Smart Sensor Based on Biofeedback to Measure Child Relaxation in Out-of-Home Care
Abstract
1. Introduction
2. Materials and Methods
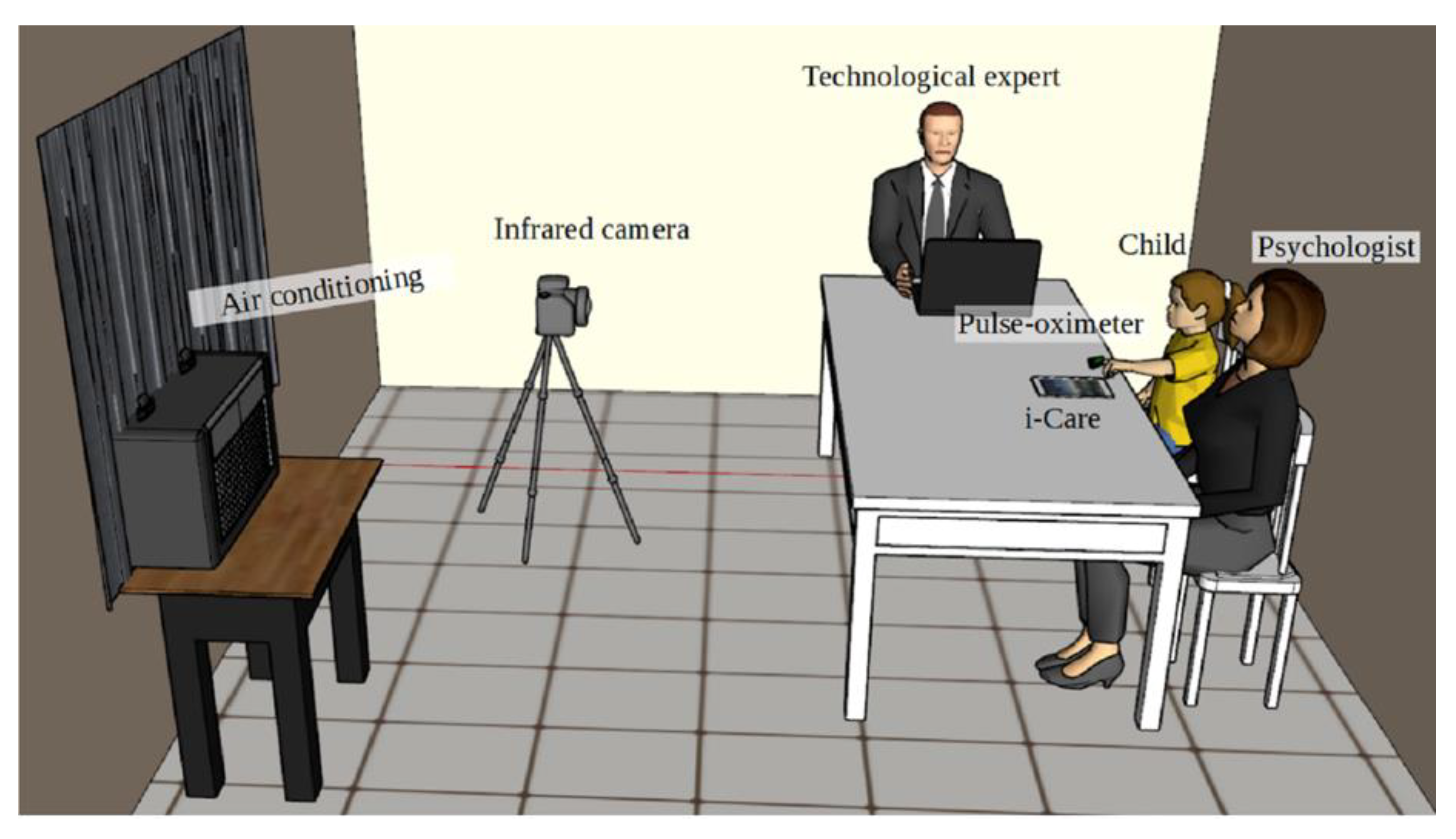
2.1. Technological Equipment
2.2. Conditioned Space
2.3. Children
2.4. i-CARE
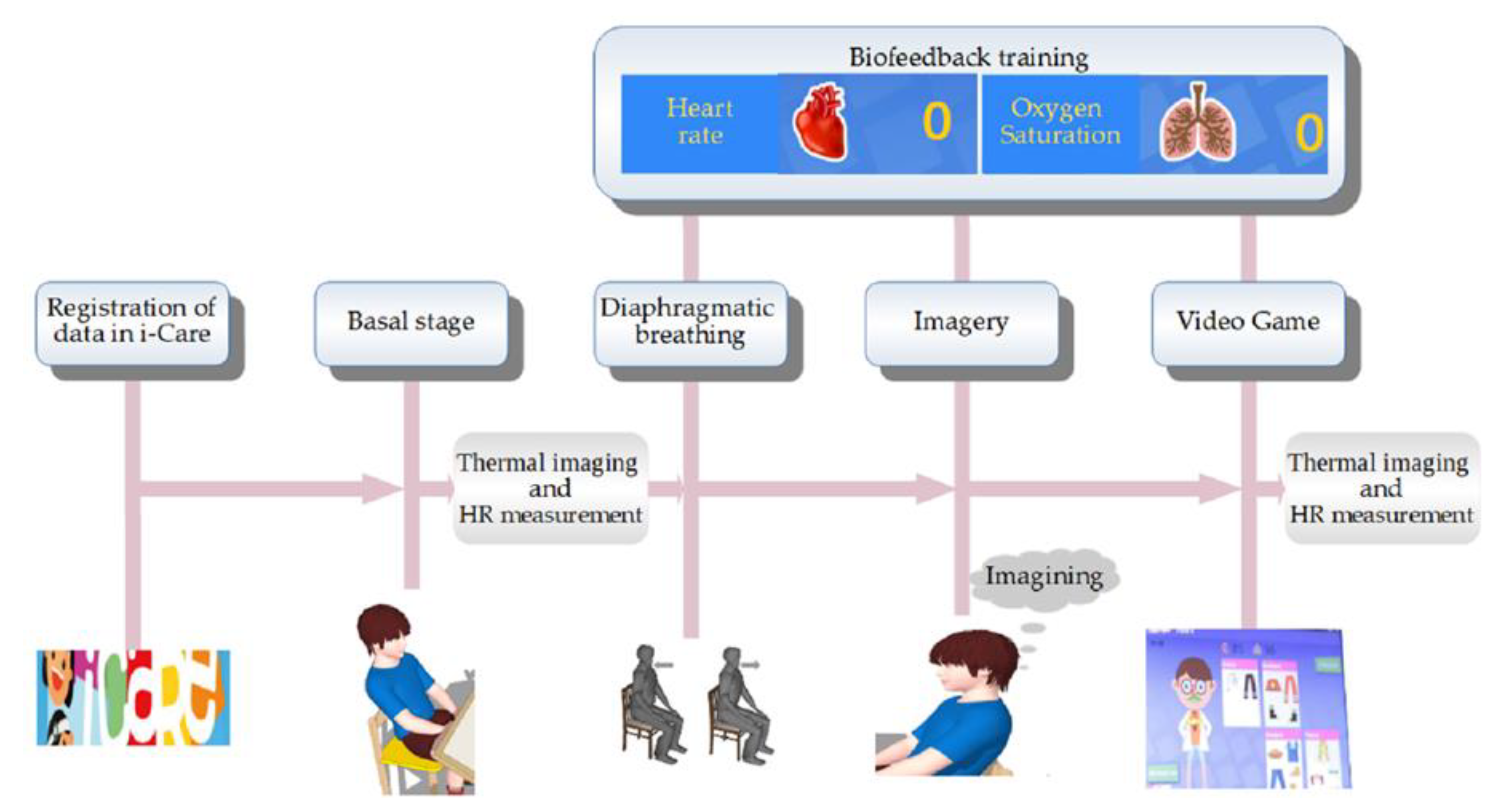
i-CARE Functions
- Phase 1. Baseline (3 min). In this phase, the child remained seated in silence, while their physiological parameters were evaluated by the smart sensor. The instructor (Psychologist) was only an observer;
- Phase 2. Training based on biofeedback (10 min). The instructor trained the child in diaphragmatic breathing and used the physiological parameters for biofeedback (visual signal on i-CARE’s screen in real time);
- Phase 3. Training in relaxation through guided imagery (10 min). On this screen, there was an ocean picture and relaxing music was played ex professo on i-CARE. The instructor trained the child on relaxation through guided imagery (therapeutic narrative), while his physiological parameters were evaluated by the smart sensor and were displayed on i-CARE’s screen;
- Phase 4. Video game (5 min). In this phase, two screens appeared with images of a female doctor, a male doctor, and different clothes, the child could choose the character to play with, and the instructor explained the activity. This phase created a virtual recreational space for the child to relax in and promoted attention, classification, and self-efficacy skills in the child. Physiological parameters were evaluated, but did not appear on the screen for a better visualization of the game.
2.5. Protocol Application
2.6. Smart Sensor
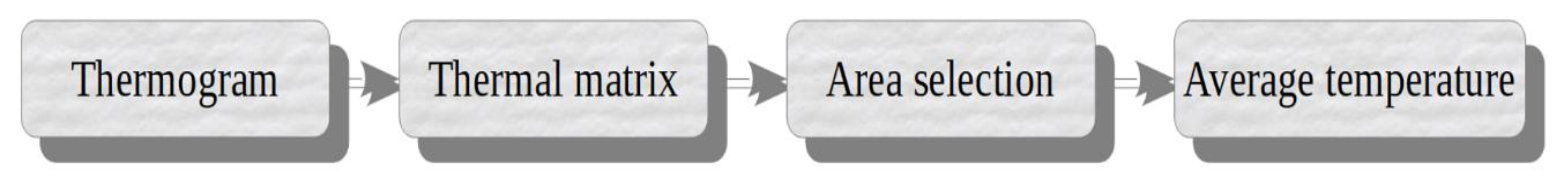
2.6.1. Thermal Image Acquisition and Processing
2.6.2. Acquisition and Processing of HR (Pulse)
2.6.3. Use of the K-NN Classifier
3. Results
3.1. Temperatures
Paired Two Sample for Means
3.2. Pulse
Paired Two Sample for Means
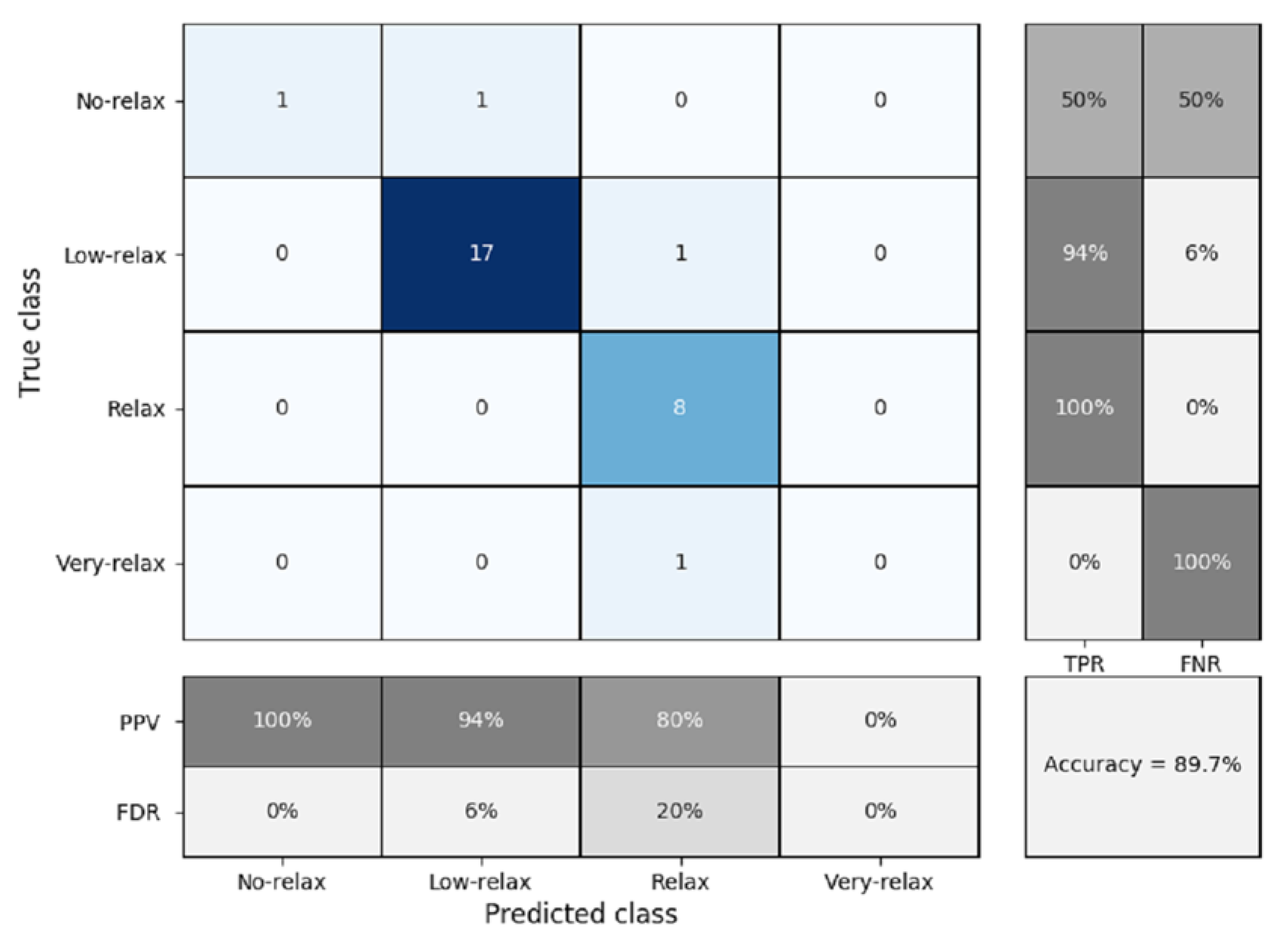
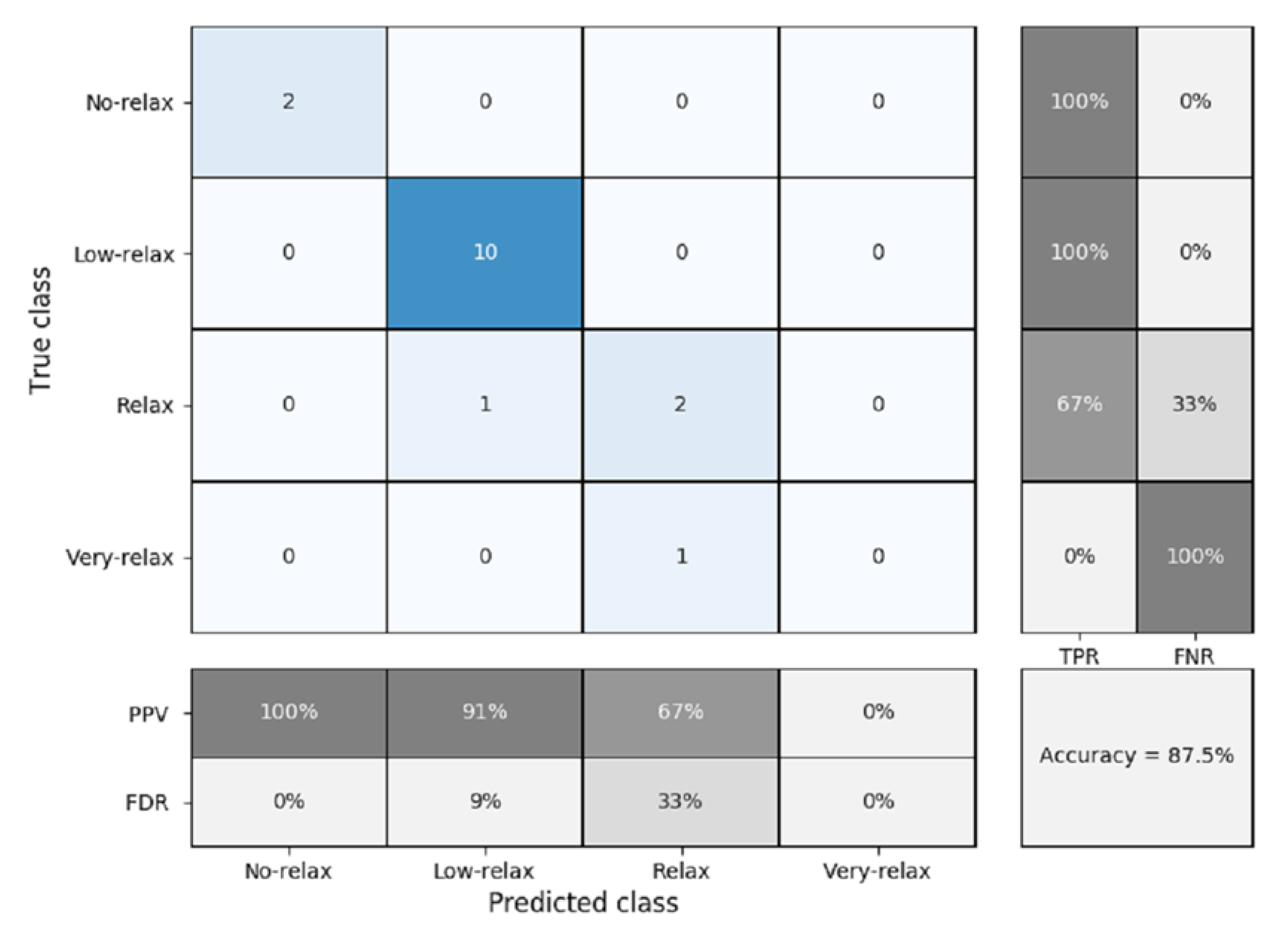
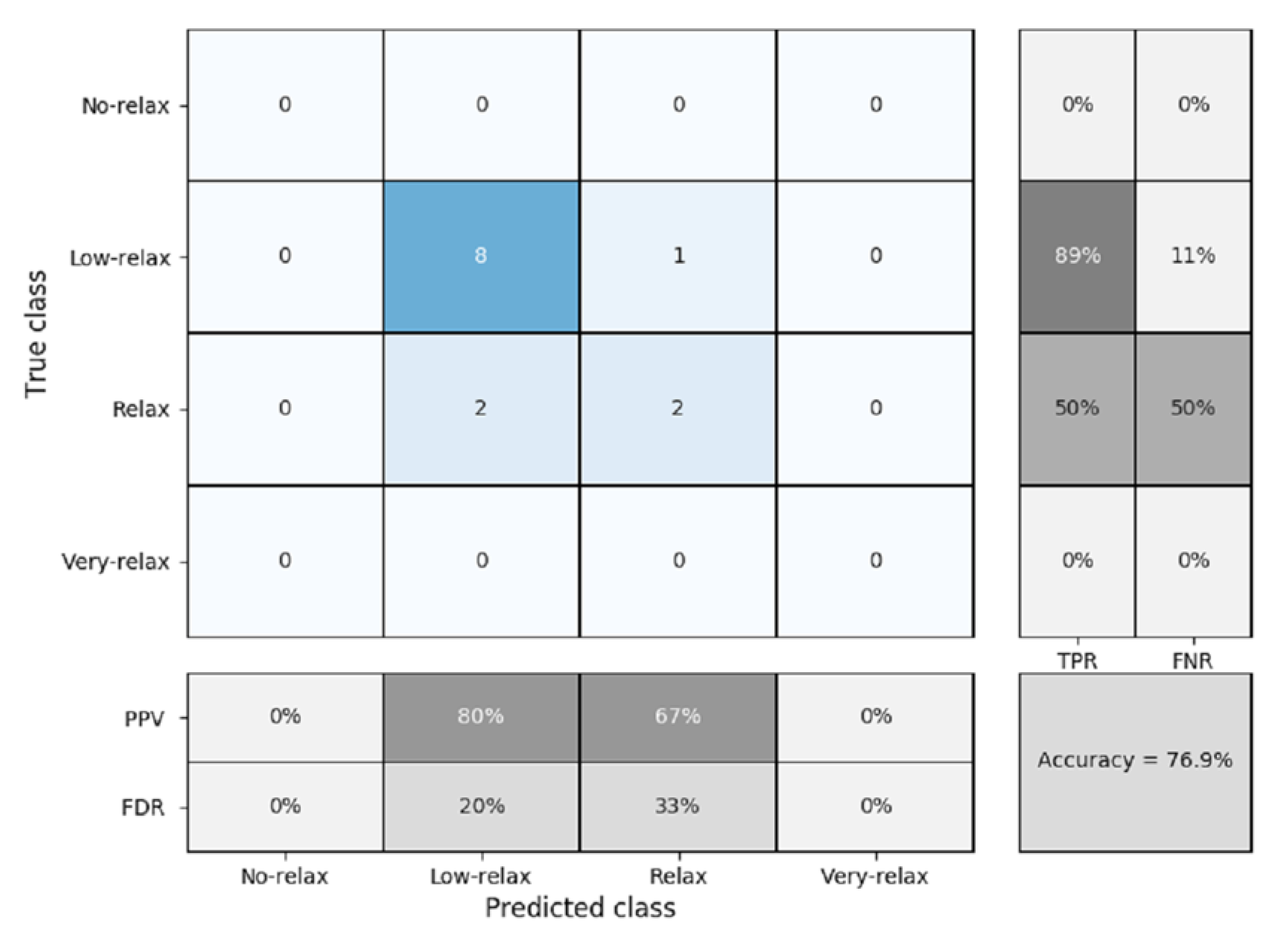
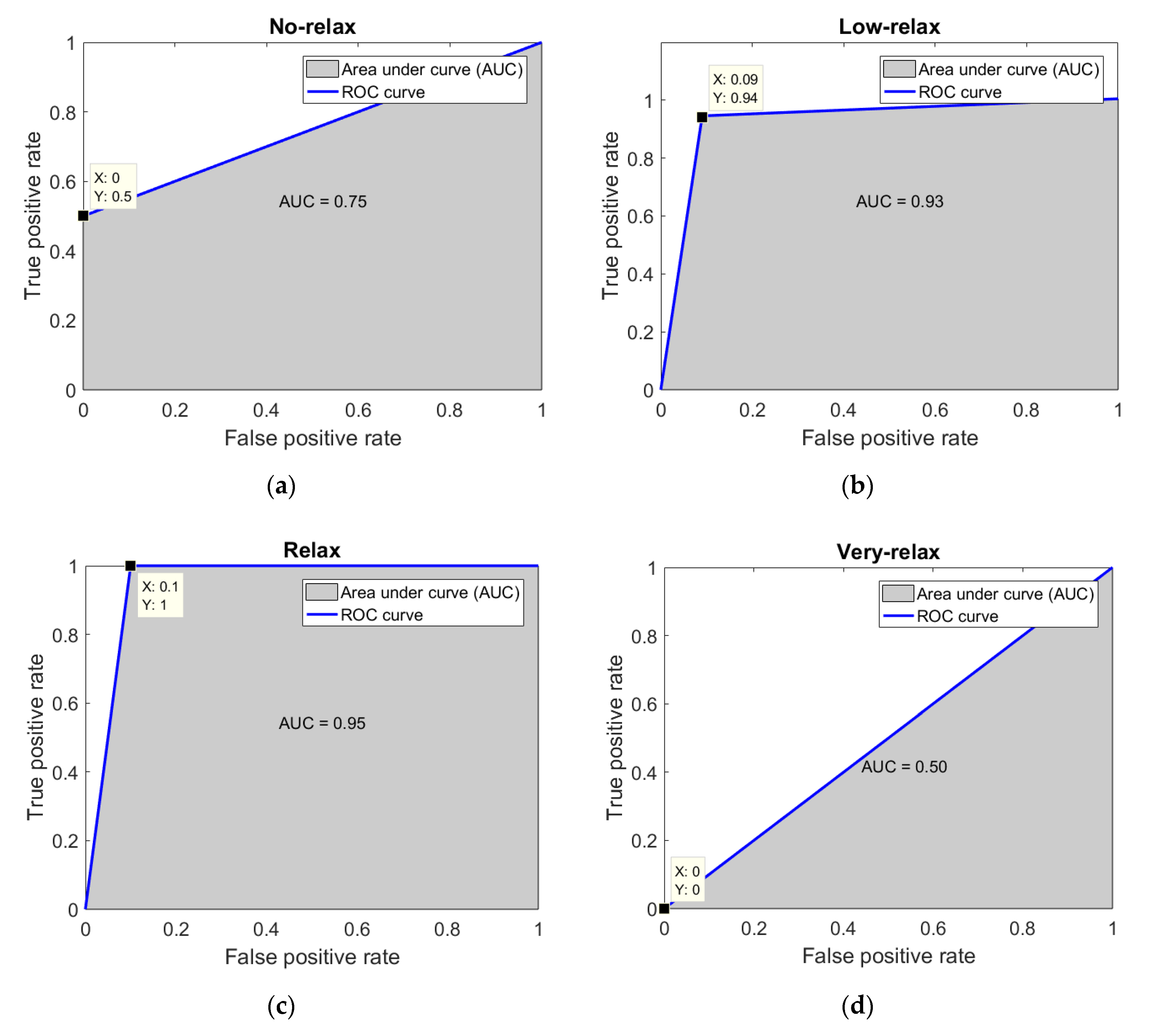
3.3. K-NN Classification
K-Fold Cross Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, B.; Barnett, L.M. Physical activity interventions to improve the health of children and adolescents in out of home care–A systematic review of the literature. Child. Youth Serv. Rev. 2020, 110, 104765. [Google Scholar] [CrossRef]
- Crawford, M. Health of children in out-of-home care: Can we do better. J. Paediatr. Child Health 2006, 42, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Kääriälä, A.; Hiilamo, H. Children in out-of-home care as young adults: A systematic review of outcomes in the Nordic countries. Child. Youth Serv. Rev. 2017, 79, 107–114. [Google Scholar] [CrossRef]
- Gross, J.J.; Jazaieri, H. Emotion, emotion regulation, and psychopathology: An affective science perspective. Clin. Psychol. Sci. 2014, 2, 387–401. [Google Scholar] [CrossRef]
- Kang, H.; Chung, I.J.; Chun, J.S.; Nho, C.R.; Woo, S. Linking Traumatic Childhood Experiences to the Physical Health of Korean Adolescents in Out-of-Home Care through Depression and Anxiety. Soc. Work Public Health 2017, 32, 122–130. [Google Scholar] [CrossRef]
- Dominick, G.M.; Saunders, R.P.; Dowda, M.; Kenison, K.; Evans, A.E. Effects of a structural intervention and implementation on physical activity among youth in residential children’s homes. Eval. Program Plan. 2014, 46, 72–79. [Google Scholar] [CrossRef]
- Kemmis-Riggs, J.; Dickes, A.; McAloon, J. Program Components of Psychosocial Interventions in Foster and Kinship Care: A Systematic Review. Clin. Child Family Psychol. Rev. 2018, 21, 13–40. [Google Scholar] [CrossRef]
- Clyman, R.B.; Harden, B.J.; Little, C. Assessment, intervention, and research with infants in out-of-home placement. Infant Ment. Health J. 2002, 23, 435–453. [Google Scholar] [CrossRef]
- Fei, Z.; Yang, E.; Li, D.D.U.; Butler, S.; Ijomah, W.; Li, X.; Zhou, H. Deep convolution network based emotion analysis towards mental health care. Neurocomputing 2020, 388, 212–227. [Google Scholar] [CrossRef]
- Oh, S.; Lee, J.Y.; Kim, D.K. The design of CNN architectures for optimal six basic emotion classification using multiple physiological signals. Sensors 2020, 20, 866. [Google Scholar] [CrossRef]
- Dzedzickis, A.; Kaklauskas, A.; Bucinskas, V. Human emotion recognition: Review of sensors and methods. Sensors 2020, 20, 592. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Yu, Y.; Chen, W.; Hua, H.; Li, Q.; Jin, J.; Xu, X. Wearable emotion recognition using heart rate data from a smart bracelet. Sensors 2020, 20, 718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, C.; Xue, W.; Hu, J.; He, Y.; Gao, M. Emotion recognition based on multichannel physiological signals with comprehensive nonlinear processing. Sensors 2018, 18, 3886. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Siddiqui, M.F.H.; Javaid, A.Y. Recognition of emotion intensities using machine learning algorithms: A comparative study. Sensors 2019, 19, 1897. [Google Scholar] [CrossRef]
- Athavipach, C.; Pan-Ngum, S.; Israsena, P. A wearable in-ear EEG device for emotion monitoring. Sensors 2019, 19, 4014. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Albarran, I.A.; Benitez-Rangel, J.P.; Osornio-Rios, R.A.; Morales-Hernandez, L.A. Human emotions detection based on a smart-thermal system of thermographic images. Infrared Phys. Technol. 2017, 81, 250–261. [Google Scholar] [CrossRef]
- Kopaczka, M.; Breuer, L.; Schock, J.; Merhof, D. A modular system for detection, tracking and analysis of human faces in thermal infrared recordings. Sensors 2019, 19, 4135. [Google Scholar] [CrossRef]
- Goulart, C.; Valadão, C.; Delisle-Rodriguez, D.; Funayama, D.; Favarato, A.; Baldo, G.; Binotte, V.; Caldeira, E.; Bastos-Filho, T. Visual and thermal image processing for facial specific landmark detection to infer emotions in a child-robot interaction. Sensors 2019, 19, 2844. [Google Scholar] [CrossRef]
- Filippini, C.; Perpetuini, D.; Cardone, D.; Chiarelli, A.M.; Merla, A. Thermal infrared imaging-based affective computing and its application to facilitate human robot interaction: A review. Appl. Sci. 2020, 10, 2924. [Google Scholar] [CrossRef]
- Fernández-Cuevas, I.; Marins, J.C.B.; Lastras, J.A.; Carmona, P.M.G.; Cano, S.P.; García-Concepción, M.Á.; Sillero-Quintana, M. Classification of factors influencing the use of infrared thermography in humans: A review. In Infrared Physics and Technology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 71, pp. 28–55. [Google Scholar]
- Piqueras-Rodríguez, J.A.; Linares-Ramos, V.; González, A.E.M.; Oblitas-Guadalupe, L.A. Emociones negativas y su impacto en la salud mental y fisica. Suma Psicol. 2009, 16, 5–112. [Google Scholar]
- Álvarez-García, C.; Yaban, Z.Ş. The effects of preoperative guided imagery interventions on preoperative anxiety and postoperative pain: A meta-analysis. Complement. Clin. Pract. 2020, 38, 101077. [Google Scholar] [CrossRef]
- Mason, E.B.; Burkhart, K.; Lazebnik, R. Adolescent Stress Management in a Primary Care Clinic. J. Pediatr. Health Care 2019, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.S.; Andrasik, F. Evaluating research in clinical biofeedback. In Biofeedback: A Practitioner’s Guide, 3rd ed.; Guilford Press: New York, NY, USA, 2003; pp. 867–880. [Google Scholar]
- Jerčić, P.; Sundstedt, V. Practicing emotion-regulation through biofeedback on the decision-making performance in the context of serious games: A systematic review. Entertain. Comput. 2019, 29, 75–86. [Google Scholar] [CrossRef]
- Kaushik, R.; Kaushik, R.M.; Mahajan, S.K.; Rajesh, V. Biofeedback assisted diaphragmatic breathing and systematic relaxation versus propranolol in long term prophylaxis of migraine. Complement. Med. 2005, 13, 165–174. [Google Scholar] [CrossRef]
- Meier, N.F.; Welch, A.S. Walking versus biofeedback: A comparison of acute interventions for stressed students. Anxiety Stress Coping 2016, 29, 463–478. [Google Scholar] [CrossRef]
- Hjelland, I.E.; Svebak, S.; Berstad, A.; Flatabø, G.; Hausken, T. Breathing exercises with vagal biofeedback may benefit patients with functional dyspepsia. Scand. J. Gastroenterol. 2007, 42, 1054–1062. [Google Scholar] [CrossRef]
- Peira, N.; Fredrikson, M.; Pourtois, G. Controlling the emotional heart: Heart rate biofeedback improves cardiac control during emotional reactions. Int. J. Psychophysiol. 2014, 91, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, Y.; Frenkel, T.I.; Schlesinger, Y.; Rousseau, S.; Hamiel, D.; Achiron, R.; Perlman, S. Visual biofeedback using transperineal ultrasound in second stage of labor. Ultrasound Obs. Gynecol. 2018, 52, 91–96. [Google Scholar] [CrossRef]
- Ramirez, P.M.; Desantis, D.; Opler, L. EEG Biofeedback Treatment of ADD: A Viable Alternative to Traditional Medical Intervention? Ann. N. Y. Acad. Sci. 2001, 931, 342–358. [Google Scholar] [CrossRef]
- Wang, T.J.; Chang, C.F.; Lou, M.F.; Ao, M.K.; Liu, C.C.; Liang, S.Y.; Wu, S.F.; Tung, H.H. Biofeedback relaxation for pain associated with continuous passive motion in Taiwanese patients after total knee arthroplasty. Res. Nurs. Health 2015, 38, 39–50. [Google Scholar] [CrossRef]
- Yu, B.; Funk, M.; Hu, J.; Feijs, L. Unwind: A musical biofeedback for relaxation assistance. Behav. Inf. Technol. 2018, 37, 800–814. [Google Scholar] [CrossRef]
- Windthorst, P.; Mazurak, N.; Kuske, M.; Hipp, A.; Giel, K.E.; Enck, P.; Nieß, A.; Zipfel, S.; Teufel, M. Heart rate variability biofeedback therapy and graded exercise training in management of chronic fatigue syndrome: An exploratory pilot study. J. Psychosom. Res. 2017, 93, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Sutarto, A.P.; Wahab, M.N.A.; Zin, N.M. Resonant breathing biofeedback training for stress reduction among manufacturing operators. Int. J. Occup. Saf. Erg. 2012, 18, 549–561. [Google Scholar] [CrossRef]
- RLande, G.; Williams, L.B.; Francis, J.L.; Gragnani, C.; Morin, M.L. Efficacy of biofeedback for post-traumatic stress disorder. Complement. Med. 2010, 8, 256–259. [Google Scholar]
- Ilgün, S.; Ovayolu, N.; Ovayolu, Ö.; Özcanli, D.; Yaǧci, F. Does biofeedback affect incontinence and quality of life in Turkish women? Int. J. Urol. Nurs. 2013, 7, 138–145. [Google Scholar] [CrossRef]
- Kopru, B.; Ergin, G.; Ebiloglu, T.; Kibar, Y. Does biofeedback therapy improve quality of life in children with lower urinary tract dysfunction: Parents’ perspective. J. Pediatr. Urol. 2020, 6, 38-e. [Google Scholar] [CrossRef]
- Amon, K.L.; Campbell, A. Can children with AD/HD learn relaxation and breathing techniques through biofeedback video games? Aust. J. Educ. Dev. Psychol. 2008, 8, 72–84. [Google Scholar]
- Mishra, J.; Anguera, J.A.; Gazzaley, A. Video Games for Neuro-Cognitive Optimization. Neuron 2016, 90, 214–218. [Google Scholar] [CrossRef]
- Jaramillo-Quintanar, D. Sistema de Visión Artificial en el Espectro Infrarrojo Térmico para Evaluación de Estrés en Niños con Cáncer. Master’s Thesis, Universidad Autónoma de Querétaro, Santiago de Querétaro, Mexico, December 2018. [Google Scholar]
- Jaramillo-Quintanar, D.; Trejo-Chávez, O.; Morales-Hernández, L.A.; Osornio-Ríos, R.A. DISEÑO Y ACONDICIONAMIENTO DE SENSOR DE MONITOREO DE FRECUENCIA CARDIACA Y OXIGENACIÓN EN LA SANGRE. In Proceedings of the 5° encuentro de jóvenes investigadores del estado de Querétaro, Queretaro, Mexico, 5 October 2017. [Google Scholar]
- Guerrero-Lbáñez, A.; Guzmán-Sandoval, V.; Flores-Cortés, C.; Trejo, B.D.; Lara, R.M.M.; Torres-Hernández, J. I-CARE: Sistema basado en tecnologías emergentes para el monitoreo remoto de variables fisiológicas del dolor en oncología pediátrica: Estudio piloto. In Proceedings of the CISCI 2014—Decima Tercera Conferencia Iberoamericana en Sistemas, Cibernetica e Informatica, Undecimo Simposium Iberoamericano en Educacion, Cibernetica e Informatica, SIECI 2014, Memorias, Mexico, 15 July 2014. [Google Scholar]
- Khan, Z.A.; Sivakumar, S.; Phillips, W.; Robertson, B. A QoS-aware routing protocol for reliability sensitive data in hospital body area networks. Procedia Comput. Sci. 2013, 19, 171–179. [Google Scholar] [CrossRef]
- Sandoval, V.G.; Pimazzoni, D.N.; Trejo, B.D.; Muñiz, J.G. Manejo psicoterapéutico del dolor: A través de la música y el tacto en neonatos: El Método ‘Sentire. Estud. Cult. Contemp. 2018, 47, 9–40. [Google Scholar]
- Jadin, M.S.; Taib, S.; Ghazali, K.H. Feature extraction and classification for detecting the thermal faults in electrical installations. Meas. J. Int. Meas. Confed. 2014, 57, 15–24. [Google Scholar] [CrossRef]
- Rajic, N. Principal component thermography for flaw contrast enhancement and flaw depth characterisation in composite structures. Compos. Struct. 2002, 58, 521–528. [Google Scholar] [CrossRef]
- Yousefi, B.; Sfarra, S.; Castanedo, C.I.; Maldague, X.P.V. Comparative analysis on thermal non-destructive testing imagery applying Candid Covariance-Free Incremental Principal Component Thermography (CCIPCT). Infrared Phys. Technol. 2017, 25, 1034–1040. [Google Scholar] [CrossRef]
- Yousefi, B.; Memarzadeh Sharifipour, H.; Eskandari, M.; Ibarra-Castanedo, C.; Laurendeau, D.; Watts, R.; Klein, M.; Maldague, X.P. Incremental low rank noise reduction for robust infrared tracking of body temperature during medical imaging. Electronics 2019, 8, 1301. [Google Scholar] [CrossRef]
- Conley, M.M.; Gastin, P.B.; Brown, H.; Shaw, C. Heart rate biofeedback fails to enhance children’s ability to identify time spent in moderate to vigorous physical activity. J. Sci. Med. Sport 2011, 14, 153–158. [Google Scholar] [CrossRef]
- Shahidi, B.; Sannes, T.; Laudenslager, M.; Maluf, K.S. Cardiovascular responses to an acute psychological stressor are associated with the cortisol awakening response in individuals with chronic neck pain. Physiol. Behav. 2015, 150, 93–98. [Google Scholar] [CrossRef]
- Bonafide, C.P.; Brady, P.W.; Keren, R.; Conway, P.H.; Marsolo, K.; Daymont, C. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics 2013, 131, E1150–E1157. [Google Scholar] [CrossRef]
- Duda, R.O.; Hart, P.E.; Stork, D.G. Pattern Classification, 2nd ed.; J. W. & Sons: Malden, MA, USA, 1999. [Google Scholar]
- Petrantonakis, P.C.; Hadjileontiadis, L.J. Emotion recognition from brain signals using hybrid adaptive filtering and higher order crossings analysis. IEEE Trans. Affect. Comput. 2010, 1, 81–97. [Google Scholar] [CrossRef]
- Verma, G.K.; Tiwary, U.S. Multimodal fusion framework: A multiresolution approach for emotion classification and recognition from physiological signals. NeuroImage 2014, 102, 162–172. [Google Scholar] [CrossRef]
- Kolodyazhniy, V.; Kreibig, S.D.; Gross, J.J.; Roth, W.T.; Wilhelm, F.H. An affective computing approach to physiological emotion specificity: Toward subject-independent and stimulus-independent classification of film-induced emotions. Psychophysiology 2011, 48, 908–922. [Google Scholar] [CrossRef]
- Davies, P.; Maconochie, I. The relationship between body temperature, heart rate and respiratory rate in children. Emerg. Med. J. 2009, 26, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Weekly, T.; Walker, N.; Beck, J.; Akers, S.; Weaver, M. A Review of Apps for Calming, Relaxation, and Mindfulness Interventions for Pediatric Palliative Care Patients. Children 2018, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Culbert, T. Perspectives on Technology-Assisted Relaxation Approaches to Support Mind-Body Skills Practice in Children and Teens: Clinical Experience and Commentary. Children 2017, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, H.; Nandi, A.; Li, M. Emotion detection from EEG recordings. In Proceedings of the 12th International Conference on Natural Computation, Fuzzy Systems and Knowledge Discovery, ICNC-FSKD, Changsha, China, 13 August 2016; pp. 1722–1727. [Google Scholar]
- Wu, C.K.; Chung, P.C.J.; Wang, C.J. Representative segment-based emotion analysis and classification with automatic respiration signal segmentation. IEEE Trans. Affect. Comput. 2012, 3, 482–495. [Google Scholar] [CrossRef]
- Shin, D.; Shin, D.; Shin, D. Development of emotion recognition interface using complex EEG/ECG bio-signal for interactive contents. Multimed. Tools Appl. 2017, 76, 11449–11470. [Google Scholar] [CrossRef]








| Age | Weight (kg) | Heart Rate (BPM) 1 |
|---|---|---|
| 6 years | 20 | 70–135 |
| 8 years | 25 | 70–135 |
| 10 years | 30 | 60–120 |
| 12 years | 40 | 60–120 |
| Number | Name | Percentage 1 |
|---|---|---|
| 1 | No-relax | 0% ≤ NR ≤ 25% |
| 2 | Low-relax | 25% < LR ≤ 50% |
| 3 | Relax | 50% < R ≤ 75% |
| 4 | Very-relax | 75% < NR ≤ 100% |
| Thermal Biomarker | P3 | ΔT 4 | ||
|---|---|---|---|---|
| Forehead | 35.05 | 35.17 | 0.003 | 0.12 |
| Left cheek | 33.69 | 33.86 | 0.000 | 0.17 |
| Right cheek | 33.69 | 33.90 | 0.000 | 0.21 |
| Chin | 33.89 | 34.23 | 0.000 | 0.34 |
| Nose | 34.69 | 34.62 | 0.227 | −0.07 |
| Maxillary | 34.60 | 34.85 | 0.003 | 0.25 |
| Indicator | P3 | ΔI 4 | ||
|---|---|---|---|---|
| 92.08 | 89.31 | 0.002 | −3.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Quintanar, D.; Cruz-Albarran, I.A.; Guzman-Sandoval, V.M.; Morales-Hernandez, L.A. Smart Sensor Based on Biofeedback to Measure Child Relaxation in Out-of-Home Care. Sensors 2020, 20, 4194. https://doi.org/10.3390/s20154194
Jaramillo-Quintanar D, Cruz-Albarran IA, Guzman-Sandoval VM, Morales-Hernandez LA. Smart Sensor Based on Biofeedback to Measure Child Relaxation in Out-of-Home Care. Sensors. 2020; 20(15):4194. https://doi.org/10.3390/s20154194
Chicago/Turabian StyleJaramillo-Quintanar, Daniel, Irving A. Cruz-Albarran, Veronica M. Guzman-Sandoval, and Luis A. Morales-Hernandez. 2020. "Smart Sensor Based on Biofeedback to Measure Child Relaxation in Out-of-Home Care" Sensors 20, no. 15: 4194. https://doi.org/10.3390/s20154194
APA StyleJaramillo-Quintanar, D., Cruz-Albarran, I. A., Guzman-Sandoval, V. M., & Morales-Hernandez, L. A. (2020). Smart Sensor Based on Biofeedback to Measure Child Relaxation in Out-of-Home Care. Sensors, 20(15), 4194. https://doi.org/10.3390/s20154194





