Abstract
Background: Obesity results from interactions between environmental factors, lifestyle, and genetics. In this scenario, nutritional genomics and nutrigenetic tests stand out, with the promise of helping patients avoid or treat obesity. This narrative review investigates whether nutrigenetic tests may help to prevent or treat obesity. Scientific studies in PubMed Science Direct were reviewed, focusing on using nutrigenetic tests in obesity. The work showed that few studies address the use of tools in obesity. However, most of the studies listed reported their beneficial effects in weight loss. Ethical conflicts were also discussed, as in most countries, there are no regulations to standardize these tools, and there needs to be more scientific knowledge for health professionals who interpret them. International Societies, such as the Academy of Nutrition and Dietetics and the Brazilian Association for the Study of Obesity and Metabolic Syndrome, do not recommend nutrigenetic tests to prevent or treat obesity, especially in isolation. Advancing nutrigenetics depends on strengthening three pillars: regulation between countries, scientific evidence with clinical validity, and professional training.
1. Introduction
Obesity is a complex chronic disease defined by excessive adiposity from exposure to obesogenic environments, psychosocial factors, and genetic variants [1]. It is a significant risk factor for the emergence of other chronic non-communicable diseases (NCDs), negatively impacting the well-being and life quality of those affected [2].
In 2019, this chronic condition contributed to approximately 5 million deaths from cardiovascular disease (CVD), diabetes, cancer, neurological disorders, chronic respiratory diseases, and digestive disorders [3]. If current trends continue, one billion adults (almost 20% of the world’s population) will be obese by 2025 [4].
Given this scenario, interventions are necessary and urgent. Many researchers try to delve deeper into understanding the complexity of the disease and which treatments would be most successful. At the 75th World Health Assembly in 2022, member states of the World Health Organization (WHO) adopted new recommendations for preventing and managing obesity, approving “The WHO Acceleration Plan to STOP Obesity”. This plan emphasizes that obesity is preventable by increasing the consumption of fruits, vegetables, and whole grains, limiting the intake of fats and sugar, and practicing regular physical activity. Furthermore, it highlights the importance of political efforts, encouraging self-care, and incorporating healthy eating and physical exercise [5].
In addition to diet and psychosocial factors, genetic factors can lead to the negative regulation of specific metabolic pathways and contribute to the onset of the disease [6]. Considering the genetic aspect, obesity can be monogenic or polygenic [4].
Monogenic obesity has a Mendelian inheritance pattern, involves genetic variations in a single gene or chromosomal region, has high penetrance, early onset, and is typically rare. On the other hand, polygenic obesity is more common than monogenic cases, results from hundreds of single nucleotide polymorphisms (SNPs) in several genes with low penetrance, and has a heritability pattern similar to other complex diseases [4].
Many genes associated with obesity are involved in regulating energy intake, lipid metabolism, adipogenesis, thermogenesis, adipokine synthesis, and transcription factors [7]. The genetic basis of polygenic obesity is diffuse, multifactorial, and non-deterministic. Many variants are distributed throughout the genome and have a small contribution to the onset of the disease, thus becoming a challenge for clinical practice. Information on existing genetic variants is necessary to characterize susceptibility to obesity [8].
Although challenging, offering personalized dietary advice based on an individual’s genetic susceptibility may be a promising strategy for preventing or treating obesity and diseases related to this chronic condition [9]. Thus, nutrigenetic tests can be an auxiliary tool in preventing and treating obesity, as they provide information on the primary SNPs, genotypes, possible predispositions to obesity, and directions for a better response to nutritional intervention [10].
Despite the existing concerns and challenges, there is great evidence for the potential of nutrigenetics in preventing and treating obesity and associated diseases [11,12,13]. This evidence points to promising perspectives regarding the use of nutrigenetic tests [14].
Considering the severity of obesity and the urgency of viable and resolute solutions for its management, nutrigenetics and precision nutrition have stood out as important prevention and treatment strategies. Thus, this narrative review provides a current overview of obesity, addressing in a conceptual, historical, and descriptive way the importance and real applicability of nutrigenetic tests in this context, highlighting their advantages and limitations.
2. Obesity: Epidemiology, Diagnosis, and Treatment
The WHO defines obesity as excess body fat resulting from a positive energy balance over time [15]. Globally, overweight and obesity affect more than 2 billion adults. By 2025, it is expected that 1 billion adults (more than 20% of the world’s population) will have obesity [4].
Excess adipose tissue not only influences the central regulation of energy homeostasis but can also become dysfunctional and predispose the individual to the development of comorbidities and complications [16], negatively impacting health and quality of life.
Both the excess and the ectopic location of body fat influence the production of adipokines and inflammatory mediators capable of altering glucose and lipid metabolism, leading to increased cardiometabolic and cancer risks and reducing life expectancy by 6 to 14 years [17]. It is estimated that 20% of all cancers can be attributed to obesity [18].
Obesity, as a complex multifactorial disease, is associated with an increased risk of developing several NCDs, such as CVD, 13 types of cancer, type 2 diabetes (DM2), and chronic respiratory diseases, including obstructive sleep apnea [19,20,21].
Cultural factors must also be taken into consideration in the etiology of obesity. For example, Asians tend to have a propensity for a lower body mass index (BMI) due to collectivistic cultures [22]. These cultures embrace cooperation and compliance with norms, unlike countries with a more individualistic culture, such as the United States (USA), where emotional and environmental triggers are the determining risk factors for weight gain [23]. A recent ecological analysis of 54 countries concluded that collectivism was significantly associated with a lower incidence of obesity in the population [24].
Diet is also a determining factor in the significant increase in obesity. Changes in the population’s dietary pattern, such as the increased consumption of ultra-processed products with low nutritional value and high levels of sodium, fats, or sugars, which have been replacing the consumption of fresh and minimally processed foods [25]. In several countries, a high consumption of ultra-processed foods has been associated with obesity due to an increased energy intake due to sugar consumption, decreased fiber consumption, and decreased protein density [26].
In Brazil, the national VIGITEL survey, a telephone survey to investigate and monitor risk factors for NCDs, detected a high consumption of ultra-processed foods by the population. Approximately 17.7% of Brazilians consume ultra-processed foods, 22.0% are men, and 14.1% are women. The consumption of these foods tended to decrease with age and was highest among schoolchildren aged 9 to 11 years old [27].
The global consumption of sweetened products and sugary drinks has grown in parallel with the obesity pandemic [28,29]. This direct association places sugar consumption as an essential risk factor for obesity [30,31]. Thus, the importance of food and nutritional education for the population, aiming to prevent diseases such as obesity, is highlighted.
Anthropometry is essential for diagnosing and assessing obesity, including body mass index (BMI), weight history, and body composition [32]. Using the BMI, it is possible to identify obesity (≥30 kg/m2), overweight (25–29.9 kg/m2), or eutrophy (18.5–24.9 kg/m2) [15]. For people with an increased BMI, waist circumference can identify increased visceral adiposity and cardiometabolic risk [16,33]. Furthermore, family history, clinical history, and biochemical data can make obesity treatment more individualized [32].
Many treatments are listed to eradicate obesity at individual and population levels. However, several have yet to succeed in the long term [34]. Behavioral and lifestyle interventions aimed at reducing energy intake and increasing energy expenditure have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations can hinder weight loss and promote weight regain [35,36].
In the USA, some medications are approved for the treatment of obesity, such as phentermine©, topiramat©, orlistat©, naltrexone©, bupropion©, liraglutide©, and semaglutide©. Often, these medications are prescribed in combination. In Europe, only orlistat©, naltrexone©, bupropion© and liraglutide© are approved [37]. Lorcaserin©, a selective serotonin 5C receptor (2-hydroxytryptamine) agonist, was recently withdrawn from the North American market due to concerns about increased cancer incidence in a cardiovascular outcome study [38]. In Brazil, three medications are approved for treating obesity: sibutramine©, orlistat©, and liraglutide© [39].
Sibutramine© blocks the reuptake of norepinephrine (NE) and serotonin (SE) and reduces food intake. Orlistat© is an analog of lipstatin, an inhibitor of gastrointestinal lipases, reducing the binding of approximately one-third of triglycerides and their absorption in the intestine. Liraglutide© is a glucagon-like peptide-1 (GLP-1) agonist, increasing the signaling of neurons synthesizing pro-opiomelanocortin and the transcript regulated by cocaine and amphetamine (POMC/CART). The medication indirectly inhibits neurotransmission in neurons that express neuropeptide Y (NPY) and agouti-related peptide (AgRP), favoring the weight loss process [40].
Endoscopic and surgical procedures also comprise an arsenal of strategies for controlling obesity. The intragastric balloon, duodenal mucosal resurfacing, and bariatric surgeries are among them. These procedures are indicated according to the degree of obesity, comorbidities, and the therapeutic effect sought [41,42].
Innovative therapeutic approaches are also researched for treating obesity and body weight regulation [37]. The new technologies of induced pluripotent stem cells (iPSC) and gene editing mediated by clustered, regularly interspaced short palindromic repeats are among them (CRISPR) [43].
Despite the various treatments available, combating obesity requires approaches that combine individual interventions with environmental and behavioral changes. Therefore, a better understanding of regional etiological differences in the prevalence of obesity can help identify the social causes of obesity and provide guidance on which intervention strategies are most promising [34]. Obesity is not caused by personal choice but rather by the relationship between an individual and their environment [34].
Thus, overweight and obesity result from an interaction between genetic and environmental factors. In this scenario, the emerging precision nutrition considers the main characteristics related to the individual (genotype, phenotype, diet, metabolic biomarkers, and intestinal microbiome) to establish personalized dietary recommendations that optimize the response to nutritional treatment. Therefore, precision nutrition is an essential complementary strategy for effectively treating obesity and its comorbidities [44].
3. Precision Nutrition and Exposome
In recent years, the concept of precision medicine or personalized medicine [45] became evident with a publication by Francis Collins [46], director of the National Institutes of Health (NIH). The director announced a new era and the creation of a national cohort aimed at recruiting and monitoring one million individuals to generate omics data [47].
Within precision medicine is precision nutrition. Precision nutrition aims to enable personalized dietary recommendations, optimize prevention, delay disease progression, and improve an individual’s health [9] through understanding the patient’s exposome.
In 2005, Wild developed the exposome concept, which comprises the totality of human exposures throughout life, from conception to the end of life [48]. This designation was improved and defined as the cumulative measure of environmental influences and associated biological responses throughout existence, including environmental exposures, diet, behavior, and endogenous processes [49].
Thus, an individual’s exposome must be considered, as well as a series of omics markers [47]. Precision nutrition must consider metabolic phenotyping using high-throughput omics technologies, such as genomics (polymorphisms and other structural genetic variants), epigenomics (DNA methylation, histone modifications, long non-coding RNA, telomere length), transcriptomics (patterns of RNA expression), proteomics (protein signatures), metabolomics (metabolite profiles), and metagenomics (intestinal microbiota composition, enterotypes), under a holistic approach comprising nutritional genomics [50].
4. Nutritional Genomics
With the Human Genome Project, nutritional genomics emerged as a field of research to assist in diagnosing, preventing, and managing chronic diseases influenced by diet [51]. Nutritional genomics studies how genes and nutrients interact and influence phenotypes, including disease risk [52]. Nutrigenomics, nutrigenetics, and nutritional epigenomics are subareas of nutritional genomics, and each studies different aspects. Nutrigenomics is a part of omics sciences that studies the influence of nutrients and diet on genes, proteins, and metabolites. Nutrigenetics studies the impact of genetic variations, mainly SNPs, on individual responses to nutrients and diet. These genetic variations can influence protein synthesis and functions, thus modifying dietary needs and metabolism, and may impact the development of diseases. Nutritional epigenomics investigates the impact of nutrients and diet on changes in the human genome that do not involve changes to the DNA sequence but affect gene expression, extending from gene activation to protein synthesis [53].
As nutritional genomics promises to transform global health and medicine, there is growing interest in the relationship between genotype and phenotype. The phenotype derives from genetic and environmental contributions [54]. Once “the” genetic variants that may predispose a trait or disease have been identified, the next challenge is to decode the genetic variation that explains heritability, in addition to the epigenetic changes [55]. Therefore, it is essential to know the genetic profile of obesity to identify how many variants are involved in this chronic condition and direct the use of nutritional genomics in patients’ treatment.
5. Genetic Aspects of Obesity
Obesity is genetically classified into monogenic or polygenic. Monogenic obesity is a rare, severe, early-onset form with a Mendelian inheritance pattern, high penetrance, and significant genetic effect [4]. In contrast, polygenic obesity is more prevalent. It has a heritability pattern derived from many variants in several genes with low penetrance [56].
Several genes associated with polygenic obesity have been found in Genome-Wide Association Studies (GWAS). Most of these genes are involved in the leptin–melanocortin pathway, which regulates food intake [56]. Other pathways are also involved in the development of polygenic obesity (Figure 1).
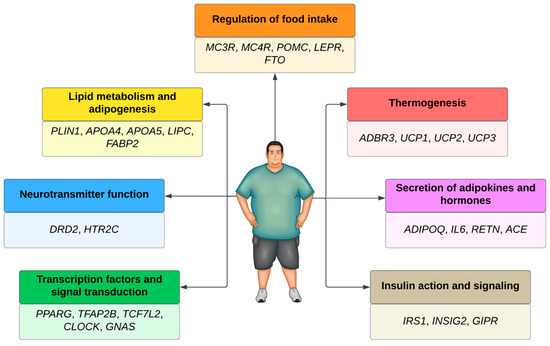
Figure 1.
Main genes and pathways associated with obesity.
Over 1100 obesity-associated loci have already been identified in approximately 60 GWAS [4]. Almost all chromosomes in the human genome (except the Y) contain at least one locus associated with body weight regulation [57]. When considering those that are BMI- and obesity-associated, more than 250 loci were identified [58].
Scientific evidence shows that, despite the discovery of many genetic loci susceptible to obesity, the size of each variant’s effect on BMI is small [59], possibly due to the influence of environmental factors. Therefore, some environmental factors, such as physical activity, diet, and smoking, have been considered in the analyses of some GWAS. However, this approach is challenging as determining the effects of these gene–environment interactions on new biological insights is a complex task. It is estimated that only 12 loci associated with obesity and influenced by environmental factors have been identified [4].
A meta-analysis identified nine loci with convincing evidence of an interaction between smoking as an environmental factor and genes associated with obesity, as evidenced by BMI and waist circumference. Thus, smoking can alter genetic susceptibility to general adiposity and body fat distribution [60].
In genes associated with obesity, the presence of thousands of genetic variants can influence the etiology of obesity. The most studied genetic variants are single nucleotide polymorphisms (SNPs), represented by a single nucleotide change concerning the reference sequence at a specific position in the genome. Scientists have found more than 600 million SNPs in human populations worldwide [61]. These minor genetic variations determine phenotypic differences between individuals [62]. Patients with SNPs in pro-opiomelanocortin (POMC) and the melanocortin 4 receptor (MC4R) are more prone to excessive body fat accumulation. Individuals with SNPs in the fat mass and obesity gene (FTO) [63] and the dopamine 2 receptor (DRD2) are more predisposed to binge eating.
SNPs in beta-adrenergic receptors 3 (ADRB3) and perilipin (PLIN) increase the predisposition to adipogenesis or lipid metabolism imbalances. SNPs in the uncoupling protein gene (UCP) lead to difficulty in energy expenditure, while SNPs in insulin receptors (ISR-2), adiponectin (ADIPOQ), and interleukin 6 (IL-6) [64] predispose patients to more significant oxidative stress in the body and make weight loss difficult. Thus, all these SNPs can influence the emergence of obesity. Genetic tests targeting SNPs have become accessible to the population, allowing genetic information to direct therapeutic strategies [65].
6. Genetic Testing (GT)
Genetic tests (GT) are those that, from a biological sample, can directly examine the DNA or RNA that constitutes a gene (direct test), observe markers inherited together with a disease-causing gene (linkage test), examine the protein products of genes (biochemical test), or examine the entire chromosome (cytogenetic test) [66].
There are several types of GT, each with a different purpose (e.g., newborn screening, carrier testing, prenatal diagnostic testing, genetic tests, predictive genetic testing, and forensic testing) [67]. Among them, diagnostic GT is used to confirm the disease of a symptomatic individual. On the other hand, predictive GT identifies genetic variations that increase a person’s risk of developing a particular disease or clinical condition. The clinical usefulness of GT depends on the evidence obtained about how much the genetic variant can contribute to the diagnosis, prognosis, or management of the disease [68].
GT generally raise concerns from consumers, healthcare professionals, and regulators. These concerns are not new and affect the individual and public health. They address ethical, legal, psychological, and clinical issues. Autonomy, confidentiality, privacy, and equity are ethical and legal aspects related to the commercialization and use of these tests, and the storage of genetic information [69].
As for the psychological impact, these tests can generate fear of discrimination, anxiety, and depression, depending on the client’s perception regarding the risk, severity, and the possibility of treatment for the diseases listed [68]. Clinical concerns are inherent to the results’ robustness and applicability [70]. Therefore, some authors agree to limit access to TG for specific clinical conditions (e.g., Alzheimer’s disease) until truly effective treatments exist [71]. However, considering the substantial benefits of genomics for personal and public health, the WHO supports disseminating and implementing genomic technologies, including GT, with ethical and legal responsibility [72].
GT regulation varies significantly between countries. Despite existing international regulations, each country has the autonomy to modify and apply them. Technological advances, medical utility, access, and societal acceptance have contributed to more permissive regulations and greater use of GT [73]. Although genetic counseling is encouraged, few countries legally require it or recognize it as a profession [74].
In the United States, federal regulations evaluate and regulate GT based on three criteria: analytical validity, clinical validity, and clinical utility. Analytical validity concerns the accuracy of the test in detecting whether a genetic variant is present. Clinical validity indicates evidence of the relationship between a genetic variant and the presence, absence, or risk of a particular disease or clinical condition. Clinical utility matches the test’s ability to contribute to better health outcomes [75,76]. The WHO discusses ethical, legal, social, and regulatory issues related to the use of genomic methods and affirms the importance of the global development of rules, technical standards, and sensible policies so that access to and ownership of data is assertive for those who use it and can use it while benefiting, or not, from the use of genomic information [15].
6.1. Direct-to-Consumer Genetic Testings (DTC-GT)
After completing the human genome project in 2003, research further advanced the investigation of genetic variations, especially SNPs, and disease risks. This fact, associated with the ease of microarray technology and GWAS, boosted the emergence and commercialization of direct-to-consumer genetic testing (DTC-GT) without needing a prescription or medical referral [77]. DTC-GT is also called “over-the-counter genetic testing”, “at-home genetic testing”, or “home DNA testing” [78]. These are not diagnostic tests but predictive and pre-symptomatic tests that assess the risk or genetic susceptibility to certain diseases or clinical conditions, such as Alzheimer’s disease, cancer, diabetes, and CVD [70]. Thus, DTC-GT allows people to access and understand their genetic information without necessarily involving a healthcare provider [78].
Predictive DTC-GT can generate uncertain results regarding the development of a clinical condition. However, it has a beneficial potential for screening, surveillance, and prevention strategies that can reduce morbidity and mortality [79,80]. They even have different clinical utility hierarchies between diseases [79]. Over the years, prior uncertainties, misunderstandings, and caution regarding predictive GT [70,81] have gradually been replaced by increased acceptance among populations in several countries [80,82,83,84,85,86].
To carry out DTC-GT, consumers purchase test kits online (most commonly), in stores [78], and by email or telephone [65]. With step-by-step instructions, consumers collect their biological sample (usually saliva), send their data by mail to the company, and, after laboratory analysis, receive their results by email or on an online platform upon account registration [78,87].
Some companies collect additional customer data and request terms of service agreements to be signed [88]. Supposing the consumer wishes to have their information used in research, a separate consent form must be signed for the company to share data with third-party collaborators [89]. However, consent forms can have a problematic reading level for a layperson, or companies can induce consent by an easy click required to finalize the order [90]. As results may be incomplete or complex to interpret [89], some companies deliver more detailed reports [91] and often offer tailored diets, nutrition supplements, meals, and exercise plans [87,92].
6.2. Nutrigenetic Tests
DTC-GT offer several services; the most common are related to ancestry, disease risks, and lifestyle, including physical activity and diet [91,93]. These tests have impacted the personalization of pharmacotherapy (pharmacogenetics) [77] and diet therapy (nutrigenetics) [73]. Nutrigenetics tests are DTC-GT focused on nutrigenetics as they evaluate genetic variations (e.g., SNPs) related to monogenic or polygenic changes and contribute to more personalized nutritional guidance. For example, using specific genotypes, it is possible to verify predisposition or susceptibility to intolerance and sensitivity to food compounds (e.g., caffeine, lactose, gluten), changes in energy and nutrient metabolism (e.g., fatty acids, folate), obesity, and dietary needs for specific vitamins and minerals [91].
Nutrigenetic tests were one of the first types of DTC-GT offered [87], and since then, they have been commercialized by several companies in several countries around the world. Philip et al. (2016) mentioned that 72 companies offered the nutrigenetic testing service, representing 30% of the categories offered by the 246 companies that provided DTC-GT [92]. According to Floris et al. (2020), 45 companies worldwide sell nutrigenetic tests [65]. Their largest concentration is in Europe (n = 21) and North America (n = 19), four of which are multinationals.
The main requirement of a nutrigenetic test is to specify a dietary recommendation that is proven beneficial to the individual. The results must be consistent and have compelling evidence in replicated studies to achieve this [94]. However, in cases of polygenic conditions or diseases, where there is a high influence between genetic and environmental factors [91], many genetic variations analyzed in nutrigenetic tests still provide inconclusive or unreliable information, limiting personalized dietary recommendations, and disagreeing on ethical issues [94]. In line with this, a recent review highlights that the lack of knowledge, skills, and evidence-based information are the main factors limiting the use of nutrigenetics in clinical practice [14]. On the other hand, these limitations are seen as opportunities for improvement [95].
Despite these limitations, people have great interest and positive attitudes towards nutrigenetic tests. A European multicenter study investigated the opinions of almost 6000 participants about nutrigenetic tests. Among the participants, 66% were willing to carry out this type of test, and 27% would like to follow a personalized diet. Most of these people had chronic conditions such as dyslipidemia, central obesity, and high levels of stress [96].
Another study in Quebec investigated the attitudes, perceptions, and concerns of 1425 individuals about nutrigenetic tests. The main advantages reported by participants regarding using nutrigenetic tests were health and disease prevention. Dietary restriction was the main disadvantage reported, but was only pointed out by a minority. The biggest concerns were access to and use of personal genetic information [10]. Interestingly, studies demonstrate that nutrigenetic tests help motivate people to adhere to the diet and incorporate a healthy lifestyle [91,97], contributing to the control of weight [14] and proactivity about health [78].
Although nutritionists are considered the best professionals to provide personalized dietary advice based on nutrigenetic testing [10], many still need to be qualified, and a higher level of education/training is necessary [98]. For this reason, and given the need for more significant scientific evidence, position statements issued by associations state that caution must be taken when interpreting and using information from the nutrigenetic tests [52,62,99]. Although individuals respond differently to a given diet, nutrient, or bioactive compound due to their genetic variations, nutrigenetic tests can assist in nutritional intervention/guidance. However, they should never be used in isolation to personalize the diet [62]. Despite the mechanisms of interactions between genes and diet that have already been revealed, scientific evidence that supports personalized nutritional recommendations through nutrigenetic tests is still scarce [94]. There is a need for more randomized clinical trials [99] to understand the targets of nutrigenetics testing.
6.3. Targets of Nutrigenetic Tests
Nutrigenetic recommendations can be classified and grouped into portfolios. These dietary recommendations are based on scientific evidence representing a basis for creating nutrigenetic standards established by different populations’ genetic and physiological characteristics worldwide [100].
The characteristics most investigated in predictive nutrigenetic tests belong to the “micronutrients” category. In 2020, thirteen companies provided DNA testing for genetic variants associated with vitamin D metabolism, ten for vitamin C, and nine for vitamins B12, A, and B6. Among minerals, iron metabolism is the most tested by four companies. Regarding macronutrient metabolism, lipid metabolism testing is provided by thirteen companies; carbohydrate metabolism and protein metabolism are tested by six companies and one company, respectively. Notably, lactose intolerance and caffeine metabolism tests are the most requested [65].
Out of 45 companies identified, only 16 declared the genes or genetic variants used in nutrigenetic predictions. Furthermore, only 50% of companies specified the dbSNP of the variants. This fact makes interpreting reports and assessing scientific reliability complex [65].
Powerful statistical tools have been created to assess the risk of having a specific phenotype, for example, the polygenic risk scores (PRS). The PRS combine multiple associated genetic variants into a single score, weighting their frequency in the population and their estimated impact on a given characteristic [101]. The PRS derived from the cumulative effect of genetic variants associated with obesity can help estimate the risk of developing this condition [102]. Thus, the PRS represent a promising advance in understanding the genetic risk of obesity [103].
In addition to the PRS, the polygenic score (PGS) is currently being discussed. Despite being considered synonymous with PRS by some, it is a more comprehensive term that includes rare or common variants and can be used when risk analysis does not apply. Furthermore, the PGS has the potential to expand understanding of the scope and role of DTC-GT [104].
6.4. Evidence from Nutrigenetics Testing in Obesity
Nutrigenetic recommendations are possible tools to complement standard dietary recommendations for preventing and controlling obesity and its comorbidities [13]. Nutrigenetic recommendations address individualized nutritional needs, considering genetic characteristics [105,106,107]. Studies have demonstrated the potential of using nutrigenetic tests to manage obesity. However, few studies describe the evidence of nutrigenetic tests marketed as DTC-GT in this context (Table 1).

Table 1.
Scientific evidence of nutrigenetic tests in obesity.
Even with few scientific studies evaluating the impact of nutrigenetic tests, such as DTC-GT, in clinical practice, there is a significant increase in commercializing these tests, whether through spontaneous demand or via professionals in private assistance [14]. Despite the positive evidence demonstrated in weight loss and weight maintenance (Table 1), more research is needed to scientifically validate commercialized nutrigenetic tests, as confirmed by some studies [105,107,108]
A systematic review aiming to evaluate the use of genetic information in dietary advice showed that incorporating GT did not significantly improve or worsen food intake compared to the control group. However, the authors emphasize that these results should be interpreted cautiously due to the limited number of studies available, heterogeneity in design, and selected genetic markers [110]. The systematic review confirms that clinical trials need to be better designed to justify the use of GT in nutritional counseling. The authors also comment that the pediatric population was outside the scope of the work, highlighting the paradigm of clinical practice complexity, which must involve genetic information, microbiome data, and omics sciences for better treatment [110].
Li and colleagues reviewed 13 studies and found that genetic counseling did not improve motivation to change dietary intake [111]. Another review evaluating 18 studies found that disclosing genetic information did not alter eating behavior [112].
Some professional associations, such as the Brazilian Association for the Study of Obesity and Metabolic Syndrome (ABESO) [32] and the Academy of Nutrition and Dietetics [52] do not endorse the use of dietary recommendations based on nutrigenetics testing in clinical practice.
Furthermore, continued education strategies must be aimed at health professionals, who need to interpret these tests better. Ethical issues concern the scientific community and nutrigenetic test consumers [14], which need more discussion and regulation.
6.5. Overview of Ethical Issues and Positions on Nutrigenetics Tests
Regarding the practical and ethical barriers related to nutrigenetics tests, Bates et al. (2005) point out that the information provided by the tests still needs to be discussed [113]. Qualitative studies in the United Kingdom, still considering the controversies surrounding the UK Biobank project, reinforced distrust in the government’s ability to regulate the use of genetic information [81].
In the case of the USA, in the early 2000s, concerns involved the potential misuse of samples, a lack of confidentiality, misuse of information by insurers and employers, discrimination based on genotype, and commercial exploitation of information and technology [114,115]. Belgium did not have specific legislation regarding GT then, and non-binding guidance documents still governed the rules and standards. In Belgium, there is no distinction between diagnostic and predictive tests; however, experienced physicians generally order predictive tests [116].
German legislation has established that a doctor can only perform a GT after providing sufficient information and appropriate genetic counseling [117]. In Italy, there are more general authorizations and guidelines depending on the type of test [97].
In China, the Ministry of Health has attempted to oversee genetic counseling by developing guidelines for clinical genetic counseling. Furthermore, China recently announced that it is developing a guide for consumers to make informed decisions about nutrigenetic tests and for companies to provide information and messages that are not misleading to the consumer [118].
There are few legislative controls regulating GT use in Canada, and the situation worsens for nutrigenetics tests. In 2017, the Parliament of Canada approved Bill S-201, the Genetic Non-Discrimination Act (GNDA), which prohibited and prevented genetic discrimination [119]. This new law aimed to protect consumers from discrimination by employers based on the results of a GT, eliminate the requirement for an individual to undergo GT, and prohibit insurance companies from requiring GT results [14]. In December 2018, the Quebec Court of Appeal considered that Sections 4 to 9 of the GNDA were ‘ultra vires’, meaning that these sections were unconstitutional and, as a consequence, the GNDA is not a valid law, and the legitimacy of the GNDA will be determined by the Supreme Court of Canada [14].
In addition to controversial ethical issues, there is the position of Academies and Societies regarding nutrigenetic tests. The Academy of Nutrition and Dietetics (USA) and the Brazilian Society of Food and Nutrition (Brazil) do not recommend the isolated use of nutrigenetic tests for the development of dietary plans and the prescription of dietary supplements [52,62,99]. ABESO also does not recommend using isolated nutrigenetic tests to treat obesity due to insufficient scientific evidence [120]. Therefore, more research should be carried out attempting to use nutrigenetic tests in each country and evaluate their use in the prevention and treatment of diseases.
7. Conclusions
Despite evidence for the benefits of nutrigenetics and nutrigenetic testing in the prevention and treatment of obesity, there are still significant challenges and ethical issues debated by regulatory and professional institutions. However, a shared conclusion is that nutrigenetic tests should not be used in isolation in the treatment of obesity but can provide important information in individual obesity management or at a public health level. It is necessary to strengthen three pillars to advance nutrigenetics: regulation, evidence, and education. Countries need to communicate with each other and evolve in their regulations on GT and the use of genetic information, including those related to nutrigenetics. More well-designed nutrigenetics studies with robust methodologies are necessary to strengthen nutrigenetics tests’ scientific evidence and clinical validity. Finally, it is essential to educate and prepare health professionals, especially nutritionists, regarding nutrigenetics so that they can safely recommend the nutrigenetic tests, interpret their results, and outline more personalized and effective diet therapy approaches for people with predispositions to or who are diagnosed with obesity.
Author Contributions
Conceptualization, L.L.-L. and A.H.d.A.M.; methodology, L.L.-L. and A.H.d.A.M.; writing—original draft preparation, M.K.R.N.D., L.L.-L. and A.H.d.A.M.; writing—review and editing, M.K.R.N.D., L.L.-L., B.L.L.M., L.F.A.-L. and A.H.d.A.M.; supervision, L.L.-L. and A.H.d.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, código: 001), for financial support and supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), process 303094/2022-2. The funders had no role in study design, data collection, analysis, publication decision, or manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision); World Health Organization: Geneva, Switzerland, 2024. Available online: https://icd.who.int/browse11/l-m/en (accessed on 24 January 2024).
- World Obesity Federation. World Obesity Atlas 2022; World Obesity Federation: London, UK, 2022; Available online: https://s3-eu-west-1.amazonaws.com/wof-files/World_Obesity_Atlas_2022.pdf (accessed on 23 October 2023).
- Chong, B.; Jayabaskaran, J.; Kong, G.; Chan, Y.H.; Chin, Y.H.; Goh, R.; Kannan, S.; Ng, C.H.; Loong, S.; Kueh, M.T.W.; et al. Trends and Predictions of Malnutrition and Obesity in 204 Countries and Territories: An Analysis of the Global Burden of Disease Study 2019. EClinicalMedicine 2023, 57, 101850. [Google Scholar] [CrossRef]
- Loos, R.; Yeo, G. The Genetics of Obesity: From Discovery to Biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- World Health Organization. WHO Acceleration Plan to Stop Obesity. Available online: https://iris.who.int/bitstream/handle/10665/370281/9789240075634-eng.pdf?sequence=1 (accessed on 23 October 2023).
- Vazquez-Vidal, I.; Desmarchelier, C.; Jones, P. Nutrigenetics of Blood Cholesterol Concentrations: Towards Personalized Nutrition. Curr. Cardiol. Rep. 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Joffe, Y.; Houghton, C. A Novel Approach to the Nutrigenetics and Nutrigenomics of Obesity and Weight Management. Cur Oncol. Rep. 2016, 18, 43. [Google Scholar] [CrossRef]
- Corrêa, T.; Quintanilha, B.; Norde, M.; Pinhel, M.; Nonino, C.; Rogero, M. Nutritional Genomics, Inflammation and Obesity. Arch. Endocrinol. Metab. 2020, 64, 205–222. [Google Scholar] [CrossRef] [PubMed]
- De Toro-Martín, J.; Arsenault, B.; Després, J.; Vohl, M. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef]
- Marcotte, B.; Cormier, H.; Garneau, V.; Robitaille, J.; Desroches, S.; Vohl, M. Nutrigenetic Testing for Personalized Nutrition: An Evaluation of Public Perceptions, Attitudes, and Concerns in a Population of French Canadians. Lifestyle Genom. 2019, 11, 155–162. [Google Scholar]
- Xiang, L.; Wu, H.; Pan, A.; Patel, B.; Xiang, G.; Qi, L.; Kaplan, R.; Hu, F.; Wylie-Rosett, J.; Qi, Q. FTO Genotype and Weight Loss in Diet and Lifestyle Interventions: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2016, 103, 1162–1170. [Google Scholar] [CrossRef]
- Keathley, J.; Garneau, V.; Marcil, V.; Mutch, D.; Robitaille, J.; Rudkowska, I.; Sofian, G.; Desroches, S.; Vohl, M. Nutrigenetics, Omega-3 and Plasma Lipids/Lipoproteins/Apolipoproteins with Evidence Evaluation Using the GRADE Approach: A Systematic Review. BMJ Open 2022, 12, e054417. [Google Scholar] [CrossRef]
- Pérez-Beltrán, Y.; Rivera-Iñiguez, I.; Gonzalez-Becerra, K.; Pérez-Naitoh, N.; Tovar, J.; Sáyago-Ayerdi, S.; Mendivil, E. Personalized Dietary Recommendations Based on Lipid-Related Genetic Variants: A Systematic Review. Front. Nutr. 2022, 9, 830283. [Google Scholar] [CrossRef]
- Horne, J.; Gilliland, J.; Madill, J.; Shelley, J. A Critical Examination of Legal and Ethical Considerations for Nutrigenetic Testing with Recommendations for Improving Regulation in Canada: From Science to Consumer. J. Law. Biosci. 2020, 7, lsaa003. [Google Scholar] [CrossRef]
- World Health Organization. WHO European Regional Obesity Report 2022; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2022.
- Wharton, S.; Lau, D.; Vallis, M.; Sharma, A.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in Adults: A Clinical Practice Guideline. Can. Med. Assoc. J. 2020, 192, E875–E891. [Google Scholar] [CrossRef]
- Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R.; Prospective Studies Collaboration. Body-Mass Index and Cause-Specific Mortality in 900 000 Adults: Collaborative Analyses of 57 Prospective Studies. Lancet 2009, 373, 1083–1096. [Google Scholar]
- Wolin, K.; Carson, K.; Colditz, G. Obesity and Cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.; Reitsma, M.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer: Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Brock, J.; Billeter, A.; Müller-Stich, B.; Herth, F. Obesity and the Lung: What We Know Today. Respiration 2020, 99, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Commodore-Mensah, Y.; Selvin, E.; Aboagye, J.; Turkson-Ocran, R.A.; Li, X.; Himmelfarb, C.D.; Ahima, R.S.; Cooper, L.A. Hypertension, Overweight/Obesity, and Diabetes among Immigrants in the United States: An Analysis of the 2010–2016 National Health Interview Survey. BMC Public Health 2018, 18, 773. [Google Scholar] [CrossRef] [PubMed]
- Brownell, K.; Kersh, R.; Ludwig, D.; Post, R.C.; Puhl, R.M.; Schwartz, M.B.; Willett, W.C. Personal Responsibility and Obesity: A Constructive Approach to a Controversial Issue. Health Aff. 2010, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Tekes, B.; Üzümcüoglu, Y.; Hoe, C.; Özkan, T. The Relationship Between Hofstede’s Cultural Dimensions, Schwartz’s Cultural Values, and Obesity. Psychol. Rep. 2018, 122, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Dinu, M.; Madarena, M.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of Ultra-Processed Foods and Health Status: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef]
- Harb, A.; Shechter, A.; Koch, P.; St-Onge, M.-P. Ultra-Processed Foods and the Development of Obesity in Adults. Eur. J. Clin. Nutr. 2023, 77, 619–627. [Google Scholar] [CrossRef]
- Ministério Da Saúde; Secretaria de Vigilância em Saúde e Ambiente; Departamento de Análise Epidemiológica e Vigilância de Doenças Não Transmissíveis. Vigitel Brasil 2023: Vigilância de Fatores de Risco e Proteção Para Doenças Crônicas Por Inquérito Telefônico; Ministério da Saúde: Brasilia, Brazil, 2023; p. 131.
- Malik, V.; Popkin, B.; Bray, G.; Després, J.; Hu, F. Sugar-Sweetened Beverages, Obesity, Type 2 Diabetes Mellitus, and Cardiovascular Disease Risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef]
- Schulze, M.; Manson, J.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages, Weight Gain, and Incidence of Type 2 Diabetes in Young and Middle-Aged Women. JAMA 2004, 292, 927–934. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.; Willett, W.; Hu, F. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Malik, V.; Hu, F. Sugar-Sweetened Beverages and Health: Where Does the Evidence Stand? Am. J. Clin. Nutr. 2011, 94, 1161–1162. [Google Scholar] [CrossRef]
- Pepe, R.; Lottenberg, A.; Fujiwara, C.; Beyruti, M.; Cintra, D.E.C.; Machado, R.M.; Rodrigues, A.; Jensen, N.S.O.; Caldas, A.; Fernandes, A.E.; et al. Posicionamento Sobre o Tratamento Nutricional do Sobrepeso e da Obesidade. ABESO 2022, 1, 260. [Google Scholar]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/ TOS Guideline for the Management of Overweight and Obesity in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.; Gilliland, J.; O’Connor, C.; Seabrook, J.; Janet, M. Change in Weight, BMI, and Body Composition in a Population-Based Intervention Versus Genetic-Based Intervention: The NOW Trial. Obesity 2020, 28, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Clemmensen, C.; Finan, B.; DiMarchi, R.; Tschöp, M. Anti-Obesity Therapy: From Rainbow Pills to Polyagonists. Pharmacol. Rev. 2018, 70, 712–746. [Google Scholar] [CrossRef] [PubMed]
- Angelidi, A.; Belanger, M.; Kokkinos, A.; Koliaki, C.; Mantzoros, C. Novel Noninvasive Approaches to the Treatment of Obesity: From Pharmacotherapy to Gene Therapy. Endocr. Rev. 2022, 43, 507–557. [Google Scholar] [CrossRef] [PubMed]
- Sharretts, J.; Galescu, O.; Gomatam, S.; Andraca-Carrera, E.; Hampp, C.; Yanoff, L. Cancer Risk Associated with Lorcaserin: The FDA’s Review of the CAMELLIA-TIMI 61 Trial. N. Engl. J. Med. 2020, 383, 1000–1002. [Google Scholar] [CrossRef] [PubMed]
- Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica. Diretrizes Brasileiras de Obesidade 2016, 4th ed.; ABESO: São Paulo, SP, Brazil, 2016; 188p. [Google Scholar]
- Cornier, M. A Review of Current Guidelines for the Treatment of Obesity. Am. J. Manag. Care. 2022, 28 (Suppl. S15), S288–S296. [Google Scholar] [PubMed]
- Telese, A.; Sehgal, V.; Magee, C.; Naik, S.; Alqahtani, S.; Lovat, L.; Haidry, R.J. Bariatric and Metabolic Endoscopy: A New Paradigm. Clin. Transl. Gastroenterol. 2021, 12, e00364. [Google Scholar] [CrossRef]
- Rubino, F.; Nathan, D.; Eckel, R.; Schauer, P.R.; Alberti, K.G.M.M.; Zimmet, P.Z.; Del Prato, S.; Ji, L.; Sadikot, S.M.; Herman, W.H.; et al. Delegates of the 2nd Diabetes Surgery Summit. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Obes. Surg. 2017, 27, 2–21. [Google Scholar] [CrossRef]
- Hinney, A.; Körner, A.; Fischer-Posovszky, P. The Promise of New Anti-Obesity Therapies Arising from Knowledge of Genetic Obesity Traits. Nat. Rev. Endocrinol. 2022, 18, 623–637. [Google Scholar] [CrossRef]
- Antwi, J. Precision Nutrition to Improve Risk Factors of Obesity and Type 2 Diabetes. Curr. Nutr. Rep. 2023, 12, 679–694. [Google Scholar] [CrossRef]
- Ramaswami, R.; Bayer, R.; Galea, S. Precision Medicine from a Public Health Perspective. Annu. Rev. Public Health 2018, 39, 153–168. [Google Scholar] [CrossRef]
- Collins, F.; Varmus, H. A New Initiative on Precision Medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Portolés, O.; Sotos-Prieto, M.; Fernández-Carrión, R.; Ramirez-Sabio, J.; Zanón-Moreno, V.; Mattei, J.; Sorlí, J.; Ordovas, J. A Guide to Applying the Sex-Gender Perspective to Nutritional Genomics. Nutrients 2018, 11, 4. [Google Scholar] [CrossRef]
- Wild, C. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef]
- Miller, G.; Jones, D. The Nature of Nurture: Refining the Definition of the Exposome. Toxicol. Sci. 2014, 137, 1–2. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Martinez, J.; Milagro, F. Holistic Integration of Omics Tools for Precision Nutrition in Health and Disease. Nutrients 2022, 14, 4074. [Google Scholar] [CrossRef]
- Fournier, T.; Poulain, J.; Jacob, M. Genômica Nutricional: (Re)Considerando as Relações Alimentação-Saúde via Religação das Ciências Sociais, Biomédicas e da Vida. Rev. Inter-Legere 2019, 2, c18336. [Google Scholar]
- Camp, K.; Trujillo, E. Position of the Academy of Nutrition and Dietetics: Nutritional Genomics. J. Acad. Nutr. Diet. 2014, 114, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, A.; Wuni, R.; Ventura, E.; Chiet, T.; Cheah, E.; Loganathan, A.; Quen, P.; Appukutty, M.; Noh, M.; Givens, I.; et al. Implementation of Nutrigenetics and Nutrigenomics Research and Training Activities for Developing Precision Nutrition Strategies in Malaysia. Nutrients 2022, 14, 5108. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.; Newsome, J.; Santos, H.; Schiller, M. Prioritization of Variants for Investigation of Genotype-Directed Nutrition in Human Superpopulations. Int. J. Mol. Sci. 2019, 20, 3516. [Google Scholar] [CrossRef]
- Steck, S.; Keku, T.; Butler, L.; Galanko, J.; Massa, B.; Millikan, R.; Sandler, R. Polymorphisms in Methionine Synthase, Methionine Synthase Reductase and Serine Hydroxymethyltransferase, Folate and Alcohol Intake, and Colon Cancer Risk. Lifestyle Genom. 2008, 1, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y. The Genetics of Obesity: A Narrative Review. Precis. Futur. Med. 2022, 6, 226–232. [Google Scholar] [CrossRef]
- Rankinen, T.; Zuberi, A.; Chagnon, Y.; Weisnagel, S.; Argyropoulos, G.; Walts, B.; Al, E. The Human Obesity Gene Map: The 2005 Update. Obesity 2006, 14, 529–644. [Google Scholar] [CrossRef]
- Ndiaye, F.; Huyvaert, M.; Ortalli, A.; Canouil, M.; Lecoeur, C.; Verbanck, M.; Lobbens, S.; Khamis, A.; Marselli, L.; Marchetti, P.; et al. The Expression of Genes in Top Obesity-Associated Loci Is Enriched in Insula and Substantia Nigra Brain Regions Involved in Addiction and Reward. Int. J. Obes. 2020, 44, 539–543. [Google Scholar] [CrossRef]
- Morais, C.; Cominetti, C. Bioetica e Testes Nutrigenéticos Preditivos. In Genômica Nutricional dos Fundamentos à Nutrição Molecular; Cominetti, C., Rogero, M., Horst, M., Eds.; Manole: Barueri, Brazil, 2017; pp. 516–521. [Google Scholar]
- Justice, A.; Winkler, T.; Al, F.M.; Feitosa, M.F.; Graff, M.; Fisher, V.A.; Young, K.; Barata, L.; Deng, X.; Czajkowski, J.; et al. Genome-Wide Meta-Analysis of 241,258 Adults Accounting for Smoking Behaviour Identifies Novel Loci for Obesity Traits. Nat. Commun. 2017, 8, 14977. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Database of Single Nucleotide Polymorphisms. dbSNP. Available online: https://www.ncbi.nlm.nih.gov/snp/ (accessed on 16 October 2023).
- Cominetti, C.; Rogero, M.; Horst, M. Brazilian Society for Food and Nutrition Position Statement: Nutrigenetic Tests. Nutrire 2017, 42, 931. [Google Scholar] [CrossRef]
- García-Solís, P.; Reyes-Bastidas, M.; Flores, K.; García, O.; Rosado, J.; Méndez-Villa, L.; Garcia, G.C.; García-Gutiérrez, D.; Kuri-García, A.; Hernández-Montiel, H.; et al. Fat Mass Obesity-Associated (FTO) (Rs9939609) and Melanocortin 4 Receptor (MC4R) (Rs17782313) SNP Are Positively Associated with Obesity and Blood Pressure in Mexican School-Aged Children. Br. J. Nutr. 2016, 116, 1834–1840. [Google Scholar] [CrossRef]
- Walley, A.; Asher, J.; Froguel, P. The Genetic Contribuiton to Non-Syndromic Human Obesity. Nat. Rev. Genet. 2009, 10, 431–442. [Google Scholar] [CrossRef]
- Floris, M.; Cano, A.; Porru, L.; Addis, R.; Cambedda, A.; Idda, M.L.; Steri, M.; Ventura, C.; Maioli, M. Direct-to-Consumer Nutrigenetics Testing: An Overview. Nutrients 2020, 12, 566. [Google Scholar] [CrossRef] [PubMed]
- Pagon, R.; Hanson, N.; Neufeld-Kaiser, W.; Covington, M. Genetic Testing. West. J. Med. 2001, 174, 344–347. [Google Scholar] [CrossRef]
- Genetic Alliance; The New York-Mid-Atlantic Consortium for Genetic and Newborn Screening Services. Understanding Genetics: A New York, Mid-Atlantic Guide for Patients and Health Professionals; Genetic Alliance: Damascus, MD, USA, 2009. [Google Scholar]
- Oliveri, S.; Ferrari, F.; Manfrinati, A.; Pravettoni, G. A Systematic Review of the Psychological Implications of Genetic Testing: A Comparative Analysis Among Cardiovascular, Neurodegenerative and Cancer Diseases. Front. Genet. 2018, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Assessing Genetic Risks; Andrews, L.B.; Fullarton, J.E.; Holtzman, N.A.; Motulsky, A.G. Assessing Genetic Risks: Implications for Health and Social Policy; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Rafi, I.; Qureshi, N.; Lucassen, A.; Modell, M.; Elmslie, F.; Kai, J.; Kirk, M.; Starey, N.; Goff, S.; Brennan, P.; et al. “Over-the-Counter” Genetic Testing: What Does It Really Mean for Primary Care? Br. J. Gen. Pract. 2009, 59, 283–287. [Google Scholar] [CrossRef]
- Inglese, S.; Lavazza, A.; Abbate, C. Crystal Ball Health Policies: A Case Against Preventive Testing For Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 842629. [Google Scholar] [CrossRef]
- World Health Organization. Accelerating Access to Genomics for Global Health: Promotion, Implementation, Collaboration, and Ethical, Legal, and Social Issues: A Report of the WHO Science Council; WHO: Geneva, Switzerland, 2022.
- Ordovas, J.; Ferguson, L.; Tai, E.; Mathers, J. Personalised Nutrition and Health. BMJ 2018, 361, bmj.k2173. [Google Scholar] [CrossRef] [PubMed]
- Ginoza, M.; Isasi, R. Regulating Preimplantation Genetic Testing across the World: A Comparison of International Policy and Ethical Perspectives. Cold Spring Harb. Perspect. Med. 2020, 10, a036681. [Google Scholar] [CrossRef] [PubMed]
- Burke, W. Genetic Tests: Clinical Validity and Clinical Utility. Curr. Protoc. Hum. Genet. 2014, 81, 9.15.1–9.15.8. [Google Scholar] [CrossRef]
- National Human Genome Research Institute. Regulation of Genetic Tests. Available online: https://www.genome.gov/about-genomics/policy-issues/Regulation-of-Genetic-Tests (accessed on 16 October 2023).
- Abul-Husn, N.; Owusu Obeng, A.; Sanderson, S.; Gottesman, O.; Scott, S. Implementation and Utilization of Genetic Testing in Personalized Medicine. Pharmgenom. Pers. Med. 2014, 7, 227–240. [Google Scholar]
- Jiang, S.; Liberti, L.; Lebo, D. Direct-to-Consumer Genetic Testing: A Comprehensive Review. Ther. Innov. Regul. Sci. 2023, 57, 1190–1198. [Google Scholar] [CrossRef]
- Evans, J.; Skrzynia, C.; Burke, W. The Complexities of Predictive Genetic Testing. BMJ 2001, 322, 1052–1056. [Google Scholar] [CrossRef]
- Xi, Q.; Jin, S.; Morris, S. Economic Evaluations of Predictive Genetic Testing: A Scoping Review. PLoS ONE 2023, 18, e0276572. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Peters, N.; Shea, J.; Armstrong, K. Attitudes and Misconceptions about Predictive Genetic Testing for Cancer Risk. Commun. Genet. 2005, 8, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Garrett, J.; Lantos, J.; Biesecker, L.; Childerhose, J.; Chung, W.; Holm, I.; Koenig, B.; McEwen, J.; Wilfond, B.; Brothers, K. Clinical Sequencing Exploratory Research (CSER) Consortium Pediatrics Working Group. Rethinking the “Open Future” Argument against Predictive Genetic Testing of Children. Genet. Med. 2019, 21, 2190–2198. [Google Scholar] [CrossRef]
- Saya, S.; McIntosh, J.; Winship, I.; Clendenning, M.; Milton, S.; Oberoi, J.; Dowty, J.; Buchanan, D.; Jenkins, M.; Emery, J. A Genomic Test for Colorectal Cancer Risk: Is This Acceptable and Feasible in Primary Care? Public Health Genom. 2020, 23, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Kvaratskhelia, E.; Chokoshvili, D.; Kvintradze, M.; Surmava, S.; Dzagoevi, K.; Borry, P.; Abzianidze, E. Public Attitudes towards the Genetic Testing in Georgia. J. Commun. Genet. 2021, 12, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Wells, I.; Simons, G.; Davenport, C.; Mallen, C.; Raza, K.; Falahee, M. Acceptability of Predictive Testing for Ischemic Heart Disease in Those with a Family History and the Impact of Results on Behavioural Intention and Behaviour Change: A Systematic Review. BMC Public Health 2022, 22, 1751. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, L.; Reddy, P. Stakeholder Knowledge and Attitudes toward the Use of Predictive Genetic Testing in South Africa. J. Community Genet. 2022, 13, 567–577. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Pietilä, A.; Iso-Touru, T.; Hopia, A.; Tahvonen, R.; Vähäkangas, K. Information Provided to Consumers about Direct-to-Consumer Nutrigenetic Testing. Public Health Genom. 2019, 22, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Murry, L.; Telford, L.; Moriarty, F. Direct-to-Consumer Genetic Testing: An Updated Systematic Review of Healthcare Professionals’ Knowledge and Views, and Ethical and Legal Concerns. Eur. J. Hum. Genet. 2022, 30, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.; Lefferts, J. Lessons Learned from Direct-to-Consumer Genetic Testing. Clin. Lab. Med. 2020, 40, 83–92. [Google Scholar] [CrossRef]
- Basch, C.; Hillyer, G.; Samuel, L.; Datuowei, E.; Cohn, B. Direct-to-Consumer Genetic Testing in the News: A Descriptive Analysis. J. Commun. Genet. 2023, 14, 63–69. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Dashti, H.; Merino, J. Nutritional Genomics and Direct-to-Consumer Genetic Testing: An Overview. Adv. Nutr. 2018, 9, 128–135. [Google Scholar] [CrossRef]
- Phillips, A. Only a Click Away—DTC Genetics for Ancestry, Health, Love…and More: A View of the Business and Regulatory Landscape. Appl. Transl. Genom. 2016, 8, 16–22. [Google Scholar] [CrossRef]
- Malgorzata, M.; Maria, S.; Michał, W. Genetic Testing-Whether to Allow Complete Freedom? Direct to Consumer Tests versus Genetic Tests for Medical Purposes. J. Appl. Genet. 2022, 63, 119–126. [Google Scholar] [CrossRef]
- Naureen, Z.; Miggiano, G.; Aquilanti, B.; Velluti, V.; Matera, G.; Gagliardi, L.; Zulian, A.; Romanelli, R.; Bertelli, M. Genetic Test for the Prescription of Diets in Support of Physical Activity. Acta Biomed. 2020, 91, e2020011. [Google Scholar]
- Roosan, D.; Wu, Y.; Tran, M.; Huang, Y.; Baskys, A.; Roosan, M.R. Opportunities to Integrate Nutrigenomics into Clinical Practice and Patient Counseling. Eur. J. Clin. Nutr. 2023, 77, 36–44. [Google Scholar] [CrossRef]
- Stewart-Knox, B.; Bunting, B.; Gilpin, S.; Parr, H.J.; Pinhão, S.; Strain, J.J.; de Almeida, M.D.V.; Gibney, M. Attitudes toward Genetic Testing and Personalised Nutrition in a Representative Sample of European Consumers. Br. J. Nutr. 2008, 101, 982–989. [Google Scholar] [CrossRef]
- Oliveri, S.; Marton, G.; Vergani, L.; Cutica, I.; Gorini, A.; Spinella, F.; Pravettoni, G. Genetic Testing Consumers in Italy: A Preliminary Investigation of the Socio-Demographic Profile, Health-Related Habits, and Decision Purposes. Front. Public Health 2020, 8, 511. [Google Scholar] [CrossRef] [PubMed]
- Kaufman-Shriqui, V.; Salem, H.; Boaz, M.; Birk, R. Knowledge and Attitudes Towards Nutrigenetics: Findings from the 2018 Unified Forces Preventive Nutrition Conference (UFPN). Nutrients 2020, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.; Monnard, C.; Ellis, A.; Rozga, M. Consensus Report of the Academy of Nutrition and Dietetics: Incorporating Genetic Testing into Nutrition Care. J. Acad. Nutr. Diet. 2021, 121, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Beltrán, Y.; González-Becerra, K.; Rivera-Iñiguez, I.; Martínez-López, E.; Ramos-Lopez, O.; Alcaraz-Mejía, M.; Rodríguez-Echevarría, R.; Sáyago-Ayerdi, S.; Mendivil, E. A Nutrigenetic Strategy for Reducing Blood Lipids and Low-Grade Inflammation in Adults with Obesity and Overweight. Nutrients 2023, 15, 4324. [Google Scholar] [CrossRef] [PubMed]
- Dudbridge, F. Power and Predictive Accuracy of Polygenic Risk Scores. PLoS Genet. 2013, 9, e003348. [Google Scholar] [CrossRef]
- Torkamani, A.; Topol, E. Polygenic Risk Scores Expand to Obesity. Cell 2019, 177, 518–520. [Google Scholar] [CrossRef]
- Joo, Y.; Actkins, K.; Pacheco, J.; Basile, A.; Carroll, R.; Crosslin, D.; Day, F.; Denny, J.C.; Edwards, D.R.V.; Hakonarson, H.; et al. A Polygenic and Phenotypic Risk Prediction for Polycystic Ovary Syndrome Evaluated by Phenome-Wide Association Studies. J. Clin. Endocrinol. Metab. 2020, 105, 1918–1936. [Google Scholar] [CrossRef]
- Park, J.; Lu, C. Polygenic Scores in the Direct-to-Consumer Setting: Challenges and Opportunities for a New Era in Consumer Genetic Testing. J. Pers. Med. 2023, 13, 573. [Google Scholar] [CrossRef]
- Arkadianos, I.; Valdes, A.; Marinos, E.; Florou, A.; Gill, R.; Grimaldi, K. Improved Weight Management Using Genetic Information to Personalize a Calorie Controlled Diet. Nutr. J. 2007, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S. Nutrigenetics and the Early Life Origins of Health and Disease: Effects of Protein Restriction. In Principles of Nutrigenetics and Nutrigenomics: Fundamentals for Individualized Nutrition; Caterina, R., Alfredo Martinez, J., Kohlmeier, M., Eds.; Elsevier: London, UK, 2019; pp. 113–119. [Google Scholar]
- Tamilvanan, J.; Kalpana, C. A Comparitive Analysis: Improved Weight Management Using Nutrigenetically Tailored Diet among Indians. J. Surv. Fish. Sci. 2023, 10, 4856–4862. [Google Scholar]
- Vranceanu, M.; Pickering, C.; Filip, L.; Pralea, I.; Sundaram, S.; Al-Saleh, A.; Popa, D.; Grimaldi, K. A Comparison of a Ketogenic Diet with a LowGI/Nutrigenetic Diet over 6 Months for Weight Loss and 18-Month Follow-Up. BMC Nutr. 2020, 6, 53. [Google Scholar] [CrossRef]
- Frankwich, K.; Egnatios, J.; Kenyon, M.; Rutledge, T.; Liao, P.; Gupta, S.; Herbst, K.; Zarrinpar, A. Differences in Weight Loss Between Persons on Standard Balanced vs Nutrigenetic Diets in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1625–1632.e1. [Google Scholar] [CrossRef]
- Robinson, K.; Rozga, M.; Braakhuis, A.; Ellis, A.; Monnard, C.; Sinley, R.; Wanner, A.; Vargas, A. Effect of Incorporating Genetic Testing Results into Nutrition Counseling and Care on Dietary Intake: An Evidence Analysis Center Systematic Review—Part I. J. Acad. Nutr. Diet. 2020, 121, 553–581.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ye, Z.; Whelan, K.; Truby, H. The Effect of Communicating the Genetic Risk of Cardiometabolic Disorders on Motivation and Actual Engagement in Preventative Lifestyle Modification and Clinical Outcome: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2016, 116, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Hollands, G.; French, D.; Griffin, S.J.; Prevost, A.T.; Sutton, S.; King, S.; Marteau, T.M. The Impact of Communicating Genetic Risks of Disease on Risk-Reducing Health Behaviour: Systematic Review with Metaanalysis. Br. Med. J. 2016, 352, i1102. [Google Scholar] [CrossRef]
- Bates, B.; Lynch, J.; Bevan, J.; Condit, C. Warranted Concerns, Warranted Outlooks: A Focus Group Study of Public Understandings of Genetic Research. Soc. Sci. Med. 2005, 60, 331–344. [Google Scholar] [CrossRef]
- Petersen, A. Securing Our Genetic Health: Engendering Trust in UK Biobank. Sociol. Health Illn. 2005, 27, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Skirton, H.; Frazier, L.; Calvin, A.; Cohen, M. A Legacy for the Children—Attitudes of Older Adults in the United Kingdom to Genetic Testing. J. Clin. Nurs. 2006, 15, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Alain, D.; Lut, M.; Christel, F. Organisation and Financing of Genetic Testing in Belgium; The Belgian Health Care Knowledge Centre: Brussels, Belgium, 2007. [Google Scholar]
- Kalokairinou, L.; Howard, H.; Slokenberga, S.; Fisher, E.; Flatscher-Thöni, M.; Hartlev, M.; van Hellemondt, R.; Juškevičius, J.; Kapelenska-Pregowska, J.; Kováč, P.; et al. Legislation of Direct-to-consumer Genetic Testing in Europe: A Fragmented Regulatory Landscape. J. Commun. Genet. 2018, 9, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liang, B.; Zhu, L.; Shen, Y.; He, L. The Rise of the Genetic Counseling Profession in China. Am. J. Med. Genet. Part C Semin. Med. Genet. 2019, 181C, 170–176. [Google Scholar] [CrossRef]
- Bill, S. An Act to Prohibit and Prevent Genetic Discrimination; Parliament of Canada: Ottawa, ON, Canada, 2017.
- Pepe, R.; Lottenberg, A.; Fujiwara, C.; Beyruti, M.; Cintra, D.; Machado, R.; Rodrigues, A.; Jensen, N.; Caldas, A.; Fernandes, A.; et al. Position Statement on Nutrition Therapy for Overweight and Obesity: Nutrition Department of the Brazilian Association for the Study of Obesity and Metabolic Syndrome (ABESO-2022). Diabetol. Metab. Syndr. 2023, 15, 124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).