The Impact of a 24-h Low and High Fermentable Oligo- Di- Mono-Saccharides and Polyol (FODMAP) Diet on Plasma Bacterial Profile in Response to Exertional-Heat Stress
Abstract
:1. Introduction
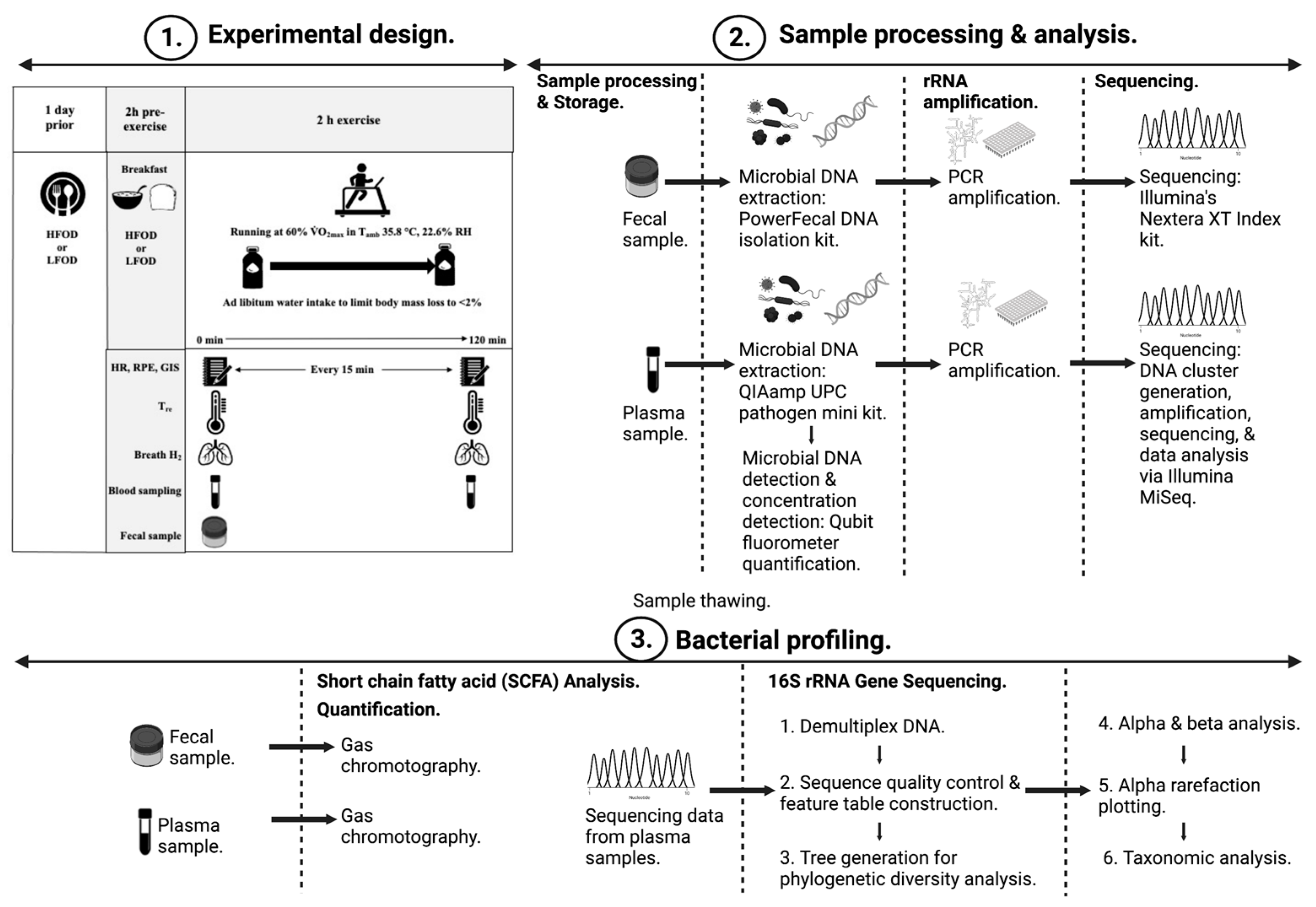
2. Methods
2.1. Participants
2.2. Experimental Procedures
2.3. Blood Sample Processing and Analysis
2.4. Plasma and Fecal Bacterial Profiling
2.5. Plasma and Fecal SCFA Analysis
2.6. Statistical Analysis
3. Results
3.1. Exertional-Heat Stress (EHS) and EIGS Markers
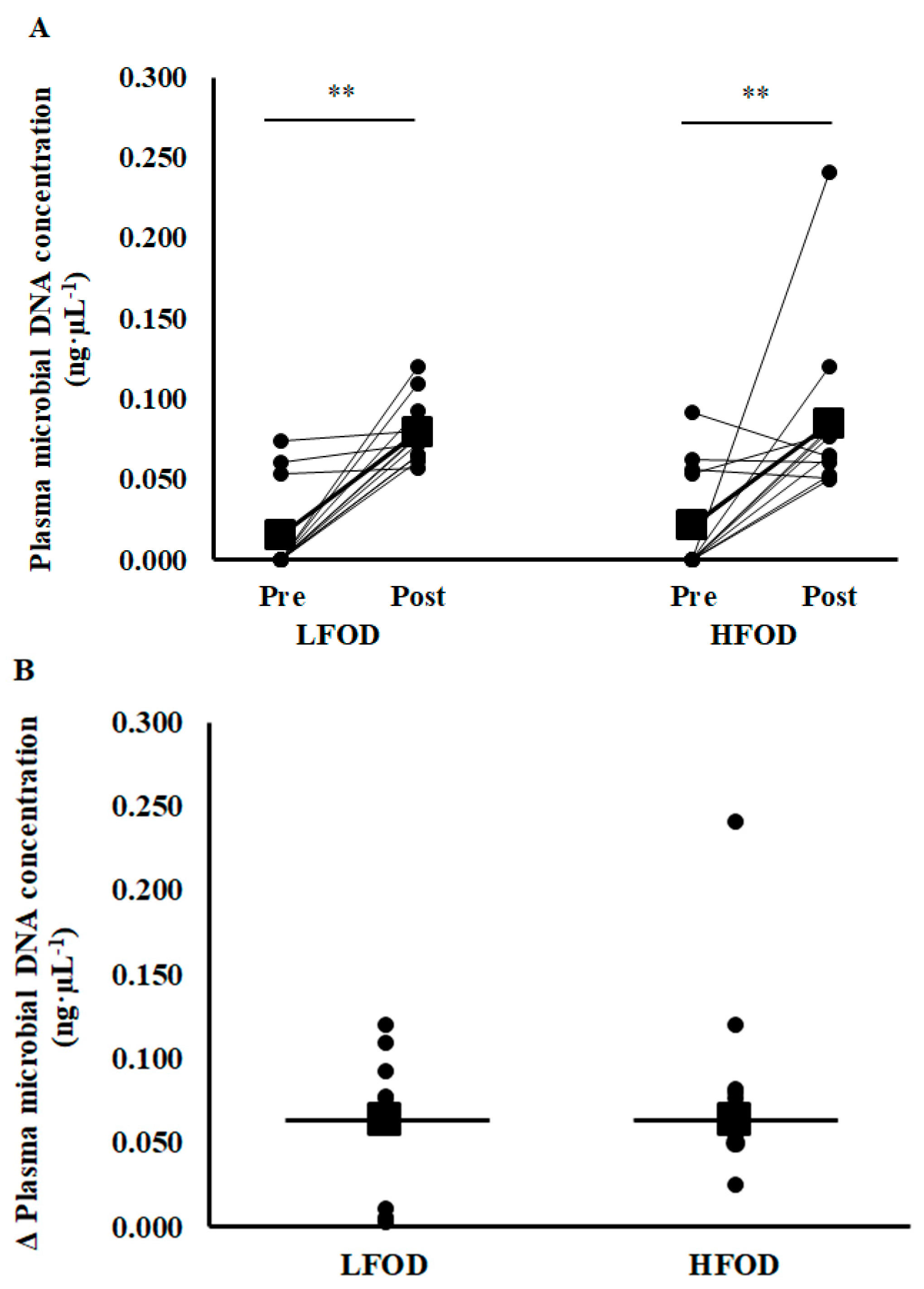
3.2. Plasma Microbial Concentration
3.3. Plasma Bacteria α-Diversity and Relative Abundance
3.4. Fecal Microbial Taxa
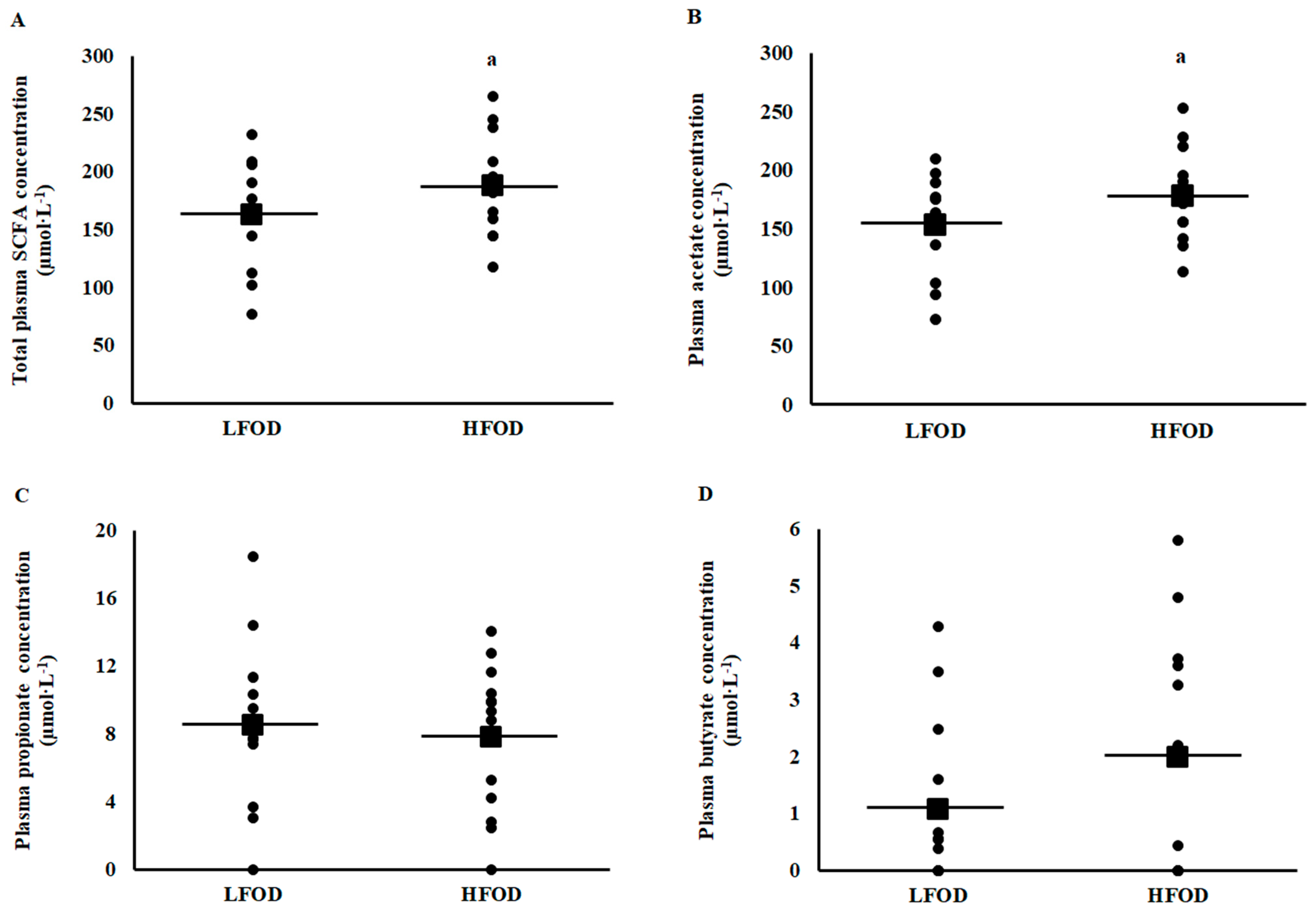
3.5. Plasma and Fecal Short Chain Fatty Acid Concentrations
4. Discussion
4.1. Plasma Microbial DNA Concentration
4.2. Plasma Bacterial Profile
4.3. Resting Pre-EHS Fecal Bacterial Profile
4.4. Fecal and Plasma Short Chain Fatty Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.; Gibson, P. Systematic review: Exercise-induced gastrointestinal syndrome—Implication for health and disease. Alim. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar]
- Costa, R.J.S.; Gaskell, S.K.; McCubbin, A.J.; Snipe, R.M.J. Exertional-heat stress associated gastrointestinal perturbations- management strategies for athletes preparing for and competing in the 2020 Tokyo Olympic Games. Temperature 2020, 7, 58–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.J.S.; Gill, S.K.; Snipe, R.M.J.; Gaskell, S.K.; Russo, I.; Burke, L.M. Assessment of exercise-associated gastrointestinal perturbations in research and practical settings: Methodological concerns and recommendations for better practice. Int. J. Sport. Nutr. Exerc. Metab. 2022, 32, 387–418. [Google Scholar] [PubMed]
- Gaskell, S.K.; Costa, R.J.S.; Lis, D.M. Exercise-induced gastrointestinal syndrome. In Clinical Sports Nutrition, 6th ed.; Burke, L., Deakin, V., Minehan, M., Eds.; McGraw-Hill Education: New York, NY, USA, 2020; Chapter 21; pp. 551–579. [Google Scholar]
- Gaskell, S.K.; Rauch, C.; Costa, R.J.S. Gastrointestinal assessment and management procedures for exercise-associated gastrointestinal symptoms. Aspetar Sports Med. J. 2021, 10, 36–44. [Google Scholar]
- Gaskell, S.K.; Rauch, C.E.; Costa, R.J.S. Gastrointestinal assessment and therapeutic intervention for the management of exercise associated gastrointestinal symptoms: A case series translational and professional practice approach. Front. Physiol. 2021, 12, 719142. [Google Scholar] [CrossRef]
- Bennett, C.J.; Henry, R.; Snipe, R.M.J.; Costa, R.J.S. Is the gut microbiota bacterial abundance and composition associated with intestinal epithelial injury, systemic inflammatory profile, and gastrointestinal symptoms in response to exertional-heat stress? J. Sci. Med. Sport 2020, 23, 1141–1153. [Google Scholar]
- Costa, R.J.S.; Camões-Costa, V.; Snipe, R.M.J.; Dixon, D.; Russo, I.; Huschtscha, Z. The impact of exercise-induced hypohydration on intestinal integrity, function, symptoms, and systemic endotoxin and inflammatory responses. J. Appl. Physiol. 2019, 126, 1281–1291. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Mika, A.; McCubbin, A. The impact of exercise modality on exercise-induced gastrointestinal syndrome and associated gastrointestinal symptoms. J. Sci. Med. Sport 2022, 25, 788–793. [Google Scholar] [CrossRef]
- Gaskell, S.K.; Rauch, C.; Parr, A.; Costa, R.J.S. Diurnal versus Nocturnal Exercise—Impact on the Gastrointestinal Tract. Med. Sci. Sport. Exerc. 2021, 53, 1056–1067. [Google Scholar] [CrossRef]
- Gaskell, S.K.; Taylor, B.; Muir, J.; Costa, R.J.S. Impact of 24-hour low and high fermentable oligo- di- mono- saccharide polyol diets on markers of exercise-induced gastrointestinal syndrome in response to exertional-heat stress. Appl. Physiol. Nutri. Metab. 2020, 45, 569–580. [Google Scholar] [CrossRef]
- Henningsen, K.; Mika, A.; Alcock, R.; Gaskell, S.K.; Parr, A.; Rauch, C.; Russo, I.; Snipe, R.M.J.; Costa, R.J.S. The increase in core body temperature in response to exertional-heat stress can predict exercise-induced gastrointestinal syndrome. Temperature 2023, 33, 230–242. [Google Scholar] [CrossRef]
- Russo, I.; Della Gatta, P.A.; Garnham, A.; Porter, J.; Burke, L.M.; Costa, R.J.S. Assessing overall exercise recovery processes using carbohydrate and carbohydrate-protein containing recovery beverages. Front. Physiol. 2021, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Della Gatta, P.A.; Garnham, A.; Porter, J.; Burke, L.M.; Costa, R.J.S. Does the nutritional composition of dairy milk based recovery beverages influence post-exercise gastrointestinal and immune status, and subsequent markers of recovery optimisation in response to high intensity interval exercise? Front. Nutr. 2021, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Della Gatta, P.A.; Garnham, A.; Porter, J.; Burke, L.M.; Costa, R.J.S. The effect of an acute “train-low” nutritional protocol on markers of recovery optimisation in endurance trained male athletes. Int. J. Sports Physiol. Perf. 2021, 16, 1764–1776. [Google Scholar]
- Snipe, R.M.J.; Costa, R.J.S. Does the temperature of water ingested during exertional-heat stress influence gastrointestinal injury, symptoms, and systemic inflammatory profile? J. Sci. Med. Sport 2018, 21, 771–776. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Khoo, A.; Kitic, C.; Gibson, P.; Costa, R.J.S. Carbohydrate and protein intake during exertional-heat stress ameliorates intestinal epithelial injury and small intestine permeability. Appl. Physiol. Nutr. Metab. 2017, 42, 1283–1292. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Khoo, A.; Kitic, C.; Gibson, P.; Costa, R.J.S. The impact of exertional-heat stress on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profile. Eur. J. Appl. Physiol. 2018, 118, 389–400. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Khoo, A.; Kitic, C.; Gibson, P.; Costa, R.J.S. The Impact of Mild Heat Stress During Prolonged Running On Gastrointestinal Integrity, Gastrointestinal Symptoms, Systemic Endotoxin and Cytokine Profiles. Int. J. Sports Med. 2018, 39, 255–263. [Google Scholar] [CrossRef]
- Young, P.; Russo, I.; Gill, P.; Muir, J.; Henry, R.; Davidson, Z.; Costa, R.J.S. Reliability of pathophysiological markers reflective of exercise-induced gastrointestinal syndrome (EIGS) in response to 2-h high-intensity interval exercise: A comprehensive methodological efficacy exploration. Front. Physiol. 2023, 14, 1063335. [Google Scholar]
- Benmassaoud, A.; Kanber, Y.; Nawar, J.; Bessissow, T. Exercise-induced ischemic colitis in an amateur marathon runner. Endoscopy 2014, 46 (Suppl. 1), E480. [Google Scholar] [CrossRef]
- Cohen, D.C.; Winstanley, A.; Engledow, A.; Windsor, A.C.; Skipworth, J.R. Marathon-induced ischemic colitis: Why running is not always good for you. Am. J. Emerg. Med. 2009, 27, 255.e5–255.e7. [Google Scholar]
- Costa, R.J.S.; Snipe, R.; Camoes-Costa, V.; Scheer, B.V.; Murray, A. The impact of gastrointestinal symptoms and dermatological injuries on nutritional intake and hydration status during ultramarathon events. Sports Med.-Open 2016, 2, 16. [Google Scholar] [PubMed] [Green Version]
- Grames, C.; Berry-Caban, C.S. Ischemic colitis in an endurance runner. Case Rep. Gastroint. Med. 2012, 2012, 356895. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.; Stellingwerff, T.; Hodgson, A.B.; Randell, R.; Pöttgen, K.; Res, P.; Jeukendrup, A.E. Nutritional intake and gastrointestinal problems during competitive endurance events. Med. Sci. Sports Exerc. 2012, 44, 344–351. [Google Scholar] [PubMed] [Green Version]
- Walter, E.; Gibson, O.R.; Stacey, M.; Hill, N.; Parsons, I.T.; Woods, D. Changes in gastrointestinal cell integrity after marathon running and exercise-associated collapse. Eur. J. Appl. Physiol. 2021, 121, 1179–1187. [Google Scholar]
- Gill, S.K.; Teixeira, A.; Rama, L.; Rosado, F.; Hankey, J.; Scheer, V.; Hemmings, K.; Ansley-Robson, P.; Costa, R.J.S. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Exerc. Immunol. Rev. 2015, 21, 114–128. [Google Scholar] [PubMed]
- Gill, S.K.; Teixeira, A.; Rosado, F.; Hankey, J.; Wright, A.; Marczak, S.; Murray, A.; Costa, R.J.S. The impact of a 24-hours ultramarathon on salivary antimicrobial protein responses. Int. J. Sports Med. 2015, 35, 966–971. [Google Scholar]
- Laitano, O.; Leon, L.R.; Roberts, W.O.; Sawka, M.N. Controversies in exertional heat stroke diagnosis, prevention, and treatment. J. Appl. Physiol. 2019, 127, 1338–1348. [Google Scholar]
- Roberts, W.O.; Armstrong, L.E.; Sawka, M.N.; Yeargin, S.W.; Heled, Y.; O’Connor, F.G. ACSM expert consensus statement on exertional heat illness: Recognition, management, and return to activity. Curr. Sports Med. Rep. 2021, 20, 470–484. [Google Scholar] [CrossRef]
- Hodgin, K.E.; Moss, M. The epidemiology of sepsis. Curr. Pharm. Des. 2008, 14, 1833–1839. [Google Scholar] [CrossRef]
- Young, P.; Rauch, C.E.; Russo, I.; Gaskell, S.K.; Davidson, Z.E.; Costa, R.J.S. Plasma endogenous endotoxin core antibody (EndoCAb) response to exertional and exertional-heat stress: Controlled-laboratory experimental exploration. Int. J. Sports Med. 2022, 43, 1023–1032. [Google Scholar] [PubMed]
- Castillo, D.J.; Rifkin, R.F.; Cowan, D.A.; Potgieter, M. The healthy human blood microbiome: Fact or fiction? Front. Cell. Infect. Microbiol. 2019, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, C.E.; Mika, A.S.; McCubbin, A.J.; Huschtscha, Z.; Costa, R.J.S. Effect of prebiotics, probiotics, and synbiotics on gastrointestinal outcomes in healthy adults and active adults at rest and in response to exercise-a systematic literature review. Front. Nutr. 2022, 9, 1003620. [Google Scholar]
- Heiman, M.L.; Greenway, F.L. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol. Metab. 2016, 5, 317–320. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnerburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Staudacher, H.M.; Rossi, M.; Kaminski, T.; Dimidi, E.; Ralph, F.S.E.; Wilson, B.; Martin, L.D.; Louis, P.; Lomer, M.C.E.; Irving, P.; et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14241. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Quiroga, J.; Lopez, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-chain fatty acids and their receptors in gut inflammation and colon cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef]
- Couto, M.R.; Goncalves, P.; Magro, F.; Martel, F. Microbiota-derived butyrate regulates intestinal inflammation: Focus on inflammatory bowel disease. Pharmacol. Res. 2020, 159, 104947. [Google Scholar] [CrossRef]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the fine-tuning of colonic homeostasis: Implications for inflammatory bowel disease. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Artis, D. Communisal bacterial at the interface of host metabolism and the immune system. Nat. Immunol. 2013, 14, 676–684. [Google Scholar]
- Mailing, L.J.; Alan, K.M.; Buford, T.W.; Fields, C.; Woods, J.A. exercise and the gut microbiome: A review of the evidence, potential mechanisms, and implications for human health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef]
- Gibson, P.R.; Halmos, E.P.; Muir, J.G. FODMAPS, prebiotics and gut health-the FODMAP hypothesis revisited. Aliment. Pharmacol. Ther. 2020, 52, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [PubMed] [Green Version]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaskell, S.K.; Costa, R.J.S. Case Study: Applying a low FODMAP dietary intervention to a female ultra-endurance runner with irritable bowel syndrome during a multi-stage ultra-marathon. Int. J. Sports Nutr. Exerc. Metab. 2019, 29, 61–67. [Google Scholar]
- Lis, D.M. Exit gluten-free and enter low FODMAPs: A novel dietary strategy to reduce gastrointestinal symptoms in athletes. Sports Med. 2019, 49 (Suppl. 1), 87–97. [Google Scholar]
- Alcock, R.; McCubbin, A.; Camões-Costa, V.; Costa, R.J.S. CASE STUDY. Nutritional support for self-sufficient multi-stage ultra-marathon: Rationed versus full energy provisions. Wilderness Envrion. Med. 2018, 29, 508–520. [Google Scholar] [CrossRef]
- Flood, T.R.; Montanari, S.; Wicks, M.; Blanchard, J.; Sharp, H.; Taylor, L.; Kuennen, M.R.; Lee, B.L. Addition of pectin-alginate to a carbohydrate beverage does not maintain gastrointestinal barrier function during exercise in hot-humid conditions better than carbohydrate ingestion alone. Appl. Physiol. Nutr. Metabol. 2020, 45, 1145–1155. [Google Scholar] [CrossRef]
- Jonvik, K.L.; Lenaerts, K.; Smeets, J.S.J.; Kolkman, J.J.; van Loon, L.J.C.; Verdijk, L.B. Sucrose but not nitrate ingestion reduces strenuous cycling-induced intestinal injury. Med. Sci. Sports Exerc. 2019, 51, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Lambert, G.P.; Broussard, L.J.; Mason, B.L.; Mauermann, W.J.; Gisolfi, C.V. Gastrointestinal permeability during exercise: Effects of aspirin and energy-containing beverages. J. Appl. Physiol. 2001, 90, 2075–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehrer, N.J.; Goes, E.; Du Gardeyn, C.; De Meirleir, K. Effect of carbohydrate on portal vein blood flow during exercise. Int. J. Sports Med. 2005, 26, 171–176. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Snipe, R.M.J.; Costa, R.J.S. Ad libitum drinking adequately supports hydration during 2 h of running in different ambient temperatures. Eur. J. Appl. Physiol. 2018, 118, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Camões-Costa, V.; Gaskell, S.K.; Porter, J.; Burke, L.M.; Costa, R.J.S. Systematic literature review: The effect of dairy milk on markers of recovery optimisation in response to endurance exercise. Int. J. Sports Sci. 2019, 9, 69–85. [Google Scholar]
- Costa, R.J.S.; Crockford, M.J.; Moore, J.P.; Walsh, N.P. Heat acclimation responses of an ultra-endurance running group preparing for hot desert based competition. Eur. J. Sport Sci. 2014, 14, S131–S141. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Alekseyenko, A.V.; Ogunrinde, E.; Li, M.; Li, Q.-Z.; Huang, L.; Tsao, B.P.; Kamen, D.L.; Oates, J.C.; Li, Z.; et al. Rigorous plasma microbiome analysis method enables disease association discovery in clinic. Front. Microbiol. 2021, 11, 613268. [Google Scholar] [CrossRef] [PubMed]
- Nikkari, S.; McLaughlin, I.J.; Bi, W.; Dodge, D.E.; Relman, D.A. Does blood of healthy subjects contain bacterial ribosomal DNA? J. Clin. Microbiol. 2001, 39, 1956–1959. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Ffrench, R.A.; Muir, J.G.; Gibson, P.R. Successful elevation of circulating acetate and propionate by dietary modulation does not alter T-regulatory cell or cytokine profiles in healthy humans: A pilot study. Eur. J. Nutr. 2020, 59, 2651–2661. [Google Scholar]
- Clarke, J.M.; Topping, D.L.; Christophersen, C.T.; Bird, A.R.; Lange, K.; Saunders, I.; Cobiac, L. Butyrate esterified to starch is released in the human gastrointestinal tract. Am. J. Clin. Nutr. 2011, 94, 1276–1283. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Chanez-Paredes, S.D.; Abtahi, S.; Kuo, W.; Turner, J.R. Differentiating between tight junction-dependant and tight junction-independent intestinal barrier loss in vivo. Methods Mol. Biol. 2021, 2367, 249–271. [Google Scholar] [PubMed]
- Agassi, R.; Czeiger, D.; Shaked, G.; Avriel, A.; Sheynin, J.; Lavrenkov, K.; Ariad, S.; Douvdevani, A. Measurement of circulating cell-free DNA levels by a simple fluorescent test in patients with breast cancer. Am. J. Clin. Pathol. 2015, 143, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Grootjans, J.; Lenaerts, K.; Buurman, W.A.; Dejong, C.H.C.; Derikx, J.P.M. Life and death at the mucosal-luminal interface: New perspectives on human intestinal ischemia-reperfusion. World J. Gastroenterol. 2016, 22, 2760–2770. [Google Scholar]
- Rehrer, N.J.; Smets, A.; Reynaert, H.; Goes, E.; De Meirleir, K. Effect of exercise on portal vein blood flow in man. Med. Sci. Sports Exerc. 2001, 33, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- van Wijck, K.; Lenaerts, K.; van Loon, L.J.; Peters, W.H.M.; Buurman, W.A.; Dejong, C.H.C. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef] [Green Version]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human disease. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.J.S.; Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.M.J.; Camões-Costa, V.; Gibson, P. Gut-training: The impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Appl. Physiol. Nutr. Metab. 2017, 42, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Snipe, R.M.J.; Costa, R.J.S. Does biological sex influence intestinal epithelial injury, small intestine permeability, gastrointestinal symptoms and systemic cytokine profile in response to exertional-heat stress? J. Sport Sci. 2018, 36, 2827–2835. [Google Scholar] [CrossRef]
- Scheppach, W.; Pomare, E.W.; Elia, M.; Cummings, J.H. The contribution of the large intestine to blood acetate in man. Clin. Sci. 1991, 80, 177–182. [Google Scholar]
- Gaskell, S.K.; Gill, P.; Muir, J.; Henry, R.; Costa, R.J.S. Impact of 24h FODMAP diet on faecal and plasma short chain fatty acid concentration and exercise-induced gastrointestinal syndrome. Nutr. Diet. 2021, 78, 7. [Google Scholar]

| LFOD | HFOD | |||||
|---|---|---|---|---|---|---|
| Pre-EHS | Post-EHS | Δ | Pre-EHS | Post-EHS | Δ | |
| Phylum | ||||||
| SEI | 0.126 (0.036) | 0.121 (0.029) | −0.005 (0.048) | 0.129 (0.033) | 0.122 (0.038) | −0.007 (0.047) |
| Actinobacteriota | 2.309 (1.187) | 3.042 (2.571) | 0.734 (2.726) | 1.874 (1.285) | 2.357 (1.493) | 0.483 (1.786) |
| Bacteroidota | 1.181 (1.537) | 0.641 (0.904) | −0.540 (1.899) | 1.192 (1.480) | 0.630 (1.097) | −0.562 (2.143) |
| Cyanobacteria | 1.310 (1.853) | 0.422 (0.556) | −0.888 (1.965) | 1.393 (3.394) | 0.912 (1.358) | −0.481 (3.915) |
| Firmicutes | 6.142 (2.004) | 6.801 (2.384) | 0.659 (2.511) | 6.669 (3.443) | 7.249 (3.175) | 0.580 (5.069) |
| Proteobacteria | 87.480 (4.951) | 87.772 (4.057) | 0.293 (4.898) | 84.796 (6.052) | 87.763 (4.402) | 2.967 (6.520) |
| Family | ||||||
| SEI | 0.228 (0.030) | 0.244 (0.017) | −0.004 (0.037) | 0.237 (0.035) | 0.231 (0.028) | −0.012 (0.035) |
| Actinobacteriota- | ||||||
| Propionibacteriaceae | 0.662 (0.612) | 0.343 (0.330) | −0.320 (0.614) | 0.689 (0.519) | 0.609 (0.431) | −0.80 (0.557) |
| Cyanobacteria- | ||||||
| Chloroplast | 1.169 (1.883) | 0.333 (0.539) | −0.836 (2.071) | 1.349 (3.409) | 0.912 (1.358) | −0.437 (3.917) |
| Firmicutes- | ||||||
| Bacillaceae | 1.928 (1.131) | 2.585 (1.651) | 0.657 (1.807) | 1.525 (1.397) | 2.246 (1.095) | 0.721 (1.552) |
| Carnobacteriaceae | 2.083 (1.322) | 2.368 (1.429) | 0.285 (1.286) | 3.261 (2.477) | 2.947 (1.395) | −0.314 (3.351) |
| Streptococcaceae | 0.518 (1.024) | 0.367 (0.436) | −0.151 (1.121) | 0.522 (0.718) | 0.761 (0.956) | 0.239 (1.212) |
| Staphylococcaceae | 0.759 (0.960) | 0.635 (0.492) | −0.125 (1.251) | 0.424 (0.549) | 0.494 (1.285) | 0.070 (1.449) |
| Proteobacteria- | ||||||
| Beijerinckiaceae | 0.380 (0.591) | 0.481 (0.524) | 0.101 (0.810) | 0.725 (1.087) | 0.672 (0.882) | −0.054 (1.374) |
| Xanthobacteraceae | 2.550 (1.448) | 1.857 (1.439) | −0.693 (2.421) | 2.881 (2.466) | 2.240 (1.602) | −0.641 (2.460) |
| Sphingomonadaceae | 4.694 (2.832) | 4.668 (2.700) | −0.026 (2.076) | 4.717 (3.919) | 3.597 (2.830) | −1.120 (3.538) |
| Comamonadaceae | 5.093 (2.120) | 6.649 (1.722) | 1.556 (2.687) | 6.317 (2.910) | 6.533 (2.893) | 0.215 (4.256) |
| Oxalobacteraceae | 1.327 (1.371) | 0.815 (0.663) | −0.513 (1.711) | 1.432 (1.746) | 0.766 (0.651) | −0.666 (1.711) |
| Yersiniceae | 15.472 (3.586) | 15.269 (3.653) | −0.203 (6.069) | 14.003 (4.061) | 16.371 (2.799) | 2.368 (4.183) |
| Moraxellaceae | 0.493 (0.577) | 0.329 (0.478) | −0.164 (0.636) | 0.936 (1.157) | 0.250 (0.298) | −0.685 (1.208) |
| Pseudomonadaceae | 54.430 (7.778) | 54.878 (4.975) | 0.447 (7.280) | 50.249 (6.352) | 53.475 (6.400) | 3.226 (9.089) |
| Genus | ||||||
| SEI | 0.195 (0.018) | 0.198 (0.015) | 0.003 (0.023) | 0.201 (0.032) | 0.201 (0.025) | −0.001 (0.045) |
| Actinobacteriota- | ||||||
| Cutibacterium | 0.628 (0.635) | 0.272 (0.256) | −0.356 (0.726) | 0.678 (0.510) | 0.572 (0.399) | −0.106 (0.508) |
| Firmicutes- | ||||||
| Bacillus | 1.551 (1.010) | 2.364 (1.500) | 0.813 (1.603) | 1.173 (1.284) | 1.884 (0.904) * | 0.711 (1.358) |
| Carnobacterium | 2.083 (1.322) | 2.366 (1.428) | 0.284 (1.283) | 3.170 (2.434) | 2.705 (1.349) | −0.465 (3.286) |
| Streptococcus | 0.518 (1.024) | 0.272 (0.393) | −0.246 (1.061) | 0.522 (0.718) | 0.761 (0.956) | 0.239 (1.212) |
| Staphylococcus | 0.644 (0.735) | 0.635 (0.492) | −0.009 (1.065) | 0.424 (0.549) | 0.491 (1.286) | 0.066 (1.449) |
| Proteobacteria- | ||||||
| Methylobacterium | 0.355 (0.541) | 0.362 (0.459) | 0.007 (0.654) | 0.725 (1.087) | 0.672 (0.882) | −0.054 (1.374) |
| Sphingomonas | 4.268 (2.608) | 4.522 (2.617) | 0.255 (2.074) | 4.430 (4.030) | 3.388 (2.898) | −1.042 (3.440) |
| Delftia | 4.626 (2.119) | 6.625 (1.758) * | 1.999 (2.854) | 5.553 (2.618) | 6.342 (2.756) | 0.789 (4.062) |
| Massilia | 1.280 (1.362) | 0.809 (0.666) | −0.472 (1.686) | 1.432 (1.746) | 0.709 (0.667) | −0.723 (1.710) |
| Serratia | 6.374 (1.437) | 4.903 (1.508) * | −1.472 (2.783) | 5.303 (2.140) | 6.143 (2.068) | 0.840 (3.005) |
| Pseudomonas | 54.430 (7.778) | 54.878 (4.975) | 0.447 (7.280) | 50.249 (6.352) | 53.475 (6.400) | 3.226 (9.089) |
| LFOD | HFOD | |
|---|---|---|
| Phylum | ||
| SEI | 0.197 (0.070) | 0.225 (0.056) |
| Actinobacteriota | 2.573 (1.451) | 5.542 (5.303) |
| Bacteroidota | 9.707 (4.486) a | 25.347 (16.395) a |
| Firmicutes | 73.854 (20.057) a | 64.648 (14.769) a |
| Proteobacteria | 9.075 (18.101) | 1.472 (2.640) |
| Verrucomicrobia | 3.905 (9.722) | 2.674 (3.183) |
| Family | ||
| SEI | 0.256 (0.034) | 0.284 (0.028) |
| Actinobacteriota | ||
| Bifidobacteriaceae | 0.483 (0.509) | 1.886 (2.423) |
| Coriobacteriaceae | 1.261 (1.154) | 2.637 (3.149) |
| Eggerthellaceae | 0.511 (0.380) | 0.879 (0.884) |
| Bacteroidota | ||
| Bacteroidaceae | 5.305 (4.418) | 8.742 (7.952) |
| Barnesiellaceae | 0.546 (0.851) | 1.264 (1.653) |
| Muribaculaceae | 0.211 (0.356) | 1.416 (1.856) |
| Prevotellaceae | 1.917 (2.322) | 9.230 (9.755) |
| Rikenellaceae | 1.217 (0.923) | 3.768 (4.131) |
| Tannerellaceae | 0.360 (0.300) | 0.616 (0.487) |
| Firmicutes | ||
| Streptococcaceae | 0.155 (0.188) | 3.420 (7.152) |
| Christensenellaceae | 3.353 (2.773) | 1.374 (1.216) |
| Clostridiaceae 1 | 1.162 (1.033) | 0.325 (0.403) |
| Lachnospiraceae | 24.878 (12.126) | 24.697 (10.165) |
| Peptostreptococcaceae | 3.816 (6.336) | 1.154 (1.323) |
| Ruminococcaceae | 34.221 (9.095) a | 26.057 (8.994) a |
| Acidaminococcaceae | 4.226 (5.596) | 5.384 (8.562) |
| Veillonellaceae | 1.152 (1.264) | 1.531 (2.323) |
| Proteobacteria | ||
| Enterobacteriaceae | 8.377 (18.253) | 0.033 (0.062) |
| Pasteurellaceae | 0.486 (0.844) | 1.139 (2.724) |
| Verrucomicrobia | ||
| Akkermansiaceae | 3.905 (9.722) | 2.673 (3.183) |
| Genus | ||
| SEI | 0.298 (0.041) | 0.298 (0.028) |
| Actinobacteriota | ||
| Bifidobacterium | 0.480 (0.170) | 1.886 (0.857) |
| Collinsella | 1.257 (0.384) | 2.637 (1.113) |
| Bacteroidota | ||
| Bacteroides | 5.305 (1.473) | 8.742 (2.811) |
| Barnesiella | 0.533 (0.279) | 1.184 (0.584) |
| Prevotella-9 | 1.081 (0.506) | 6.288 (3.058) |
| Prevotellaceae-NK3B31 | 0.032 (0.021) | 2.553 (2.451) |
| Alistipes | 1.090 (0.245) | 3.758 (1.458) |
| Firmicutes | ||
| Streptococcus | 0.153 (0.063) | 3.407 (2.531) |
| Christensenellaceae R-7 | 3.335 (0.924) | 1.351 (0.430) |
| Clostridium sensu stricto-1 | 1.161 (0.344) | 0.322 (0.141) |
| Agathobacter | 3.274 (1.191) | 2.150 (1.141) |
| Anaerostipes | 0.617 (0.241) | 1.493 (0.689) |
| Blautia | 3.460 (0.820) | 5.400 (2.022) |
| Coprococcus-2 | 0.771 (0.277) | 0.395 (0.157) |
| Coprococcus-3 | 0.627 (0.160) | 0.657 (0.252) |
| Dorea | 1.408 (0.284) | 1.125 (0.367) |
| Fusicatenibacter | 1.008 (0.357) | 1.063 (0.650) |
| Lachnospiraceae-NK4A136 | 1.722 (0.580) | 0.604 (0.268) |
| Roseburia | 6.056 (1.971) | 3.692 (1.072) |
| Eubacterium-eligens | 0.414 (0.164) | 1.732 (1.306) |
| Eubacterium-hallii | 0.662 (0.163) | 1.036 (0.353) |
| Ruminococcus-torques | 1.016 (0.395) | 0.919 (0.284) |
| Intestinibacter | 2.125 (1.857) | 0.322 (0.248) |
| Romboutsia | 1.245 (0.447) | 0.759 (0.309) |
| Faecalibacterium | 13.505 (3.900) | 6.647 (3.359) |
| Ruminiclostridium-6 | 0.485 (0.217) | 0.884 (0.598) |
| Ruminococcaceae-NK4A214 | 0.990 (0.454) | 1.385 (0.473) |
| Ruminococcaceae-UCG-002 | 3.612 (0.768) | 4.329 (1.717) |
| Ruminococcaceae-UCG-010 | 0.714 (0.302) | 0.291 (0.137) |
| Ruminococcaceae-UCG-014 | 1.715 (0.496) | 1.492 (0.883) |
| Ruminococcus-1 | 0.994 (0.493) | 1.201 (0.589) |
| Ruminococcus-2 | 1.396 (0.649) | 0.630 (0.355) |
| Subdoligranulum | 5.584 (2.869) | 4.045 (1.278) |
| Eubacterium-coprostanoligenes | 1.757 (0.479) | 1.508 (0.523) |
| Phascolarctobacterium | 4.129 (1.874) | 5.384 (3.027) |
| Veillonella | 0.746 (0.297) | 0.260 (0.115) |
| Proteobacteria | ||
| Escherichia-Shigella | 6.263 (5.913) | 0.006 (0.004) |
| Klebsiella | 1.671 (1.339) | 0.025 (0.021) |
| Haemophilus | 0.461 (0.281) | 0.975 (0.801) |
| Verrucomicrobia | ||
| Akkermansia | 3.905 (3.241) | 2.673 (1.125) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaskell, S.K.; Henningsen, K.; Young, P.; Gill, P.; Muir, J.; Henry, R.; Costa, R.J.S. The Impact of a 24-h Low and High Fermentable Oligo- Di- Mono-Saccharides and Polyol (FODMAP) Diet on Plasma Bacterial Profile in Response to Exertional-Heat Stress. Nutrients 2023, 15, 3376. https://doi.org/10.3390/nu15153376
Gaskell SK, Henningsen K, Young P, Gill P, Muir J, Henry R, Costa RJS. The Impact of a 24-h Low and High Fermentable Oligo- Di- Mono-Saccharides and Polyol (FODMAP) Diet on Plasma Bacterial Profile in Response to Exertional-Heat Stress. Nutrients. 2023; 15(15):3376. https://doi.org/10.3390/nu15153376
Chicago/Turabian StyleGaskell, Stephanie K., Kayla Henningsen, Pascale Young, Paul Gill, Jane Muir, Rebekah Henry, and Ricardo J. S. Costa. 2023. "The Impact of a 24-h Low and High Fermentable Oligo- Di- Mono-Saccharides and Polyol (FODMAP) Diet on Plasma Bacterial Profile in Response to Exertional-Heat Stress" Nutrients 15, no. 15: 3376. https://doi.org/10.3390/nu15153376





