Validation of a Sensor-Based Gait Analysis System with a Gold-Standard Motion Capture System in Patients with Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
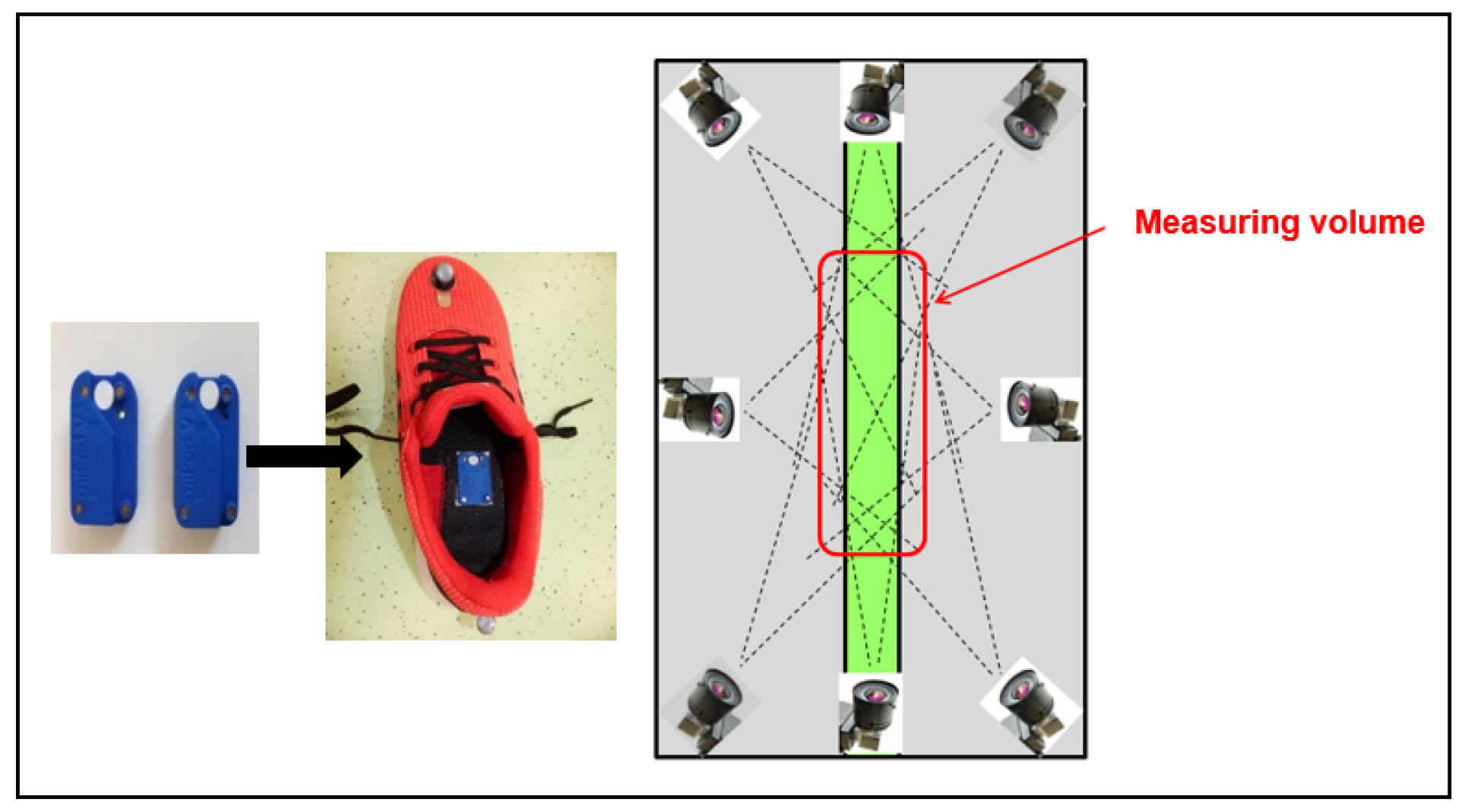
2.2. Study Procedure
2.3. Statistical Analysis
3. Results
3.1. Correlation of MGL and MC Gait Parameters
3.2. Clinical Relevance of Gait Analysis Systems—Comparison with Control Group
4. Discussion
4.1. Sensor-Based Gait Analysis System Provides Valid Results in PD Patients
4.2. Shuffling Gait Parameters
4.3. Clinical Relevance of Sensor-Based Gait Parameters
4.4. Use Cases for Gold Standard and Mobile Systems
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.W.; Song, Y.S.; Kim, H.; Ku, B.D.; Lee, W.W. Alteration of tremor dominant and postural instability gait difficulty subtypes during the progression of Parkinson’s disease: Analysis of the PPMI cohort. Front. Neurol. 2019, 10, 471. [Google Scholar] [CrossRef] [Green Version]
- Amadori, K.; Püllen, R.; Steiner, T. Gangstörungen im Alter (Gait disorders in the elderly). Der Nervenarzt 2014, 85, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Gaßner, H.; Sanders, P.; Dietrich, A.; Marxreiter, F.; Eskofier, B.M.; Winkler, J.; Klucken, J. Clinical relevance of standardized mobile gait tests. Reliability analysis between gait recordings at hospital and home in Parkinson’s disease: A pilot study. J. Parkinson’s Dis. 2020, 10, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Wang, R.-L.; Liou, D.-J.; Shaw, J.-S. Gait disorders in Parkinson’s disease: Assessment and management. Int. J. Gerontol. 2013, 7, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Pau, M.; Corona, F.; Pili, R.; Casula, C.; Guicciardi, M.; Cossu, G.; Murgia, M. Quantitative assessment of gait parameters in people with Parkinson’s disease in laboratory and clinical setting: Are the measures interchangeable? Neurol. Int. 2018, 10, 7729. [Google Scholar] [CrossRef] [Green Version]
- Götz-Neumann, K. Gehen Verstehen: Ganganalyse in der Physiotherapie, 4th ed.; Thieme: Stuttgart, Germany, 2016. [Google Scholar]
- Perry, J.; Burnfield, J. Gait Analysis—Normal and Pathological Function, 2nd ed.; SLACK Incorporated: Thorofare, NJ, USA, 2010. [Google Scholar]
- Eskofier, B.; Lee, S.; Baron, M.; Simon, A.; Martindale, C.; Gaßner, H.; Klucken, J. An overview of smart shoes in the internet of health things: Gait and mobility assessment in health promotion and disease monitoring. Appl. Sci. 2017, 7, 986. [Google Scholar] [CrossRef] [Green Version]
- Del Din, S.; Godfrey, A.; Mazzà, C.; Lord, S.; Rochester, L. Free-living monitoring of Parkinson’s disease: Les-sons from the field. Mov. Disord. 2016, 31, 1293–1313. [Google Scholar] [CrossRef]
- Klucken, J.; Barth, J.; Kugler, P.; Schlachetzki, J.; Henze, T.; Marxreiter, F.; Kohl, Z.; Steidl, R.; Hornegger, J.; Eskofier, B.; et al. Unbiased and mobile gait analysis detects motor impairment in Parkinson’s disease. PLoS ONE 2013, 8, e56956. [Google Scholar] [CrossRef]
- Gaßner, H.; Raccagni, C.; Eskofier, B.M.; Klucken, J.; Wenning, G.K. The diagnostic scope of sensor-based gait analysis in atypical parkinsonism: Further observations. Front. Neurol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Schlachetzki, J.C.M.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J.; et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS ONE 2017, 12, e0183989. [Google Scholar] [CrossRef]
- Kluge, F.; Gaßner, H.; Hannink, J.; Pasluosta, C.; Klucken, J.; Eskofier, B.M. Towards mobile gait analysis: Concurrent validity and test-retest reliability of an inertial measurement system for the assessment of spatio-temporal gait parameters. Sensors 2017, 17, 1522. [Google Scholar] [CrossRef]
- Monticone, M.; Ambrosini, E.; Laurini, A.; Rocca, B.; Foti, C. In-patient multidisciplinary rehabilitation for Parkinson’s disease: A randomized controlled trial. Mov. Disord. 2015, 30, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Rochester, L.; Espay, A.J. Multidisciplinary rehabilitation in Parkinson’s disease: A mile-stone with future challenges. Mov. Disord. 2015, 30, 1011–1013. [Google Scholar] [CrossRef]
- Ferrazzoli, D.; Ortelli, P.; Zivi, I.; Cian, V.; Urso, E.; Ghilardi, M.F.; Maestri, R.; Frazzitta, G. Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: A randomised controlled study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Barth, J.; Oberndorfer, C.; Pasluosta, C.; Schülein, S.; Gassner, H.; Reinfelder, S.; Kugler, P.; Schuldhaus, D.; Winkler, J.; Klucken, J.; et al. Stride segmentation during free walk movements using multi-dimensional subsequence dynamic time warping on inertial sensor data. Sensors 2015, 15, 6419–6440. [Google Scholar] [CrossRef] [PubMed]
- Rampp, A.; Barth, J.; Schülein, S.; Gaßmann, K.-G.; Klucken, J.; Eskofier, B.M. Inertial sensor-based stride parameter calculation from gait sequences in geriatric patients. IEEE Trans. Biomed. Eng. 2015, 62, 1089–1097. [Google Scholar] [CrossRef]
- Gaßner, H.; Marxreiter, F.; Steib, S.; Kohl, Z.; Schlachetzki, J.C.M.; Adler, W.; Eskofier, B.M.; Pfeifer, K.; Winkler, J.; Klucken, J. Gait and cognition in Parkinson’s disease: Cognitive impairment is inadequately reflected by gait performance during dual task. Front. Neurol. 2017, 8, 550. [Google Scholar] [CrossRef] [Green Version]
- Kanzler, C.M.; Barth, J.; Rampp, A.; Schlarb, H.; Rott, F.; Klucken, J.; Eskofier, B.M. Inertial sensor based and shoe size independent gait analysis including heel and toe clearance estimation. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 5424–5427. [Google Scholar]
- Baudet, A.; Morisset, C.; d’Athis, P.; Maillefert, J.-F.; Casillas, J.-M.; Ornetti, P.; Laroche, D. Cross-talk correction method for knee kinematics in gait analysis using principal component analysis (PCA): A new proposal. PLoS ONE 2014, 9, e102098. [Google Scholar] [CrossRef]
- McCahill, J.; Stebbins, J.; Theologis, T. Use of the Oxford Foot Model in clinical practice. J. Foot. Ankle. Res. 2008, 1, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Mariani, B.; Rochat, S.; Büla, C.J.; Aminian, K. Heel and toe clearance estimation for gait analysis using wireless inertial sensors. IEEE Trans. Biomed. Eng. 2012, 59, 3162–3168. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Pistacchi, M.; Gioulis, M.; Sanson, F.; de Giovannini, E.; Filippi, G.; Rossetto, F.; Zambito Marsala, S. Gait analysis and clinical correlations in early Parkinson’s disease. Funct. Neurol. 2017, 32, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Roth, N.; Ghassemi, N.H.; Hannink, J.; Seel, T.; Klucken, J.; Gassner, H.; Eskofier, B.M. Development and clinical validation of inertial sensor-based gait-clustering methods in Parkinson’s disease. J. Neuroeng. Rehabil. 2019, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef] [Green Version]

| Validation Group PD (n = 37) | Control Group (n = 13) | p | |
|---|---|---|---|
| Age (years) | 70.8 ± 9.3 | 72.2 ± 28.8 | 0.575 |
| Gender (m:f) | 20:17 | 8:6 | 0.843 ° |
| UPDRS-III (score) | 19.9 ± 9.0 | na | na |
| H&Y (score) | 3.1 ± 0.7 | na | na |
| 1; 1.5 | 1 | na | na |
| 2; 2.5 | 5 | na | na |
| 3; 3.5 | 21 | na | na |
| 4 | 12 | na | na |
| MMST (score) | 28.4 ± 1.8 | na | na |
| Gait (without:with wheeled walker) | 25:12 | 14:0 | 0.046 ° |
| ICC | 95% Confidence Interval | p | αc * | ||
|---|---|---|---|---|---|
| Minimum | Maximum | ||||
| Bradykinesia/short steps | |||||
| gait speed | 0.986 | 0.975 | 0.992 | <0.001 | <0.001 |
| stride length | 0.985 | 0.974 | 0.992 | <0.001 | <0.001 |
| stride time | 0.966 | 0.942 | 0.981 | <0.001 | <0.001 |
| rel. stance time | 0.964 | 0.938 | 0.980 | <0.001 | <0.001 |
| rel. swing time | 0.964 | 0.938 | 0.980 | <0.001 | <0.001 |
| Shuffling gait | |||||
| HS | 0.822 | 0.162 | 0.943 | <0.001 | <0.001 |
| TO | 0.903 | 0.505 | 0.967 | <0.001 | <0.001 |
| TC | 0.900 | 0.425 | 0.967 | <0.001 | <0.001 |
| System | Group Comparison | ||||
|---|---|---|---|---|---|
| Mean | std | p | αc * | ||
| Bradykinesia/short steps | |||||
| gait speed [m/s] | MGL | 0.98 | 0.32 | 0.780 | 0.780 |
| MC | 0.98 | 0.32 | |||
| stride length [m] | MGL | 1.14 | 0.29 | 0.122 | 0.195 |
| MC | 1.13 | 0.28 | |||
| stride time [s] | MGL | 1.20 | 0.16 | 0.003 | 0.006 |
| MC | 1.19 | 0.16 | |||
| stance time [%] | MGL | 65.40 | 2.47 | 0.192 | 0.219 |
| MC | 65.14 | 2.68 | |||
| swing time [%] | MGL | 34.60 | 2.47 | 0.192 | 0.219 |
| MC | 34.86 | 2.68 | |||
| Shuffling gait | |||||
| HS [°] | MGL | 8.97 | 5.52 | <0.001 | <0.001 |
| MC | 16.49 | 7.00 | |||
| TO [°] | MGL | 61.27 | 11.84 | <0.001 | <0.001 |
| MC | 52.95 | 9.01 | |||
| TC [cm] | MGL | 5.87 | 2.77 | <0.001 | <0.001 |
| MC | 8.32 | 2.99 | |||
| Group Comparison | Effect Size | p (Benjamini–Hochberg Post Hoc Test) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p | αc | z | r | c–II | αc * c–II | C–III | αc * c–III | c–IV | αc * c–IV | ||
| Bradykinesia/short steps | |||||||||||
| gait speed [m/s] | MGL | 0.002 | 0.003 | 3.784 | 0.53 | 0.172 | 0.356 | 0.007 | 0.011 | <0.001 | <0.001 |
| MC | 0.001 | 0.002 | 3.880 | 0.54 | 0.151 | 0.243 | 0.005 | 0.010 | <0.001 | <0.001 | |
| stride length [m] | MGL | 0.001 | 0.003 | 3.917 | 0.55 | 0.262 | 0.356 | 0.023 | 0.031 | <0.001 * | <0.001 |
| MC | 0.001 | 0.002 | 3.926 | 0.55 | 0.247 | 0.247 | 0.024 | 0.024 | <0.001 | <0.001 | |
| stride time [s] | MGL | 0.025 | 0.002 | −2.798 | 0.39 | 0.267 | 0.356 | 0.005 | 0.001 | 0.005 | 0.005 |
| MC | 0.008 | 0.008 | −2.958 | 0.41 | 0.201 | 0.247 | 0.003 | 0.001 | 0.003 | 0.003 | |
| stance time [%] | MGL | 0.044 | 0.044 | −2.832 | 0.40 | 0.464 | 0.464 | 0.159 | 0.159 | 0.005 | 0.005 |
| MC | 0.002 | 0.027 | −3.766 | 0.53 | 0.029 | 0.116 | 0.020 | 0.023 | <0.001 | <0.001 | |
| swing time [%] | MGL | 0.044 | 0.044 | 2.832 | 0.40 | 0.464 | 0.464 | 0.159 | 0.159 | 0.005 | 0.005 |
| MC | 0.002 | 0.027 | 3.766 | 0.53 | 0.029 | 0.116 | 0.020 | 0.023 | <0.001 | <0.001 | |
| Shuffling gait | |||||||||||
| HS [°] | MGL | 0.001 | 0.003 | 3.740 | 0.52 | 0.011 | 0.088 | 0.001 | 0.004 | <0.001 | <0.001 |
| MC | 0.001 | 0.002 | 3.856 | 0.54 | 0.060 | 0.158 | 0.003 | 0.010 | <0.001 | <0.001 | |
| TO [°] | MGL | 0.002 | 0.003 | 3.701 | 0.52 | 0.204 | 0.356 | 0.004 | 0.010 | <0.001 | <0.001 |
| MC | 0.003 | 0.003 | 3.463 | 0.48 | 0.217 | 0.247 | 0.005 | 0.010 | 0.001 | 0.001 | |
| TC [cm] | MGL | <0.001 | <0.001 | 4.370 | 0.61 | 0.024 | 0.096 | 0.001 | 0.004 | <0.001 | <0.001 |
| MC | 0.001 | 0.002 | 4.209 | 0.69 | 0.079 | 0.158 | 0.008 | 0.013 | <0.001 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakob, V.; Küderle, A.; Kluge, F.; Klucken, J.; Eskofier, B.M.; Winkler, J.; Winterholler, M.; Gassner, H. Validation of a Sensor-Based Gait Analysis System with a Gold-Standard Motion Capture System in Patients with Parkinson’s Disease. Sensors 2021, 21, 7680. https://doi.org/10.3390/s21227680
Jakob V, Küderle A, Kluge F, Klucken J, Eskofier BM, Winkler J, Winterholler M, Gassner H. Validation of a Sensor-Based Gait Analysis System with a Gold-Standard Motion Capture System in Patients with Parkinson’s Disease. Sensors. 2021; 21(22):7680. https://doi.org/10.3390/s21227680
Chicago/Turabian StyleJakob, Verena, Arne Küderle, Felix Kluge, Jochen Klucken, Bjoern M. Eskofier, Jürgen Winkler, Martin Winterholler, and Heiko Gassner. 2021. "Validation of a Sensor-Based Gait Analysis System with a Gold-Standard Motion Capture System in Patients with Parkinson’s Disease" Sensors 21, no. 22: 7680. https://doi.org/10.3390/s21227680
APA StyleJakob, V., Küderle, A., Kluge, F., Klucken, J., Eskofier, B. M., Winkler, J., Winterholler, M., & Gassner, H. (2021). Validation of a Sensor-Based Gait Analysis System with a Gold-Standard Motion Capture System in Patients with Parkinson’s Disease. Sensors, 21(22), 7680. https://doi.org/10.3390/s21227680









