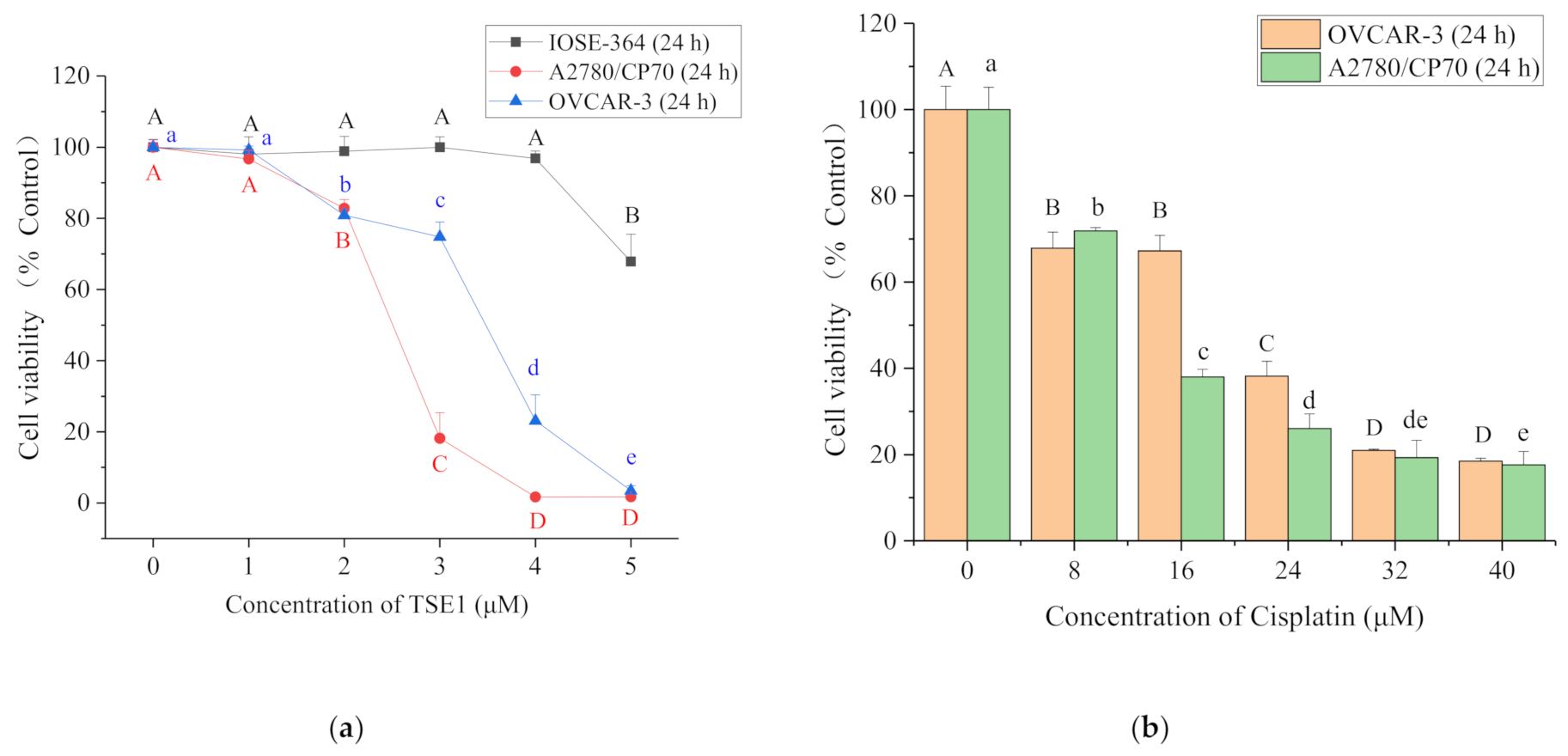
2.1. Cytotoxicity of TSE1 on OVCAR-3, A2780/CP70 and IOSE-364 Cells
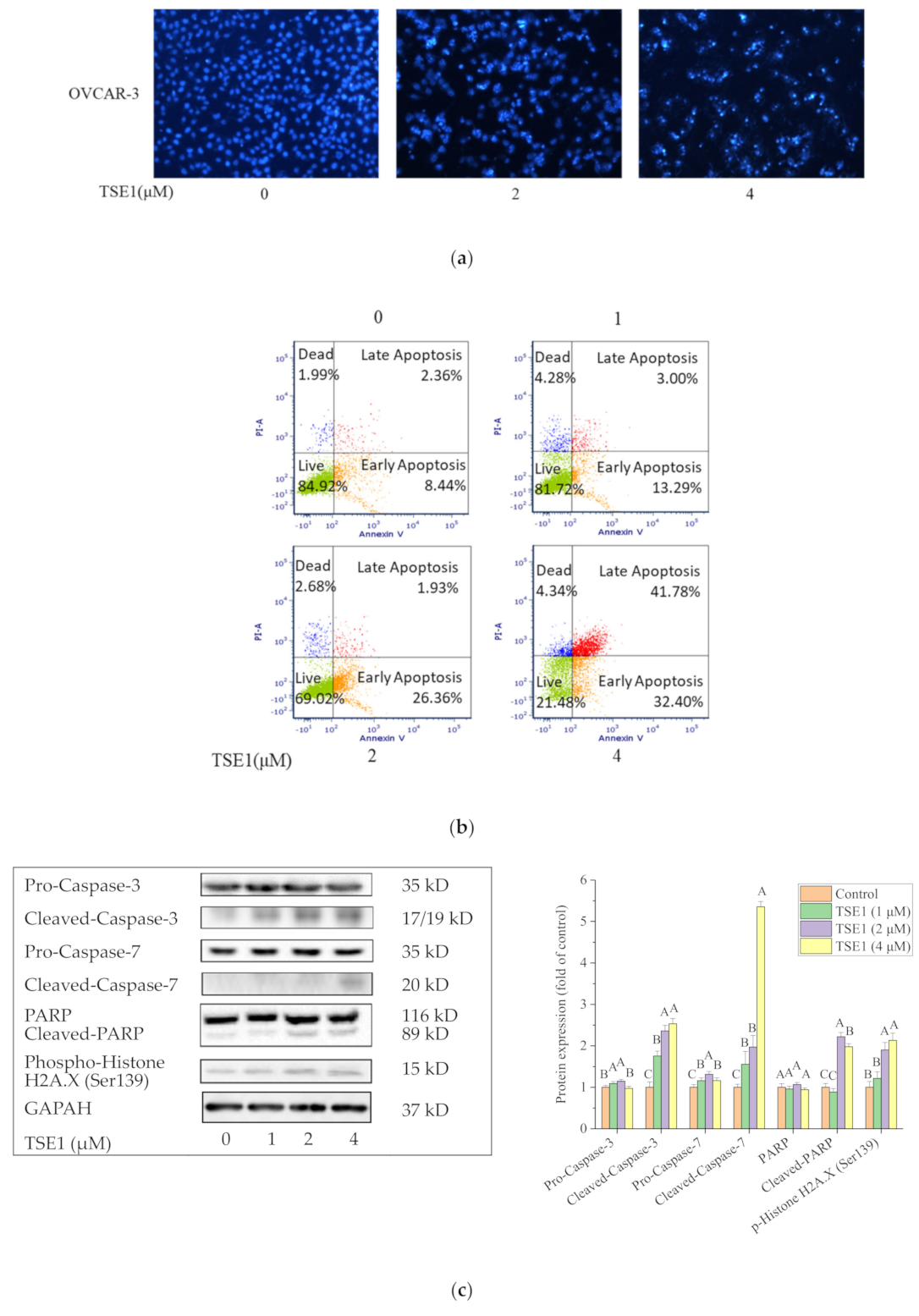
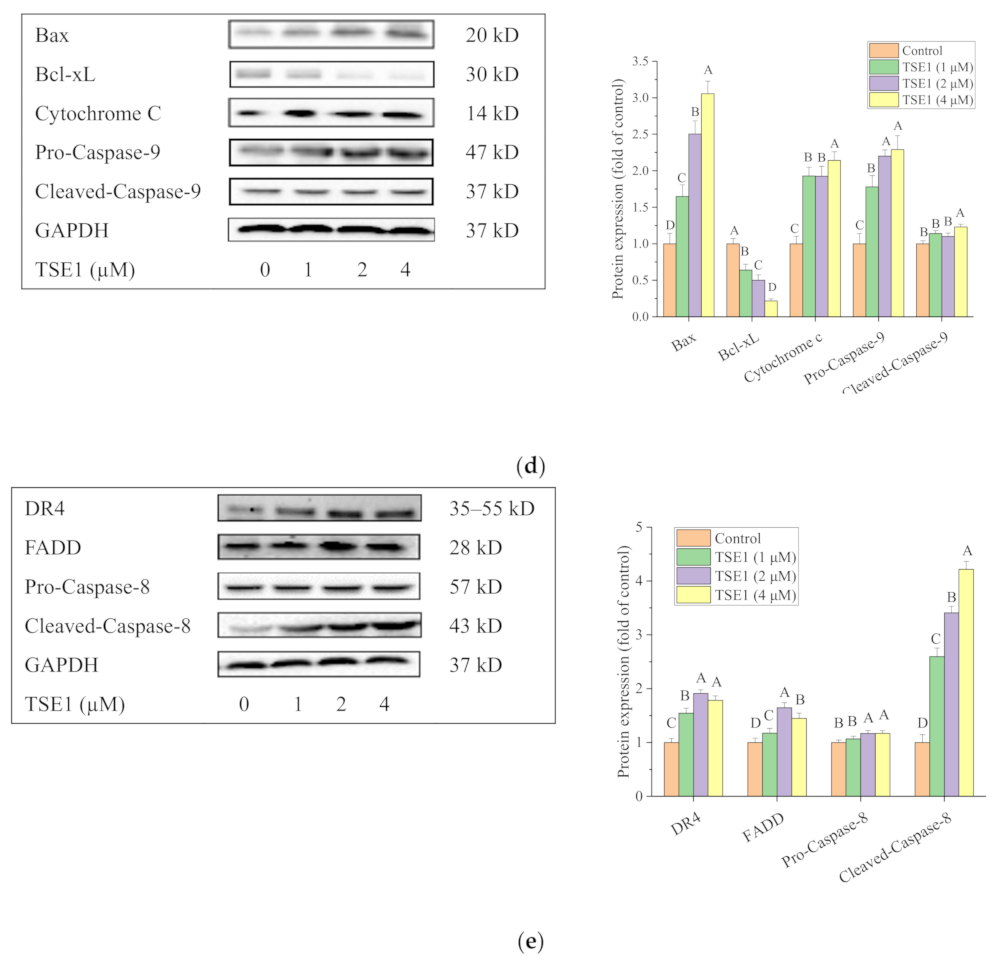
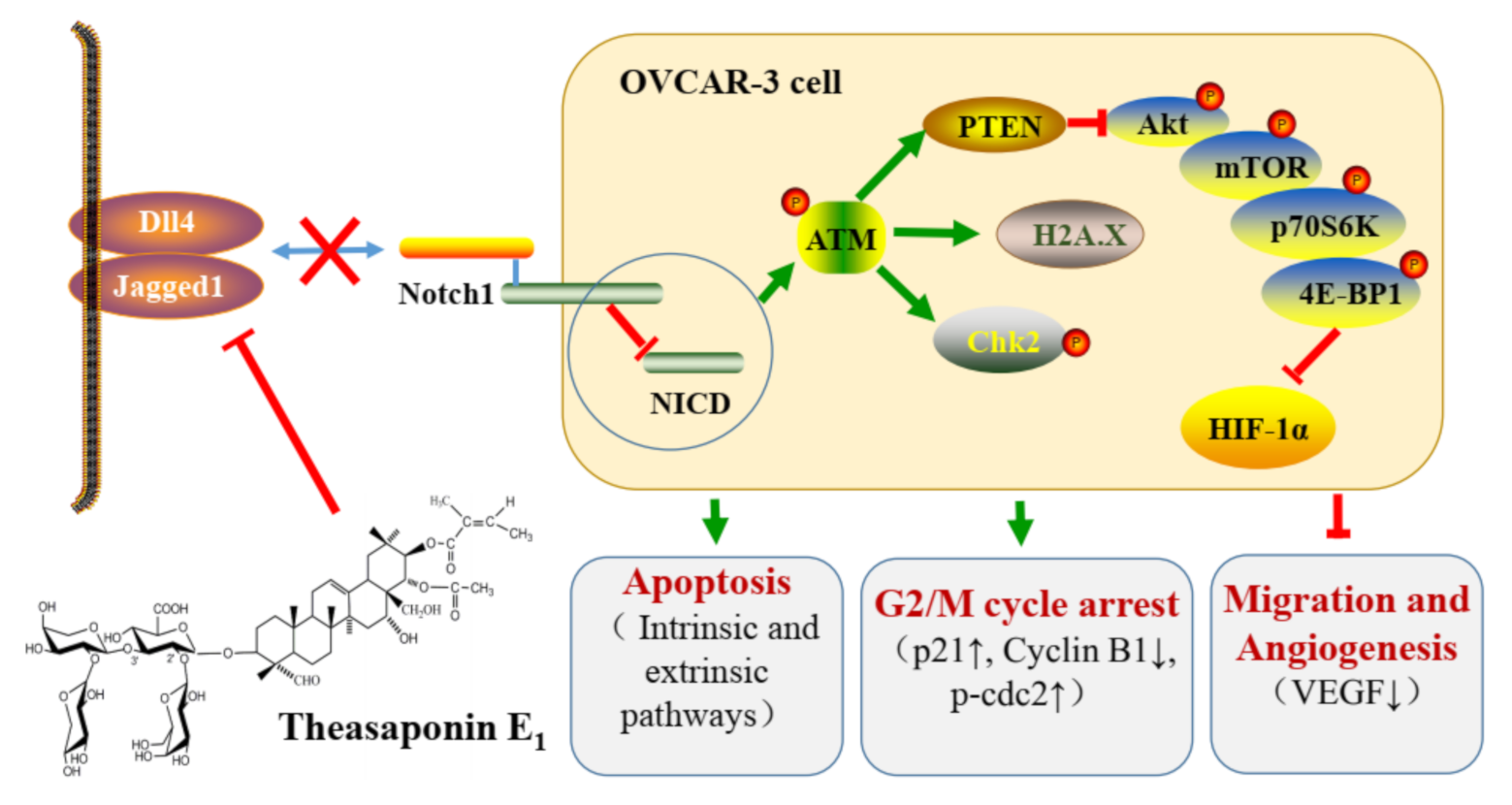
2.2. TSE1 Induced Apoptosis in OVCAR-3 Cells
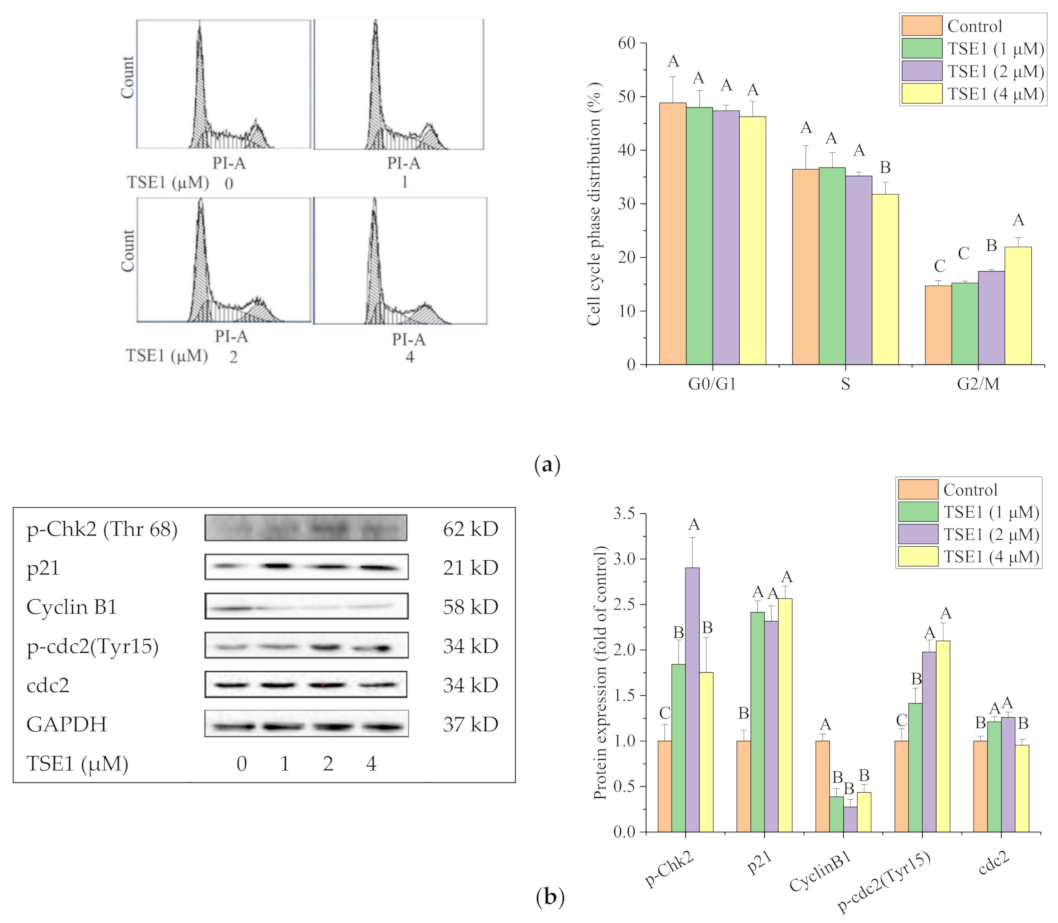
2.3. TSE1 Induces G2/M Cell Cycle Arrest in OVCAR-3 Cell
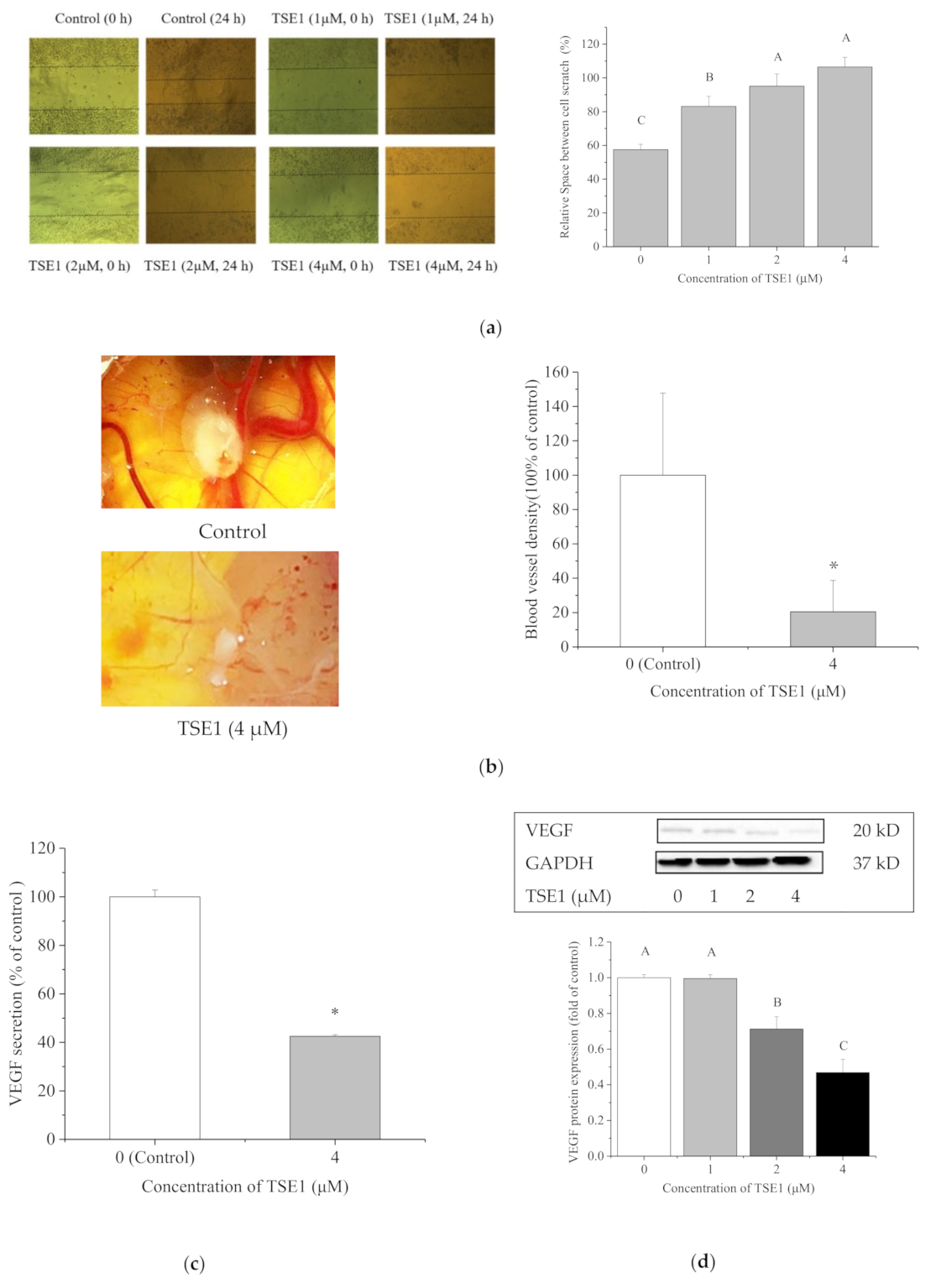
2.4. TSE1 Inhibited the Migration and Angiogenesis
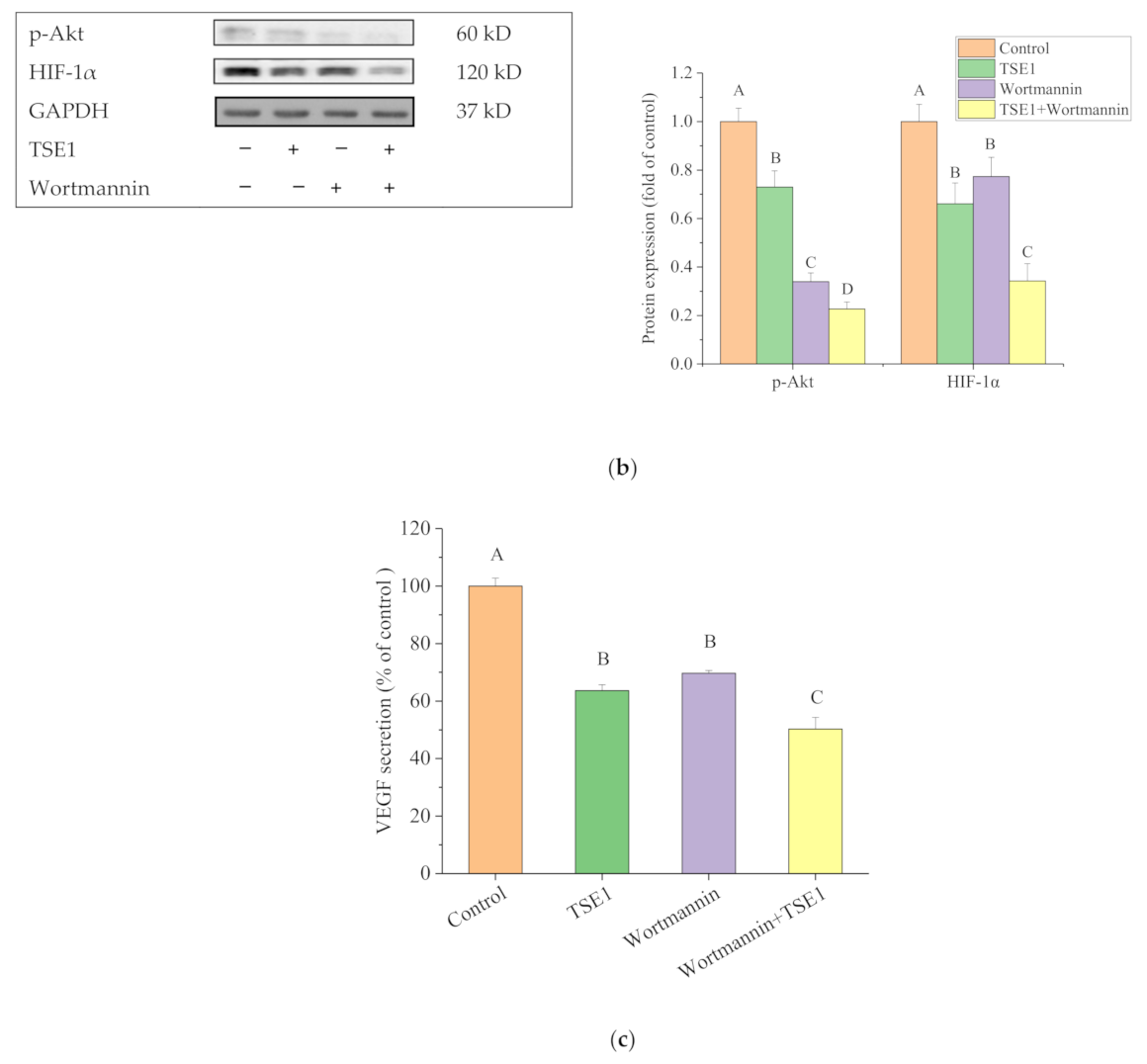
2.5. Effect of TSE1 on ATM/PTEN/Akt/mTOR/HIF-1α Pathway
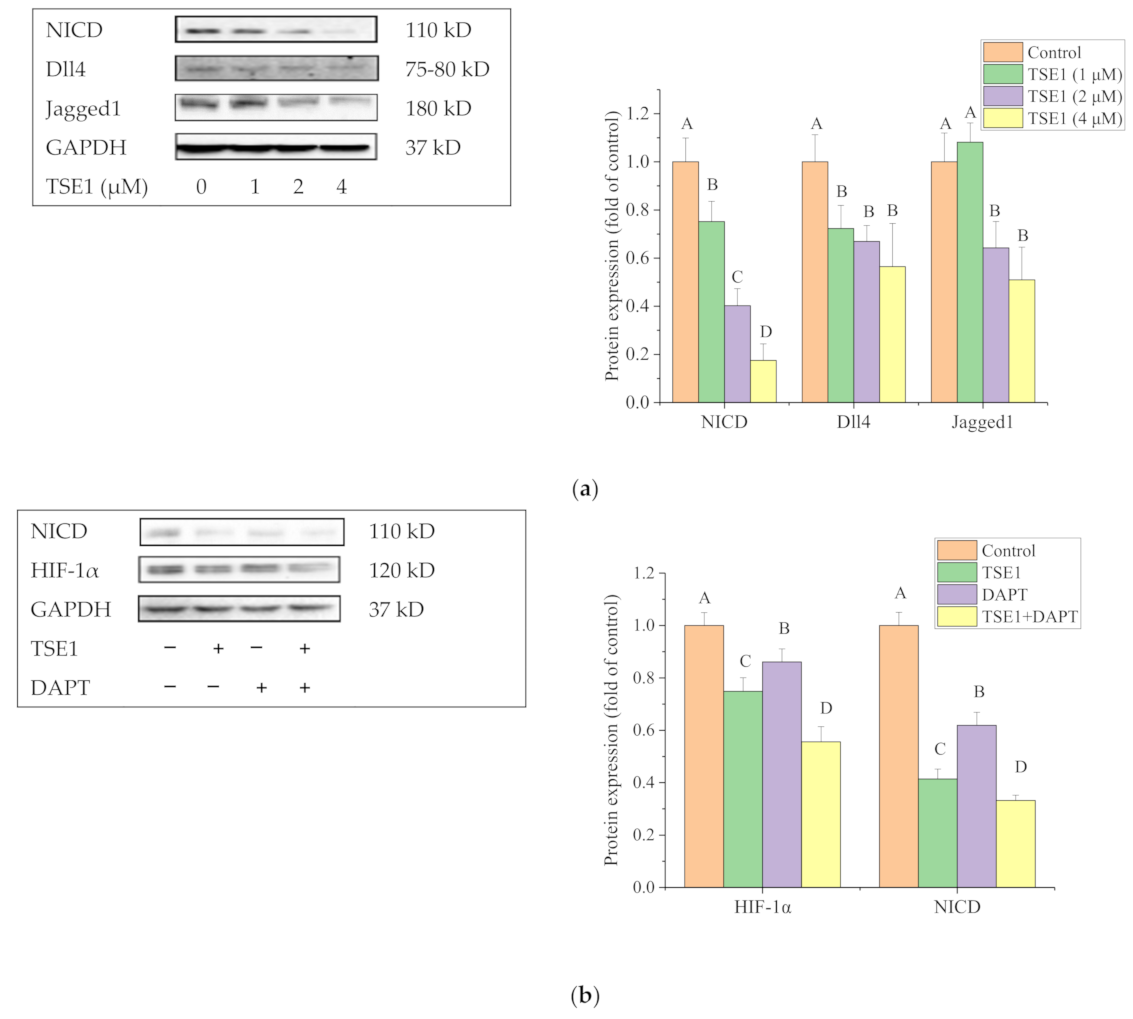
2.6. Effect of TSE1 on Dll4 and Jagged1-Mediated Notch 1 Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Viability Assay
4.3. Hoechst 33342 Staining Assay
4.4. Flow Cytometry Analysis for Apoptosis and Cell Cycle
4.5. Wound Healing Assay
4.6. Chicken Chorioallantoic Membrane (CAM) Assay
4.7. Enzyme Linked Immunosorbent Assay (ELISA)
4.8. Western Blot Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability: Samples of the compounds TSE1 are available from the authors. |
References
- Mirza-Aghazadeh-Attari, M.; Ostadian, C.; Saei, A.A.; Mihanfar, A.; Darband, S.G.; Sadighparvar, S.; Kaviani, M.; Kafil, H.S.; Yousefi, B.; Majidinia, M. DNA damage response and repair in ovarian cancer: Potential targets for therapeutic strategies. DNA Repair 2019, 80, 59–84. [Google Scholar] [CrossRef]
- Lee, A.W.; Navajas, E.E.; Liu, L.H. Clear differences in ovarian cancer incidence and trends by ethnicity among Asian Americans. Cancer Epidemiol. 2019, 61, 142–149. [Google Scholar] [CrossRef]
- Chornokur, G.; Amankwah, E.K.; Schildkraut, J.M.; Phelan, C.M. Global ovarian cancer health disparities. Gynecol. Oncol. 2013, 129, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zong, Z.H.; Liu, Y.; Chen, S.; Sheng, X.J.; Zhao, Y. CEMIP promotes ovarian cancer development and progression via the PI3K/AKT signaling pathway. Biomed. Pharmacother. 2019, 114, 108787. [Google Scholar] [CrossRef] [PubMed]
- Mullen, M.M.; Kuroki, L.M.; Thaker, P.H. Novel treatment options in platinum-sensitive recurrent ovarian cancer: A review. Gynecol. Oncol. 2019, 152, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.M.; Liu, P.S.; Ma, X.M.; Ma, X.X.; Zhu, L.H.; Lin, Y.K.; You, Y.J.; Yu, W.H.; Ma, D.P.; Sun, C.Y.; et al. TRIM50 acts as a novel Src suppressor and inhibits ovarian cancer progression. BBA Mol. Cell Res. 2019, 1866, 1412–1420. [Google Scholar] [CrossRef]
- Samper, K.G.; Marker, S.C.; Bayon, P.; MacMillan, S.N.; Keresztes, I.; Palacios, O.; Wilson, J.J. Anticancer activity of hydroxy- and sulfonamide-azobenzene platinum(II) complexes in cisplatin-resistant ovarian cancer cells. J. Inorg. Biochem. 2017, 174, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, F.; Jang, K.; Yoon, H.; Hew, K.E.; Kim, M.; Azzam, D.J.; Sun, J.; Zhao, D.K.; Ince, T.A.; Liu, W.B.; et al. Dual Src and MEK Inhibition Decreases Ovarian Cancer Growth and Targets Tumor Initiating Stem-Like Cells. Clin. Cancer Res. 2018, 24, 4874–4886. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Greer, Y.; Lipkowitz, S.; Takebe, N. Novel apoptosis-inducing agents for the treatment of cancer, a new arsenal in the toolbox. Cancers 2019, 11, 1087. [Google Scholar] [CrossRef]
- Yang, Y.N.; Li, S.; Sun, Y.T.; Zhang, D.; Zhao, Z.Y.; Liu, L. Reversing platinum resistance in ovarian cancer multicellular spheroids by targeting Bcl-2. Oncotargets Ther. 2019, 12, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Salem, M.L.; Gouida, M.S.; El-Azab, K.M. Comparative expression of caspases and annexin V in benign and malignant ovarian tumors. J. Cancer Res. Ther. 2018, 14, 1042–1048. [Google Scholar] [PubMed]
- Yan, X.Y.; Zhong, X.R.; Yu, S.H.; Zhang, L.C.; Liu, Y.N.; Zhang, Y.; Sun, L.K.; Su, J. p62 aggregates mediated Caspase 8 activation is responsible for progression of ovarian cancer. J. Cell Mol. Med. 2019, 23, 4030–4042. [Google Scholar] [CrossRef]
- Kim, M.; Hernandez, L.; Annunziata, C.M. Caspase 8 expression may determine the survival of women with ovarian cancer. Cell Death Dis. 2016, 7, e2045. [Google Scholar] [CrossRef]
- Schmitt, J.; Matei, D. Targeting angiogenesis in ovarian cancer. Cancer Treat. Rev. 2012, 38, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Mangala, L.S.; Mooberry, L.; Bayraktar, E.; Dasari, S.K.; Ma, S.L.; Ivan, C.; Court, K.A.; Rodriguez-Aguayo, C.; Bayraktar, R.; et al. Identifying and targeting angiogenesis-related microRNAs in ovarian cancer. Oncogene 2019, 38, 6095–6108. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Xu, T.E.; Zheng, L.F.; Li, G.L. Angiogenesis inhibitors for the treatment of ovarian cancer: An updated systematic review and meta-analysis of randomized controlled trials. Int. J. Gynecol. Cancer 2018, 28, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.; Anttila, M.; Rautiainen, S.; Arponen, O.; Hamalainen, K.; Kononen, M.; Vanninen, R.; Sallinen, H. Dynamic contrast-enhanced perfusion parameters in ovarian cancer: Good accuracy in identifying high HIF-1 alpha expression. PLoS ONE 2019, 14, e0221340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhao, C.Y.; Zhou, Y.T.; Zheng, J.; Gao, S.J.; Lu, Y. HIF-1 alpha binding to AEG-1 promoter induced upregulated AEG-1 expression associated with metastasis in ovarian cancer. Cancer Med. 2017, 6, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Long, F.Y.; Liu, W.X.; Jia, P.; Wang, H.F.; Jiang, G.; Wang, T. HIF-1 alpha-induced autophagy contributes to cisplatin resistance in ovarian cancer cells. Pharmazie 2018, 73, 533–536. [Google Scholar] [PubMed]
- Ai, Z.H.; Lu, Y.; Qiu, S.B.; Fan, Z. Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism. Cancer Lett. 2016, 373, 36–44. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, C.Y.; Ye, N.; Liu, Z.Q.; Wold, E.A.; Chen, H.Y.; Wild, C.; Shen, Q.; Zhou, J. Discovery and development of natural product oridonin-inspired anticancer agents. Eur. J. Med. Chem. 2016, 122, 102–117. [Google Scholar] [CrossRef]
- Jeepipallia, S.P.K.; Du, B.; Sabitaliyevich, U.Y.; Xu, B. New insights into potential nutritional effects of dietary saponins in protecting against the development of obesity. Food Chem. 2020, 318, 126474. [Google Scholar] [CrossRef]
- Xu, X.H.; Li, T.; Fong, C.M.V.; Chen, X.P.; Chen, X.J.; Wang, Y.T.; Huang, M.Q.; Lu, J.J. Saponins from chinese medicines as anticancer agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef]
- Guo, N.; Tong, T.T.; Ren, N.; Tu, Y.Y.; Li, B. Saponins from seeds of Genus Camellia: Phytochemistry and bioactivity. Phytochemistry 2018, 149, 42–55. [Google Scholar] [CrossRef]
- Li, N.; Ma, Z.J.; Chu, Y.; Wang, Y.; Li, X. Phytochemical analysis of the triterpenoids with cytotoxicity and QR inducing properties from the total tea seed saponin of Camellia sinensis. Fitoterapia 2013, 84, 321–325. [Google Scholar] [CrossRef]
- Jia, L.Y.; Xia, H.L.; Chen, Z.D.; Compton, C.; Bucur, H.; Sawant, D.A.; Rankin, G.O.; Li, B.; Tu, Y.Y.; Chen, Y.C. Anti-proliferation effect of theasaponin E-1 on the ALDH-positive ovarian cancer stem-like cells. Molecules 2018, 23, 1469. [Google Scholar] [CrossRef]
- Zhao, P.X.; Li, M.S.; Chen, Y.; He, C.C.; Zhang, X.J.; Fan, T.; Yang, T.; Lu, Y.; Lee, R.J.; Ma, X.; et al. Selenium-doped calcium carbonate nanoparticles loaded with cisplatin enhance efficiency and reduce side effects. Int. J. Pharm. 2019, 570, 118638. [Google Scholar] [CrossRef] [PubMed]
- Shirjang, S.; Mansoori, B.; Asghari, S.; Duijf, P.H.G.; Mohammadi, A.; Gjerstorff, M.; Baradaran, B. MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radic. Biol. Med. 2019, 139, 1–15. [Google Scholar] [CrossRef]
- Derakhshan, A.; Chen, Z.; Van Waes, C. Therapeutic small molecules target inhibitor of apoptosis proteins in cancers with deregulation of extrinsic and intrinsic cell death pathways. Clin. Cancer Res. 2017, 23, 1379–1387. [Google Scholar] [CrossRef]
- Yang, S.; Mao, Y.J.; Zhang, H.J.; Xu, Y.; An, J.; Huang, Z.W. The chemical biology of apoptosis: Revisited after 17 years. Eur. J. Med. Chem. 2019, 177, 63–75. [Google Scholar] [CrossRef]
- Seo, J.; Kim, M.W.; Bae, K.H.; Lee, S.C.; Song, J.; Lee, E.W. The roles of ubiquitination in extrinsic cell death pathways and its implications for therapeutics. Biochem. Pharmacol. 2019, 162, 21–40. [Google Scholar] [CrossRef]
- Sachan, R.; Kundu, A.; Jeon, Y.; Choi, W.S.; Yoon, K.; Kim, I.S.; Kwak, J.H.; Kim, H.S. Afrocyclamin A, a triterpene saponin, induces apoptosis and autophagic cell death via the PI3K/Akt/mTOR pathway in human prostate cancer cells. Phytomedicine 2018, 51, 139–150. [Google Scholar] [CrossRef]
- Zong, J.F.; Wang, D.X.; Jiao, W.T.; Zhang, L.; Bao, G.H.; Ho, C.T.; Hou, R.Y.; Wan, X.C. Oleiferasaponin C-6 from the seeds of Camellia oleifera Abel.: A novel compound inhibits proliferation through inducing cell-cycle arrest and apoptosis on human cancer cell lines in vitro. RSC Adv. 2016, 6, 91386–91393. [Google Scholar] [CrossRef]
- Mo, S.S.; Xiong, H.; Shu, G.W.; Yang, X.Z.; Wang, J.X.; Zheng, C.Y.; Xiong, W.; Mei, Z.N. Phaseoloideside E, a novel natural triterpenoid saponin identified from entada phaseoloides, induces apoptosis in Ec-109 esophageal cancer cells through reactive oxygen species generation. J. Pharmacol. Sci. 2013, 122, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Stucki, M. Histone H2A.X Tyr142 phosphorylation: A novel sWItCH for apoptosis? DNA Repair 2009, 8, 873–876. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Q.; Li, B.; Xie, J.M.; Yang, X.D.; Zhao, K.; Wu, Y.; Ye, Z.Y.; Chen, Z.R.; Qin, Z.H.; et al. Escin-induced DNA damage promotes escin-induced apoptosis in human colorectal cancer cells via p62 regulation of the ATM/gamma H2AX pathway. Acta Pharmacol. Sin. 2018, 39, 1645–1660. [Google Scholar] [CrossRef] [PubMed]
- Iness, A.N.; Litovchick, L. MuvB: A key to cell cycle control in ovarian cancer. Front. Oncol. 2018, 8, 223. [Google Scholar] [CrossRef]
- Shin, S.S.; Hwang, B.; Muhammad, K.; Gho, Y.; Song, J.H.; Kim, W.J.; Kim, G.; Moon, S.K. Nimbolide represses the proliferation, migration, and invasion of bladder carcinoma cells via Chk2-mediated G2/M phase cell cycle arrest, altered signaling pathways, and reduced transcription factors-associated MMP-9 expression. Evid. Based Complement. Altern. 2019, 2019, 3753587. [Google Scholar] [CrossRef]
- Gogineni, V.R.; Nalla, A.K.; Gupta, R.; Dinh, D.H.; Klopfenstein, J.D.; Rao, J.S. Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells. Cancer Lett. 2011, 313, 64–75. [Google Scholar] [CrossRef]
- Garrido, M.P.; Torres, I.; Vega, M.; Romero, C. Angiogenesis in Gynecological Cancers: Role of neurotrophins. Front. Oncol. 2019, 9, 913. [Google Scholar] [CrossRef]
- Caporarello, N.; Lupo, G.; Olivieri, M.; Cristaldi, M.; Cambria, M.T.; Salmeri, M.; Anfuso, C.D. Classical VEGF, Notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions (Review). Mol. Med. Rep. 2017, 16, 4393–4402. [Google Scholar] [CrossRef]
- Han, Y.Q.; Pan, L.Y.; Ran, S.; Song, Y.; Sun, F.F.; Wang, Y.Z.; Hong, Y. Rhizoma Paridis saponins ameliorates hepatic fibrosis in rats by downregulating expression of angiogenesis-associated growth factors. Mol. Med. Rep. 2019, 19, 3548–3554. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Sha, D.J.; Wang, S.L.; Li, C.S.; Qian, J.; Wang, J.Q.; Zhao, Y.; Zhang, J.H.; Cheng, H.Y.; Yang, H.; et al. Panaxatriol saponins promotes angiogenesis and enhances cerebral perfusion after ischemic stroke in rats. BMC Complement. Altern. Med. 2017, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.B.; Zhao, Y.; Guo, X.; Zhang, L.X.; Li, P.Y.; Fu, T.H.; Wang, W.D.; Yin, Y.X.; Chen, G.L.; Liu, J.P. Chiisanoside, a triterpenoid saponin, exhibits anti-tumor activity by promoting apoptosis and inhibiting angiogenesis. RSC Adv. 2017, 7, 41640–41650. [Google Scholar] [CrossRef]
- Rajasekar, J.; Perumal, M.K.; Vallikannan, B. A critical review on anti-angiogenic property of phytochemicals. J. Nutr. Biochem. 2019, 71, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Fan, L.L.; Gao, Q.S.; Silwal, B.M.; Ren, M.L.; Shen, Y.; Qu, W.L. Targeted therapy of ovarian cancer with angiogenesis inhibitors. Curr. Drug Targets 2017, 18, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Redfern, A.; Agarwal, V.; Thompson, E.W. Hypoxia as a signal for prison breakout in cancer. Curr. Opin. Clin. Nutr. 2019, 22, 250–263. [Google Scholar] [CrossRef]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef]
- Mi, C.L.; Ma, J.; Shi, H.; Li, J.; Wang, F.; Lee, J.J.; Jin, X.J. 4′,6-Dihydroxy-4-methoxyisoaurone inhibits the HIF-1 alpha pathway through inhibition of Akt/mTOR/p70S6K/4E-BP1 phosphorylation. J. Pharmacol. Sci. 2014, 125, 193–201. [Google Scholar] [CrossRef]
- Ataie-Kachoie, P.; Pourgholami, M.H.; Bahrami-B, F.; Badar, S.; Morris, D.L. Minocycline attenuates hypoxia-inducible factor-1 alpha expression correlated with modulation of p53 and AKT/mTOR/p70S6K/4E-BP1 pathway in ovarian cancer: In vitro and in vivo studies. Am. J. Cancer Res. 2015, 5, 575–588. [Google Scholar]
- Huang, J.L.; Gao, L.K.; Li, B.S.; Liu, C.; Hong, S.S.; Min, L.; Hong, L. Knockdown of hypoxia-inducible factor 1 alpha (HIF-1 alpha) promotes autophagy and inhibits phosphatidylinositol 3-Kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway in ovarian cancer cells. Med. Sci. Monit. 2019, 25, 4250–4263. [Google Scholar] [CrossRef]
- Zhang, M.F.; Hagan, C.T.; Min, Y.Z.; Foley, H.; Tian, X.; Yang, F.F.; Mi, Y.; Au, K.M.; Medik, Y.; Roche, K.; et al. Nanoparticle co-delivery of wortmannin and cisplatin synergistically enhances chemoradiotherapy and reverses platinum resistance in ovarian cancer models. Biomaterials 2018, 169, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, T.; Burguillos, M.A.; Lopez-Lluch, G.; Navas, P.; Herrador, M.; Gonzalez, I.; Pinero, J. Enhanced induction of apoptosis in a radio-resistant bladder tumor cell line by combined treatments with X-rays and wortmannin. Radiat. Environ. Biophys. 2008, 47, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Kantidze, O.L.; Velichko, A.K.; Luzhin, A.V.; Petrova, N.V.; Razin, S.V. Synthetically lethal interactions of ATM, ATR, and DNA-PKcs. Trends Cancer 2018, 4, 755–768. [Google Scholar] [CrossRef]
- Abdel-Fatah, T.M.; Arora, A.; Moseley, P.; Coveney, C.; Perry, C.; Johnson, K.; Kent, C.; Ball, G.; Chan, S.; Madhusudan, S. ATM, ATR and DNA-PKcs expressions correlate to adverse clinical outcomes in epithelial ovarian cancers. BBA Clin. 2014, 2, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, P.; Yang, L.N.; Liu, Y.; Wang, Y.; Liu, M.M.; Qi, Z.H.; Meng, J.; Shi, T.Y.; Yang, G.; et al. Wip1 suppresses ovarian cancer metastasis through the ATM/AKT/Snail mediated signaling. Oncotarget 2016, 7, 29359–29370. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Zhang, Y.Y.; Zhu, B.L.; Feng, F.Z.; Zhang, H.T.; Yan, H.; Zhou, B. MiR-203a-3p regulates the biological behaviors of ovarian cancer cells through mediating the Akt/GSK-3 beta/Snail signaling pathway by targeting ATM. J. Ovarian Res. 2019, 12, 60. [Google Scholar] [CrossRef]
- Ali, R.; Alabdullah, M.; Miligy, I.; Normatova, M.; Babaei-Jadidi, R.; Nateri, A.S.; Rakha, E.A.; Madhusudan, S. ATM regulated PTEN degradation is XIAP E3 ubiquitin ligase mediated in p85alpha deficient cancer cells and influence platinum sensitivity. Cells 2019, 8, 1271. [Google Scholar] [CrossRef]
- Chen, J.; Bai, M.; Ning, C.; Xie, B.; Zhang, J.; Liao, H.; Xiong, J.; Tao, X.; Yan, D.; Xi, X.; et al. Gankyrin facilitates follicle-stimulating hormone-driven ovarian cancer cell proliferation through the PI3K/AKT/HIF-1 alpha/cyclin D1 pathway. Oncogene 2016, 35, 2506–2517. [Google Scholar] [CrossRef]
- Ma, Y.C.; Su, N.; Shi, X.J.; Zhao, W.; Ke, Y.; Zi, X.L.; Zhao, N.M.; Qin, Y.H.; Zhao, H.W.; Liu, H.M. Jaridonin-induced G2/M phase arrest in human esophageal cancer cells is caused by reactive oxygen species-dependent Cdc2-tyr15 phosphorylation via ATM-Chk1/2-Cdc25C pathway. Toxicol. Appl. Pharm. 2015, 282, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Aster, J.C.; Pear, W.S.; Blacklow, S.C. The varied roles of Notch in cancer. Annu. Rev. Pathol. Mech. 2017, 12, 245–275. [Google Scholar] [CrossRef]
- Groeneweg, J.W.; Foster, R.; Growdon, W.B.; Verheijen, R.H.M.; Rueda, B.R. Notch signaling in serous ovarian cancer. J. Ovarian Res. 2014, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.L.; Kunnimalaiyaan, M.; Drenzek, J.; Seiler, N. Notch 1 signaling is active in ovarian cancer. Gynecol. Oncol. 2010, 117, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, W.; Hu, L.M.; Previs, R.A.; Dalton, H.J.; Yang, X.Y.; Sun, Y.J.; McGuire, M.; Rupaimoole, R.; Nagaraja, A.S.; et al. Dll4 inhibition plus aflibercept markedly reduces ovarian tumor growth. Mol. Cancer Ther. 2016, 15, 1344–1352. [Google Scholar] [CrossRef]
- Yang, J.; Xing, H.; Lu, D.H.; Wang, J.; Li, B.S.; Tang, J.M.; Gu, F.Q.; Hong, L. Role of Jagged1/STAT3 signalling in platinum-resistant ovarian cancer. J. Cell Mol. Med. 2019, 23, 4005–4018. [Google Scholar] [CrossRef] [PubMed]
- Vermezovic, J.; Adamowicz, M.; Santarpia, L.; Rustighi, A.; Forcato, M.; Lucano, C.; Massimiliano, L.; Costanzo, V.; Bicciato, S.; Del Sal, G.; et al. Notch is a direct negative regulator of the DNA-damage response. Nat. Struct. Mol. Biol. 2015, 22, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, J.H.; Woo, J.S. Resveratrol induces cell death through ROS-dependent downregulation of Notch1/PTEN/Akt signaling in ovarian cancer cells. Mol. Med. Rep. 2019, 19, 3353–3360. [Google Scholar] [CrossRef]
- Shen, F.H.; Xiong, Z.W.; Kong, J.M.; Wang, L.; Cheng, Y.S.; Jin, J.; Huang, Z.Y. Triptolide impairs thioredoxin system by suppressing Notch1-mediated PTEN/Akt/Txnip signaling in hepatocytes. Toxicol. Lett. 2019, 300, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Poddar, S.; Kesharwani, D.; Datta, M. miR-449a regulates insulin signalling by targeting the Notch ligand, Jag1 in skeletal muscle cells. Cell Commun. Signal. 2019, 17, 84. [Google Scholar] [CrossRef]
- Li, S.R.; Ren, B.; Shi, Y.; Gao, H.; Wang, J.W.; Xin, Y.; Huang, B.; Liao, S.C.; Yang, Y.P.; Xu, Z.X.; et al. Notch1 inhibition enhances DNA damage induced by cisplatin in cervical cancer. Exp. Cell Res. 2019, 376, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Gong, L.H.; Ou, R.Y.; Zheng, Z.Z.; Chen, J.Y.; Xie, F.F.; Huang, X.X.; Qiu, J.; Zhang, W.J.; Jiang, Q.W.; et al. Sequential combination therapy of ovarian cancer with cisplatin and gamma-secretase inhibitor MK-0752. Gynecol. Oncol. 2016, 140, 537–544. [Google Scholar] [CrossRef]
- Böttger, S.; Melzig, M.F. The influence of saponins on cell membrane cholesterol. Bioorg. Med. Chem. 2013, 21, 7118–7124. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jia, L.; Wu, J.; Liu, Y.; Kang, H.; Liu, X.; Li, P.; He, P.; Tu, Y.; Li, B. Simultaneous determination and quantification of triterpene saponins from Camellia sinensis seeds using UPLC-PDA-QTOF-MS/MS. Molecules 2019, 24, 3794. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Tong, T.; Ren, N.; Rankin, G.O.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Theasaponin E1 Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis. Molecules 2021, 26, 1681. https://doi.org/10.3390/molecules26061681
Li B, Tong T, Ren N, Rankin GO, Rojanasakul Y, Tu Y, Chen YC. Theasaponin E1 Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis. Molecules. 2021; 26(6):1681. https://doi.org/10.3390/molecules26061681
Chicago/Turabian StyleLi, Bo, Tuantuan Tong, Ning Ren, Gary O. Rankin, Yon Rojanasakul, Youying Tu, and Yi Charlie Chen. 2021. "Theasaponin E1 Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis" Molecules 26, no. 6: 1681. https://doi.org/10.3390/molecules26061681
APA StyleLi, B., Tong, T., Ren, N., Rankin, G. O., Rojanasakul, Y., Tu, Y., & Chen, Y. C. (2021). Theasaponin E1 Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis. Molecules, 26(6), 1681. https://doi.org/10.3390/molecules26061681