Novel Biomarkers of Bone Metabolism
Abstract
1. Introduction
2. Novel Bone Markers
2.1. Receptor Activator of Nuclear Factor Kappa B Ligand and Osteoprotegerin
2.2. Sclerostin and Dickkopf1
2.3. Periostin
2.4. Sphingosine-1-Phosphate
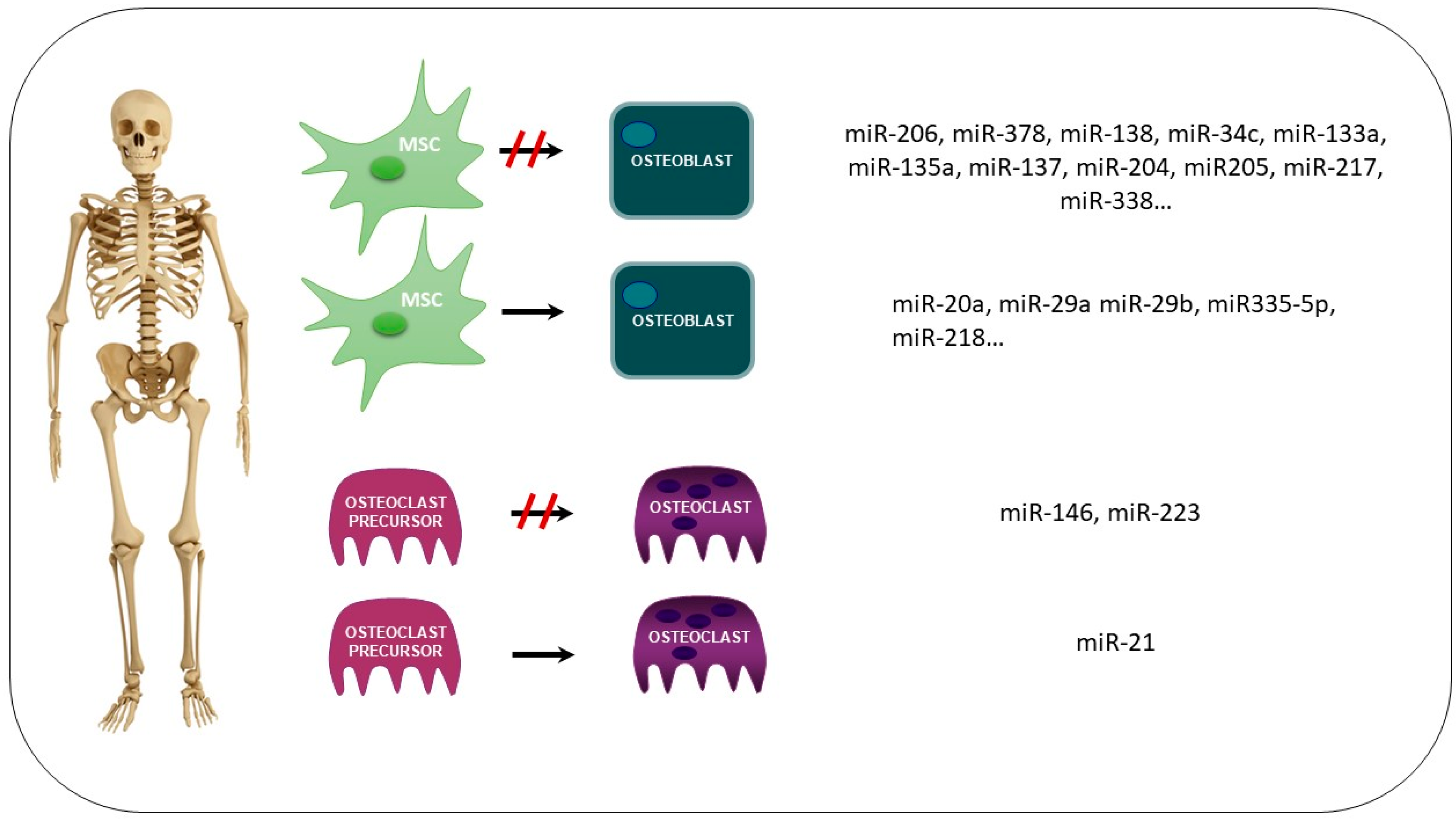
2.5. microRNAs (miRs)
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef]
- Alford, A.I.; Kozloff, K.M.; Hankenson, K.D. Extracellular matrix networks in bone remodeling. Int. J. Biochem. Cell Biol. 2015, 65, 20–31. [Google Scholar] [CrossRef]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar]
- Zhou, R.; Guo, Q.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.; Luo, X. Endocrine role of bone in the regulation of energy metabolism. Bone Res. 2021, 9, 25. [Google Scholar] [CrossRef]
- Shao, J.; Wang, Z.; Yang, T.; Ying, H.; Zhang, Y.; Liu, S. Bone Regulates Glucose Metabolism as an Endocrine Organ through Osteocalcin. Int. J. Endocrinol. 2015, 2015, 967673. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, L.; Wang, Z.; Zhao, X.; Zou, J. Endocrine Regulation of Extra-skeletal Organs by Bone-derived Secreted Protein and the effect of Mechanical Stimulation. Front. Cell Dev. Biol. 2021, 9, 778015. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yu, X. FGF23 Actions in CKD-MBD and in Other Organs during CKD. Curr. Med. Chem. 2022, 30, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; Zemel, B. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.-P.; Chevalley, T.; Ferrari, S.; Rizzoli, R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud Publica Mex. 2009, 51 (Suppl. S1), S5–S17. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.A. Assessing bone health in children and adolescents. Indian. J. Endocrinol. Metab. 2012, 16 (Suppl. S2), S205–S212. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; Amato, A.; Drid, P.; Korovljev, D.; Vasto, S.; Baldassano, S. The Impact of Diet and Physical Activity on Bone Health in Children and Adolescents. Front. Endocrinol. 2021, 12, 704647. [Google Scholar] [CrossRef] [PubMed]
- Office of the Surgeon General. Reports of the Surgeon General. In Bone Health and Osteoporosis: A Report of the Surgeon General; Office of the Surgeon General (US): Rockville, MD, USA, 2004. [Google Scholar]
- Covic, A.; Vervloet, M.; Massy, Z.; Torres, P.U.; Goldsmith, D.; Brandenburg, V.; Mazzaferro, S.; Evenepoel, P.; Bover, J.; Apetrii, M.; et al. Bone and mineral disorders in chronic kidney disease: Implications for cardiovascular health and ageing in the general population. Lancet Diabetes Endocrinol. 2018, 6, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Desbiens, L.-C.; Goupil, R.; Madore, F.; Mac-Way, F. Incidence of fractures in middle-aged individuals with early chronic kidney disease: A population-based analysis of CARTaGENE. Nephrol. Dial. Transplant. 2020, 35, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, P.; Nickolas, T.L. The young, the uremic and the broken. Nephrol. Dial. Transplant. 2020, 35, 1649–1651. [Google Scholar] [CrossRef] [PubMed]
- Vilaca, T.; Salam, S.; Schini, M.; Harnan, S.; Sutton, A.; Poku, E.; Allen, I.E.; Cummings, S.R.; Eastell, R. Risks of Hip and Nonvertebral Fractures in Patients With CKD G3a-G5D: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2020, 76, 521–532. [Google Scholar] [CrossRef]
- Genant, H.K.; Cooper, C.; Poor, G.; Reid, I.; Ehrlich, G.; Kanis, J.; Nordin, B.E.C.; Barrett-Connor, E.; Black, D.; Bonjour, J.-P.; et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos. Int. 1999, 10, 259–264. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Watts, N.B. New guidelines for the prevention and treatment of osteoporosis. South. Med. J. 2009, 102, 175–179. [Google Scholar] [CrossRef]
- Pisani, P.; Renna, M.D.; Conversano, F.; Casciaro, E.; Di Paola, M.; Quarta, E.; Muratore, M.; Casciaro, S. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J. Orthop. 2016, 7, 171–181. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef]
- Chan, J.J.; Cupples, L.A.; Kiel, D.P.; O’Donnell, C.J.; Hoffmann, U.; Samelson, E.J. QCT Volumetric Bone Mineral Density and Vascular and Valvular Calcification: The Framingham Study. J. Bone Miner. Res. 2015, 30, 1767–1774. [Google Scholar] [CrossRef]
- Kiel, D.P.; Kauppila, L.I.; Cupples, L.A.; Hannan, M.T.; O’Donnell, C.J.; Wilson, P.W.F. Bone loss and the progression of abdominal aortic calcification over a 25 year period: The Framingham Heart Study. Calcif. Tissue Int. 2001, 68, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Leow, K.; Szulc, P.; Schousboe, J.T.; Kiel, D.P.; Teixeira-Pinto, A.; Shaikh, H.; Sawang, M.; Sim, M.; Bondonno, N.; Hodgson, J.M.; et al. Prognostic Value of Abdominal Aortic Calcification: A Systematic Review and Meta-Analysis of Observational Studies. J. Am. Heart Assoc. 2021, 10, e017205. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Arfai, K.; Liu, X.; Sayre, J.; Gilsanz, V. Aortic calcification and the risk of osteoporosis and fractures. J. Clin. Endocrinol. Metab. 2004, 89, 4246–4253. [Google Scholar] [CrossRef] [PubMed]
- Naves, M.; García, M.R.; López, J.B.D.; Alonso, C.G.; Andía, J.B.C. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos. Int. 2008, 19, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Coen, G.; Ballanti, P.; Mantella, D.; Manni, M.; Lippi, B.; Pierantozzi, A.; Di Giulio, S.; Pellegrino, L.; Romagnoli, A.; Simonetti, G.; et al. Bone turnover, osteopenia and vascular calcifications in hemodialysis patients. A histomorphometric and multislice CT study. Am. J. Nephrol. 2009, 29, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Cascio, V.L.; Bertoldo, F.; Gambaro, G.; Gasperi, E.; Furlan, F.; Colapietro, F.; Cascio, C.L.; Campagnola, M. Urinary galactosyl-hydroxylysine in postmenopausal osteoporotic women: A potential marker of bone fragility. J. Bone Miner. Res. 1999, 14, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Forsprecher, J.; Wang, Z.; Goldberg, H.A.; Kaartinen, M.T. Transglutaminase-mediated oligomerization promotes osteoblast adhesive properties of osteopontin and bone sialoprotein. Cell Adh Migr. 2011, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Holm, E.; Gleberzon, J.S.; Liao, Y.; Sørensen, E.S.; Beier, F.; Hunter, G.K.; Goldberg, H.A. Osteopontin mediates mineralization and not osteogenic cell development in vitro. Biochem. J. 2014, 464, 355–364. [Google Scholar] [CrossRef]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159. [Google Scholar] [CrossRef]
- Cannata-Andia, J.B.; Roman-Garcia, P.; Hruska, K. The connections between vascular calcification and bone health. Nephrol. Dial. Transplant. 2011, 26, 3429–3436. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C.; et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546. [Google Scholar] [CrossRef]
- Takegahara, N.; Kim, H.; Choi, Y. RANKL biology. Bone 2022, 159, 116353. [Google Scholar] [CrossRef]
- Andreo-López, M.C.; Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. The Influence of the Mediterranean Dietary Pattern on Osteoporosis and Sarcopenia. Nutrients 2023, 15, 3224. [Google Scholar] [CrossRef]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: A follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Carmen Naranjo, M.; Garcia, I.; Bermudez, B.; Lopez, S.; Cardelo, M.P.; Abia, R.; Muriana, F.J.G.; Montserrat-de la Paz, S. Acute effects of dietary fatty acids on osteclastogenesis via RANKL/RANK/OPG system. Mol. Nutr. Food Res. 2016, 60, 2505–2513. [Google Scholar] [CrossRef]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes. Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Schoppet, M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004, 292, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-López, N.; Panizo, S.; Alonso-Montes, C.; Martínez-Arias, L.; Avello, N.; Sosa, P.; Dusso, A.S.; Cannata-Andía, J.B.; Naves-Díaz, M. High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 934–941. [Google Scholar] [CrossRef]
- Panizo, S.; Cardus, A.; Encinas, M.; Parisi, E.; Valcheva, P.; López-Ongil, S.; Coll, B.; Fernandez, E.; Valdivielso, J.M. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ. Res. 2009, 104, 1041–1048. [Google Scholar] [CrossRef]
- Helas, S.; Goettsch, C.; Schoppet, M.; Zeitz, U.; Hempel, U.; Morawietz, H.; Hofbauer, L.C. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am. J. Pathol. 2009, 175, 473–478. [Google Scholar] [CrossRef]
- Samelson, E.J.; Miller, P.D.; Christiansen, C.; Daizadeh, N.S.; Grazette, L.; Anthony, M.S.; Egbuna, O.; Wang, A.; Siddhanti, S.R.; Cheung, A.M.; et al. RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J. Bone Miner. Res. 2014, 29, 450–457. [Google Scholar] [CrossRef]
- Azizieh, F.Y.; Shehab, D.; Al Jarallah, K.; Gupta, R.; Raghupathy, R. Circulatory Levels of RANKL, OPG, and Oxidative Stress Markers in Postmenopausal Women With Normal or Low Bone Mineral Density. Biomark. Insights 2019, 14, 1177271919843825. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Lu, J.; Xu, J. Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arthritis-induced osteoporosis. Rheumatol. Int. 2012, 32, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Fadda, S.; Hamdy, A.; Abulkhair, E.; Elsify, H.M.; Mostafa, A. Serum levels of osteoprotegerin and RANKL in patients with rheumatoid arthritis and their relation to bone mineral density and disease activity. Egypt. Rheumatol. 2015, 37, 1–6. [Google Scholar] [CrossRef]
- Nitta, K.; Akiba, T.; Uchida, K.; Otsubo, S.; Takei, T.; Yumura, W.; Kabaya, T.; Nihei, H. Serum osteoprotegerin levels and the extent of vascular calcification in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1886–1889. [Google Scholar] [CrossRef] [PubMed]
- Osorio, A.; Ortega, E.; Torres, J.M.; Sanchez, P.; Ruiz-Requena, E. Biochemical markers of vascular calcification in elderly hemodialysis patients. Mol. Cell Biochem. 2013, 374, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, A.H.; Shamsara, J.; Nazemi, S.; Ghadirzadeh, S.; Shahsavand, S.; Ramezani, M. Evaluation of RANKL/OPG Serum Concentration Ratio as a New Biomarker for Coronary Artery Calcification: A Pilot Study. Thrombosis 2012, 2012, 306263. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yao, Q.; Guo, S.; Ma, S.; Dong, Y.; Xin, H.; Wang, H.; Liu, L.; Chang, W.; Zhang, Y. Type 2 diabetes with hypertensive patients results in changes to features of adipocytokines: Leptin, Irisin, LGR4, and Sfrp5. Clin. Exp. Hypertens 2019, 41, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef]
- Pereira, L.A.L.; Meng, C.; Amoedo, M.A.G.; Pinto, M.T.D.S.C.; Mendes, F.; Marques, M.A.M.P.; Weigert, A.L.L. Etelcalcetide controls secondary hyperparathyroidism and raises sclerostin levels in hemodialysis patients previously uncontrolled with cinacalcet. Nefrología 2023, 43, 197–203. [Google Scholar] [CrossRef]
- Kulkarni, N.; Halladay, D.; Miles, R.; Gilbert, L.; Frolik, C.; Galvin, R.; Martin, T.; Gillespie, M.; Onyia, J. Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell Biochem. 2005, 95, 1178–1190. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Mödder, U.I.; Hoey, K.A.; Amin, S.; McCready, L.K.; Achenbach, S.J.; Riggs, B.L.; Khosla, S. Relation of Age, Gender, and Bone Mass to Circulating Sclerostin Levels in Women and Men. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 373–379. [Google Scholar] [CrossRef]
- Asadipooya, K.; Abdalbary, M.; Ahmad, Y.; Kakani, E.; Monier-Faugere, M.-C.; El-Husseini, A. Bone Quality in CKD Patients: Current Concepts and Future Directions—Part I. Kidney Dis. 2021, 7, 268–277. [Google Scholar] [CrossRef]
- Gomes, T.S.; Aoike, D.T.; Baria, F.; Graciolli, F.G.; Moyses, R.M.; Cuppari, L. Effect of Aerobic Exercise on Markers of Bone Metabolism of Overweight and Obese Patients with Chronic Kidney Disease. J. Ren. Nutr. 2017, 27, 364–371. [Google Scholar] [CrossRef]
- Ardawi, M.-S.M.; Al-Kadi, H.; Rouzi, A.; Qari, M.H. Determinants of serum sclerostin in healthy pre- and postmenopausal women. J. Bone Miner. Res. 2011, 26, 2812–2822. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Dong, Z.; Hui, Z.; Aifei, W.; Lianfu, D.; Youjia, X. Bone Sclerostin and Dickkopf-related protein-1 are positively correlated with bone mineral density, bone microarchitecture, and bone strength in postmenopausal osteoporosis. BMC Musculoskelet. Disord. 2021, 22, 480. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-H.; Lin, W.-H.; Chao, J.-Y.; Wu, A.-B.; Tseng, C.-C.; Chang, Y.-T.; Liou, H.-H.; Wang, M.-C. Serum sclerostin levels are positively related to bone mineral density in peritoneal dialysis patients: A cross-sectional study. BMC Nephrol. 2019, 20, 266. [Google Scholar] [CrossRef] [PubMed]
- Gorter, E.A.; Reinders, C.R.; Krijnen, P.; Appelman-Dijkstra, N.M.; Schipper, I.B. Serum sclerostin levels in osteoporotic fracture patients. Eur. J. Trauma. Emerg. Surg. 2022, 48, 4857–4865. [Google Scholar] [CrossRef]
- Lu, J.-W.; Syu, R.-J.; Wang, C.-H.; Hsu, B.-G.; Tsai, J.-P. Serum Sclerostin Level Is Negatively Associated with Bone Mineral Density in Hemodialysis Patients. Medicina 2022, 58, 385. [Google Scholar] [CrossRef]
- Jean, G.; Chazot, C.; Bresson, E.; Zaoui, E.; Cavalier, E. High Serum Sclerostin Levels Are Associated with a Better Outcome in Haemodialysis Patients. Nephron 2016, 132, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.-S.M.; Rouzi, A.; Al-Sibiani, S.; Al-Senani, N.S.; Qari, M.H.; Mousa, S. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: The Center of Excellence for Osteoporosis Research Study. J. Bone Miner. Res. 2012, 27, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Magalhaes, J.; Mendonca, L.; Neto, R.; Santos, J.; Carvalho, C.G.; Oliveira, A.; Beco, A.; Frazao, J. Evaluation of Renal Osteodystrophy and Serum Bone-Related Biomarkers in a Peritoneal Dialysis Population. J. Bone Miner. Res. 2022, 37, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Guan, L.; Zhang, Y.; Yu, S.; Cao, B.; Ji, Y. Sclerostin as a new key factor in vascular calcification in chronic kidney disease stages 3 and 4. Int. Urol. Nephrol. 2016, 48, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- De Maré, A.; Opdebeeck, B.; Neven, E.; D’Haese, P.C.; Verhulst, A. Sclerostin Protects Against Vascular Calcification Development in Mice. J. Bone Miner. Res. 2022, 37, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ginsberg, C.; Seifert, M.; Agapova, O.; Sugatani, T.; Register, T.C.; Freedman, B.I.; Monier-Faugere, M.-C.; Malluche, H.; Hruska, K.A. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J. Am. Soc. Nephrol. 2014, 25, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-López, N.; Martínez-Arias, L.; Fernández-Villabrille, S.; Ruiz-Torres, M.P.; Dusso, A.; Cannata-Andía, J.B.; European Renal Osteodystrophy (EUROD) Workgroup. Role of the RANK/RANKL/OPG and Wnt/β-Catenin Systems in CKD Bone and Cardiovascular Disorders. Calcif. Tissue Int. 2021, 108, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef]
- Hwang, E.Y.; Jeong, M.S.; Park, E.-K.; Kim, J.H.; Jang, S.B. Structural characterization and interaction of periostin and bone morphogenetic protein for regulation of collagen cross-linking. Biochem. Biophys. Res. Commun. 2014, 449, 425–431. [Google Scholar] [CrossRef]
- Bonnet, N.; Standley, K.N.; Bianchi, E.N.; Stadelmann, V.; Foti, M.; Conway, S.J.; Ferrari, S.L. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J. Biol. Chem. 2009, 284, 35939–35950. [Google Scholar] [CrossRef]
- Bonnet, N.; Conway, S.J.; Ferrari, S.L. Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc. Natl. Acad. Sci. USA 2012, 109, 15048–15053. [Google Scholar] [CrossRef]
- Gossiel, F.; Scott, J.R.; Paggiosi, M.; Naylor, K.; McCloskey, E.V.; Peel, N.F.; Walsh, J.S.; Eastell, R. Effect of Teriparatide Treatment on Circulating Periostin and Its Relationship to Regulators of Bone Formation and BMD in Postmenopausal Women with Osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 1302–1309. [Google Scholar] [CrossRef]
- Khan, R.A.; Bhandari, U.; Kapur, P.; Jain, A.; Farah, F. Effects of rosuvastatin (added to hypocaloric diet) on serum periostin, adiponectin, proinflammtory cytokines levels and hepatic steatosis in non-alcoholic fatty liver disease patients with dyslipidemia. Clin. Epidemiol. Glob. Health 2019, 7, 53–59. [Google Scholar] [CrossRef]
- Rousseau, J.C.; Sornay-Rendu, E.; Bertholon, C.; Chapurlat, R.; Garnero, P. Serum periostin is associated with fracture risk in postmenopausal women: A 7-year prospective analysis of the OFELY study. J. Clin. Endocrinol. Metab. 2014, 99, 2533–2539. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Rhee, Y.; Kim, C.H.; Baek, K.H.; Min, Y.-K.; Kim, D.-Y.; Ahn, S.H.; Kim, H.; Lee, S.H.; Lee, S.-Y.; et al. Plasma periostin associates significantly with non-vertebral but not vertebral fractures in postmenopausal women: Clinical evidence for the different effects of periostin depending on the skeletal site. Bone 2015, 81, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Savvides, M.; Sakellariou, G.T.; Gkiomisi, A.; Papatheodorou, A.; Terpos, E. Circulating periostin levels do not differ between postmenopausal women with normal and low bone mass and are not affected by zoledronic acid treatment. Horm. Metab. Res. 2014, 46, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, J.-J.; Wang, X.-D.; Sun, X.-J.; Li, M.; Wei, Q.; Ren, L.-Q.; Liu, N.-F. Periostin promotes arterial calcification through PPARγ-related glucose metabolism reprogramming. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H2222–H2239. [Google Scholar] [CrossRef]
- Sun, X.-J.; Ma, W.-Q.; Zhu, Y.; Liu, N.-F. POSTN promotes diabetic vascular calcification by interfering with autophagic flux. Cell Signal 2021, 83, 109983. [Google Scholar] [CrossRef] [PubMed]
- Alesutan, I.; Henze, L.A.; Boehme, B.; Luong, T.T.D.; Zickler, D.; Pieske, B.; Eckardt, K.-U.; Pasch, A.; Voelkl, J. Periostin Augments Vascular Smooth Muscle Cell Calcification via β-Catenin Signaling. Biomolecules 2022, 12, 1157. [Google Scholar]
- Ryu, J.; Kim, H.J.; Chang, E.-J.; Huang, H.; Banno, Y.; Kim, H.-H. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006, 25, 5840–5851. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Kikuta, J.; Shimazu, Y.; Meier-Schellersheim, M.; Germain, R.N. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. 2010, 207, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Lee, S.H.; Ahn, S.H.; Kim, H.-M.; Kim, B.-J.; Koh, J.-M. The circulating sphingosine-1-phosphate level predicts incident fracture in postmenopausal women: A 3.5-year follow-up observation study. Osteoporos. Int. 2016, 27, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.-S.M.; Rouzi, A.A.; Al-Senani, N.S.; Qari, M.H.; Elsamanoudy, A.Z.; Mousa, S.A. High Plasma Sphingosine 1-phosphate Levels Predict Osteoporotic Fractures in Postmenopausal Women: The Center of Excellence for Osteoporosis Research Study. J. Bone Metab. 2018, 25, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, S.Y.; Lee, Y.S.; Kim, B.J.; Lim, K.H.; Cho, E.H.; Kim, G.S. Higher circulating sphingosine 1-phosphate levels are associated with lower bone mineral density and higher bone resorption marker in humans. J. Clin. Endocrinol. Metab. 2012, 97, E1421–E1428. [Google Scholar] [CrossRef]
- Alam, M.S.; Getz, M.; Safeukui, I.; Yi, S.; Tamez, P.; Shin, J.; Haldar, K. Genomic expression analyses reveal lysosomal, innate immunity proteins, as disease correlates in murine models of a lysosomal storage disorder. PLoS ONE 2012, 7, e48273. [Google Scholar] [CrossRef]
- Morris, T.G.; Borland, S.J.; Clarke, C.J.; Wilson, C.; Hannun, Y.A.; Ohanian, V.; Canfield, A.E.; Ohanian, J. Sphingosine 1-phosphate activation of ERM contributes to vascular calcification. J. Lipid Res. 2018, 59, 69–78. [Google Scholar] [CrossRef]
- Fernández-Pisonero, I.; López, J.; Onecha, E.; Dueñas, A.I.; Maeso, P.; Crespo, M.S.; Román, J.A.S.; García-Rodríguez, C. Synergy between sphingosine 1-phosphate and lipopolysaccharide signaling promotes an inflammatory, angiogenic and osteogenic response in human aortic valve interstitial cells. PLoS ONE 2014, 9, e109081. [Google Scholar] [CrossRef]
- Goettsch, C.; Rauner, M.; Pacyna, N.; Hempel, U.; Bornstein, S.R.; Hofbauer, L.C. miR-125b regulates calcification of vascular smooth muscle cells. Am. J. Pathol. 2011, 179, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Hutcheson, J.D.; Aikawa, E. MicroRNA in cardiovascular calcification: Focus on targets and extracellular vesicle delivery mechanisms. Circ. Res. 2013, 112, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Panizo, S.; Naves-Díaz, M.; Carrillo-López, N.; Martínez-Arias, L.; Fernández-Martín, J.L.; Ruiz-Torres, M.P.; Cannata-Andía, J.B.; Rodríguez, I. MicroRNAs 29b, 133b, and 211 Regulate Vascular Smooth Muscle Calcification Mediated by High Phosphorus. J. Am. Soc. Nephrol. 2016, 27, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, T.; Taipaleenmäki, H.; Stenvang, J.; Abdallah, B.M.; Ditzel, N.; Nossent, A.Y.; Bak, M.; Kauppinen, S.; Kassem, M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 6139–6144. [Google Scholar] [CrossRef]
- Inose, H.; Ochi, H.; Kimura, A.; Fujita, K.; Xu, R.; Sato, S.; Iwasaki, M.; Sunamura, S.; Takeuchi, Y.; Fukumoto, S.; et al. A microRNA regulatory mechanism of osteoblast differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 20794–20799. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Q.; Gordon, J.A.; Beloti, M.M.; Croce, C.M.; Wijnen AJ, V.; Stein, J.L.; Lian, J.B. A network connecting Runx2, SATB2, and the miR-23a~27a~24-2 cluster regulates the osteoblast differentiation program. Proc. Natl. Acad. Sci. USA 2010, 107, 19879–19884. [Google Scholar] [CrossRef] [PubMed]
- Kahai, S.; Lee, S.-C.; Lee, D.Y.; Yang, J.; Li, M.; Wang, C.-H.; Jiang, Z.; Zhang, Y.; Peng, C.; Yang, B.B. MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. PLoS ONE 2009, 4, e7535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, R.L.; Croce, C.M.; Stein, J.L.; Lian, J.B.; Van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar] [CrossRef]
- Li, Z.; Hassan, M.Q.; Jafferji, M.; Aqeilan, R.I.; Garzon, R.; Croce, C.M.; Lian, J.B. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J. Biol. Chem. 2009, 284, 15676–15684. [Google Scholar] [CrossRef]
- Kapinas, K.; Kessler, C.; Ricks, T.; Gronowicz, G.; Delany, A.M. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J. Biol. Chem. 2010, 285, 25221–25231. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, W.; He, M.; Xie, W.; Lv, Q.; Wan, G.; Li, G.; Wang, H.; Lu, G.; Hu, X.; et al. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol. 2011, 8, 829–838. [Google Scholar] [CrossRef]
- Sugatani, T.; Vacher, J.; Hruska, K.A. A microRNA expression signature of osteoclastogenesis. Blood 2011, 117, 3648–3657. [Google Scholar] [CrossRef]
- Nakasa, T.; Shibuya, H.; Nagata, Y.; Niimoto, T.; Ochi, M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum. 2011, 63, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Hruska, K.A. MicroRNA-223 is a key factor in osteoclast differentiation. J. Cell Biochem. 2007, 101, 996–999. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Moore, B.T.; Peng, X.-H.; Fang, X.; Lappe, J.M.; Recker, R.R.; Xiao, P. MiR-133a in human circulating monocytes: A potential biomarker associated with postmenopausal osteoporosis. PLoS ONE 2012, 7, e34641. [Google Scholar] [CrossRef] [PubMed]
- Kelch, S.; Balmayor, E.R.; Seeliger, C.; Vester, H.; Kirschke, J.S.; van Griensven, M. miRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients. Sci. Rep. 2017, 7, 15861. [Google Scholar] [CrossRef]
- Kocijan, R.; Muschitz, C.; Geiger, E.; Skalicky, S.; Baierl, A.; Dormann, R.; Plachel, F.; Feichtinger, X.; Heimel, P.; Fahrleitner-Pammer, A.; et al. Circulating microRNA Signatures in Patients With Idiopathic and Postmenopausal Osteoporosis and Fragility Fractures. J. Clin. Endocrinol. Metab. 2016, 101, 4125–4134. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Fu, Q.; Zhang, J. Plasma miRNA levels correlate with sensitivity to bone mineral density in postmenopausal osteoporosis patients. Biomarkers 2014, 19, 553–556. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, D.; Pan, N.; Sun, N.; Wang, Q.; Fan, J.; Zhou, P.; Zhu, W.; Jiang, L. Identification of miR-194-5p as a potential biomarker for postmenopausal osteoporosis. PeerJ 2015, 3, e971. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Selvamurugan, N. MicroRNAs: Synthesis, Gene Regulation and Osteoblast Differentiation. Curr. Issues Mol. Biol. 2013, 15, 7–18. [Google Scholar]
- Chao, C.-T.; Han, D.-S.; Huang, J.-W. Circulating microRNA-125b Levels Are Associated With the Risk of Vascular Calcification in Healthy Community-Dwelling Older Adults. Front. Cardiovasc. Med. 2021, 8, 624313. [Google Scholar] [CrossRef]
- Chao, C.-T.; Liu, Y.-P.; Su, S.-F.; Yeh, H.-Y.; Chen, H.-Y.; Lee, P.-J.; Chen, W.-J.; Lee, Y.-M.; Huang, J.-W.; Chiang, C.-K.; et al. Circulating MicroRNA-125b Predicts the Presence and Progression of Uremic Vascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Villabrille, S.; Martín-Carro, B.; Martín-Vírgala, J.; Alonso-Montes, C.; Palomo-Antequera, C.; García-Castro, R.; López-Ongil, S.; Dusso, A.S.; Fernández-Martín, J.L.; Naves-Díaz, M.; et al. MicroRNA-145 and microRNA-486 are potential serum biomarkers for vascular calcification. Nephrol. Dial. Transplant. 2023, 38, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Hackl, M.; Heilmeier, U.; Weilner, S.; Grillari, J. Circulating microRNAs as novel biomarkers for bone diseases—Complex signatures for multifactorial diseases? Mol. Cell. Endocrinol. 2016, 432, 83–95. [Google Scholar] [CrossRef] [PubMed]
| BIOMARKERS OF BONE REMODELING |
| Classic Markers |
| Markers of bone homeostasis |
| Calcium (Ca) |
| Phosphorus (P) |
| Parathyroid hormone (PTH) |
| 1,25(OH)2D3 (calcitriol) |
| Fibroblast growth factor 23 (FGF23) |
| Klotho |
| Markers of bone formation |
| Total alkaline phosphatase (ALP) |
| Bone-specific alkaline phosphatase (BALP) |
| Osteocalcin (*) |
| Type I procollagen extension peptides (P1NP and P1CP) |
| Bone Resorption Markers |
| Urinary calcium excretion |
| Urinary hydroxyproline excretion |
| Urinary hydroxylysine excretion |
| Urinary pyridinoline and deoxypyridinoline excretion |
| Tartrate resistant acid phosphatase (TRACP) |
| Amino and carboxyl terminal cross-linked telopeptides of type 1 collagen |
| Cathepsin K (CatK). |
| Osteopontin (OPN) |
| Novel Bone Markers |
| Receptor Activator of NFkappa B ligand (RANKL) and osteoprotegerin (OPG) |
| Sclerostin (Sost) and Dickkopf1 (Dkk1) |
| Periostin |
| Sphingosine-1-phosphate (S1P) |
| MicroRNAs (miRs) |
| Characteristics of a Good Biomarker of Bone Remodeling |
|---|
| Ease of sample collection. |
| Non-invasive determination. |
| Specificity to bone metabolism. |
| Strong correlation with reference techniques in remodeling analysis (bone histomorphometry, bone biopsy, radiographs, and studies involving isotopes with labeled calcium). |
| Responsiveness to treatments of diseases affecting bone metabolism. |
| OPG—RANKL Levels | Subjects | General Results of the Studies | |
|---|---|---|---|
| Bone mass | Low serum OPG High serum RANKL/OPG | Postmenopausal women (low BMD versus normal BMD) [45]. | Consensus |
| Low serum OPG High serum RANKL | Rheumatoid arthritis patients (osteoporotic versus normal BMD) [46,47]. | ||
| Vascular calcification | High serum OPG | CKD patients (with vascular calcification versus no vascular calcification) [48,49]. | Controversial |
| High serum RANKL/OPG | Ischemic coronary disease (with arterial calcification versus no calcification) [50]. |
| Sphingosine-1-Phosphate (S1P) | |
|---|---|
| Osteoblast | Survival and migration |
| RANKL synthesis | |
| Osteoclast | Osteoclast precursors dynamic migration: |
| Receptor S1PR1: positive chemotaxis | |
| Receptor S1PR2: negative chemotaxis (chemorepulsion) | |
| miRNA | Target Gen | Population Studied | |
|---|---|---|---|
| Vascular calcification inhibitors | miR-16 | VEGFA | CKD patients |
| miR-17 | VEGFA | CKD patients | |
| miR-106b | VEGFA | CKD patients | |
| miR-125b | Osterix | Older adults CKD patients | |
| miR-145 | KLF-4, Osterix, α-actin | General population CKD patients | |
| miR-155 | Akt-FOXO3a, AT1R | CKD patients | |
| miR-211 | RUNX2 | CKD patients | |
| miR-378a-3p | CTGF | Older adults CKD patients | |
| miR-486 | RUNX2, Osterix | General population | |
| Vascular calcification promotors | miR-29a | DKK1 | CKD patients |
| miR-29b | ACVR2A, CTNNBIP1 | CKD patients | |
| miR-221 | Pit-1 | CKD patients | |
| miR-223 | Mef2c, Rhob, NFIA | Obstructive coronary disease patients CKD patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Villabrille, S.; Martín-Carro, B.; Martín-Vírgala, J.; Rodríguez-Santamaria, M.d.M.; Baena-Huerta, F.; Muñoz-Castañeda, J.R.; Fernández-Martín, J.L.; Alonso-Montes, C.; Naves-Díaz, M.; Carrillo-López, N.; et al. Novel Biomarkers of Bone Metabolism. Nutrients 2024, 16, 605. https://doi.org/10.3390/nu16050605
Fernández-Villabrille S, Martín-Carro B, Martín-Vírgala J, Rodríguez-Santamaria MdM, Baena-Huerta F, Muñoz-Castañeda JR, Fernández-Martín JL, Alonso-Montes C, Naves-Díaz M, Carrillo-López N, et al. Novel Biomarkers of Bone Metabolism. Nutrients. 2024; 16(5):605. https://doi.org/10.3390/nu16050605
Chicago/Turabian StyleFernández-Villabrille, Sara, Beatriz Martín-Carro, Julia Martín-Vírgala, Mª del Mar Rodríguez-Santamaria, Francisco Baena-Huerta, Juan Rafael Muñoz-Castañeda, José Luis Fernández-Martín, Cristina Alonso-Montes, Manuel Naves-Díaz, Natalia Carrillo-López, and et al. 2024. "Novel Biomarkers of Bone Metabolism" Nutrients 16, no. 5: 605. https://doi.org/10.3390/nu16050605
APA StyleFernández-Villabrille, S., Martín-Carro, B., Martín-Vírgala, J., Rodríguez-Santamaria, M. d. M., Baena-Huerta, F., Muñoz-Castañeda, J. R., Fernández-Martín, J. L., Alonso-Montes, C., Naves-Díaz, M., Carrillo-López, N., & Panizo, S. (2024). Novel Biomarkers of Bone Metabolism. Nutrients, 16(5), 605. https://doi.org/10.3390/nu16050605







