Effect of Nuts on Markers of Inflammation and Oxidative Stress: A Narrative Review
Abstract
1. Introduction
2. Nuts and Inflammation and Oxidative Stress
2.1. Evidence from Cohort Studies
2.2. Evidence from RCTs
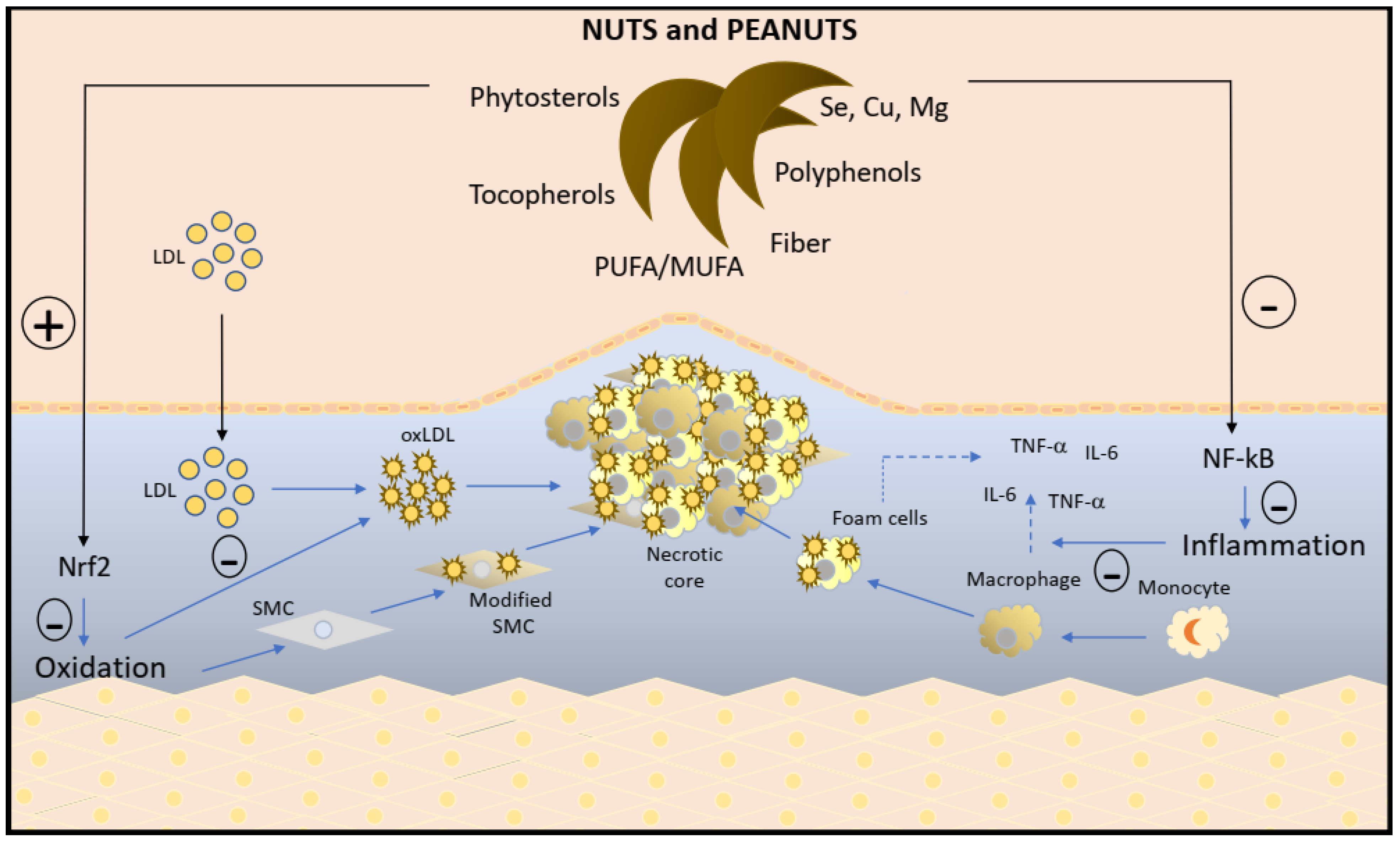
3. Nuts: Antioxidant and Anti-Inflammatory Mechanisms
4. Scope for the Future
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Non Communicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 3 December 2022).
- Seyedsadjadi, N.; Grant, R. The Potential Benefit of Monitoring Oxidative Stress and Inflammation in the Prevention of Non-Communicable Diseases (NCDs). Antioxidants 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian Dietary Patterns and Mortality in Adventist Health Study. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Associations between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.; Clifton, P.M. Nuts and Cardio-Metabolic Disease: A Review of Meta-Analyses. Nutrients 2018, 10, 1935. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Li, J.; Hu, F.B.; Salas-Salvadó, J.; Tobias, D.K. Effects of walnut consumption on blood lipids and other cardiovascular disease risk factors: An updated meta-analyses and systematic review of controlled trials. Am. J. Clin. Nutr. 2018, 108, 174–187. [Google Scholar] [CrossRef]
- Xia, J.-Y.; Yu, J.-H.; Xu, D.-F.; Yang, C.; Xia, H.; Sun, G.-J. The Effects of Peanuts and Tree Nuts on Lipid Profile in Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized, Controlled-Feeding Clinical Studies. Front. Nutr. 2021, 8, 765571. [Google Scholar] [CrossRef]
- Blanco Mejia, S.; Kendall, C.W.C.; Viguiliouk, E.; Augustin, L.S.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Maroleanu, A.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of tree nuts on metabolic syndrome criteria: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2014, 4, e004660. [Google Scholar] [CrossRef]
- Khalili, L.; A-Elgadir, T.M.E.; Mallick, A.K.; El Enshasy, H.A.; Sayyed, R.Z. Nuts as a Part of Dietary Strategy to Improve Metabolic Biomarkers: A Narrative Review. Front. Nutr. 2022, 9, 881843. [Google Scholar] [CrossRef]
- Ros, E.; Singh, A.; O’Keefe, J.H. Nuts: Natural Pleiotropic Nutraceuticals. Nutrients 2021, 13, 3269. [Google Scholar] [CrossRef]
- Lorenzon dos Santos, J.; Quadros, A.S.; Weschenfelder, C.; Garofallo, S.B.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.-Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef]
- Sabaté, J.; Wien, M. Nuts, blood lipids and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2010, 19, 131–136. [Google Scholar]
- Rajaram, S.; Cofán, M.; Sala-Vila, A.; Haddad, E.; Serra-Mir, M.; Bitok, E.; Roth, I.; Freitas-Simoes, T.M.; Kaur, A.; Valls-Pedret, C.; et al. Effects of Walnut Consumption for 2 Years on Lipoprotein Subclasses among Healthy Elders: Findings from the WAHA randomized controlled trial. Circulation 2021, 144, 1083–1085. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Sheehan, D.; Meade, G.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2011, 360, 1–16. [Google Scholar] [CrossRef]
- Lockyer, S.; de la Hunty, A.E.; Steenson, S.; Spiro, A.; Stanner, S.A. Walnut consumption and health outcomes with public health relevance—A systematic review of cohort studies and randomized controlled trials published from 2017 to present. Nutr. Rev. 2022, 81, 26–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guasch-Ferré, M.; Hu, Y.; Li, Y.; Hu, F.B.; Rimm, E.B.; Manson, J.E.; Rexrode, K.M.; Sun, Q. Nut Consumption in Relation to Cardiovascular Disease Incidence and Mortality among Patients with Diabetes Mellitus. Circ. Res. 2019, 124, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Brennan, A.M.; Wedick, N.M.; Mantzoros, C.; Rifai, N.; Hu, F.B. Regular Consumption of Nuts Is Associated with a Lower Risk of Cardiovascular Disease in Women with Type 2 Diabetes. J. Nutr. 2009, 139, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Williams, C.J.; Manson, J.E.; Meigs, J.B.; Hu, F.B. Adherence to the Mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. Am. J. Clin. Nutr. 2006, 84, 328–335. [Google Scholar] [CrossRef]
- Jiang, R.; Jacobs, D.R., Jr.; Mayer-Davis, E.; Szklo, M.; Herrington, D.; Jenny, N.S.; Kronmal, R.; Barr, R.G. Nut and Seed Consumption and Inflammatory Markers in the Multi-Ethnic Study of Atherosclerosis. Am. J. Epidemiol. 2006, 163, 222–231. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; De Curtis, A.; Costanzo, S.; Bracone, F.; Persichillo, M.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Nut consumption is inversely associated with both cancer and total mortality in a Mediterranean population: Prospective results from the Moli-sani study. Br. J. Nutr. 2015, 114, 804–811. [Google Scholar] [CrossRef]
- Yu, Z.; Malik, V.S.; Keum, N.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S.; Bao, Y. Associations between nut consumption and inflammatory biomarkers. Am. J. Clin. Nutr. 2016, 104, 722–728. [Google Scholar] [CrossRef]
- Neale, E.P.; Tapsell, L.C.; Guan, V.; Batterham, M.J. The effect of nut consumption on markers of inflammation and endothelial function: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017, 7, e016863. [Google Scholar] [CrossRef]
- Xiao, Y.; Xia, J.; Ke, Y.; Cheng, J.; Yuan, J.; Wu, S.; Lv, Z.; Huang, S.; Kim, J.H.; Wong, S.Y.-S.; et al. Effects of nut consumption on selected inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2018, 54, 129–143. [Google Scholar] [CrossRef]
- Fatahi, S.; Daneshzad, E.; Lotfi, K.; Azadbakht, L. The effects of almond consumption on inflammatory biomarkers in adults: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2021, 13, 1462–1475. [Google Scholar] [CrossRef]
- Mateș, L.; Popa, D.-S.; Rusu, M.E.; Fizeșan, I.; Leucuța, D. Walnut Intake Interventions Targeting Biomarkers of Metabolic Syndrome and Inflammation in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2022, 11, 1412. [Google Scholar] [CrossRef]
- Asbaghi, O.; Hadi, A.; Campbell, M.S.; Venkatakrishnan, K.; Ghaedi, E. Effects of pistachios on anthropometric indices, inflammatory markers, endothelial function and blood pressure in adults: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2021, 126, 718–729. [Google Scholar] [CrossRef]
- Godos, J.; Giampieri, F.; Micek, A.; Battino, M.; Forbes-Hernández, T.Y.; Quiles, J.L.; Paladino, N.; Falzone, L.; Grosso, G. Effect of Brazil nuts on selenium status, blood lipids, and biomarkers of oxidative stress and inflammation: A systematic review and meta-analysis of randomized controlled trials. Antioxidants 2022, 11, 403. [Google Scholar] [CrossRef]
- Brown, R.; Ware, L.; Tey, S.L. Effects of Hazelnut Consumption on Cardiometabolic Risk Factors and Acceptance: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2880. [Google Scholar] [CrossRef]
- Berryman, C.E.; West, S.G.; Fleming, J.A.; Bordi, P.L.; Kris-Etherton, P.M. Effects of Daily Almond Consumption on Cardiometabolic Risk and Abdominal Adiposity in Healthy Adults with Elevated LDL-Cholesterol: A Randomized Controlled Trial. J. Am. Heart Assoc. 2015, 4, e000993. [Google Scholar] [CrossRef]
- Rajaram, S.; Connell, K.M.; Sabaté, J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: A randomised, controlled, crossover study. Br. J. Nutr. 2010, 103, 907–912. [Google Scholar] [CrossRef]
- Palacios, O.M.; Maki, K.C.; Xiao, D.; Wilcox, M.L.; Dicklin, M.R.; Kramer, M.; Trivedi, R.; Burton-Freeman, B.; Edirisinghe, I. Effects of Consuming Almonds on Insulin Sensitivity and Other Cardiometabolic Health Markers in Adults with Prediabetes. J. Am. Coll. Nutr. 2020, 39, 397–406. [Google Scholar] [CrossRef]
- Madan, J.; Desai, S.; Moitra, P.; Salis, S.; Agashe, S.; Battalwar, R.; Mehta, A.; Kamble, R.; Kalita, S.; Phatak, A.G.; et al. Effect of Almond Consumption on Metabolic Risk Factors—Glucose Metabolism, Hyperinsulinemia, Selected Markers of Inflammation: A Randomized Controlled Trial in Adolescents and Young Adults. Front. Nutr. 2021, 8, 668622. [Google Scholar] [CrossRef]
- Jung, H.; Chen, C.-Y.O.; Blumberg, J.B.; Kwak, H.-K. The effect of almonds on vitamin E status and cardiovascular risk factors in Korean adults: A randomized clinical trial. Eur. J. Nutr. 2018, 57, 2069–2079. [Google Scholar] [CrossRef]
- Hou, Y.-Y.; Ojo, O.; Wang, L.-L.; Wang, Q.; Jiang, Q.; Shao, X.-Y.; Wang, X.-H. A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 1565. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Cofán, M.; Rajaram, S.; Sala-Vila, A.; Valls-Pedret, C.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.M.; Bitok, E.; Sabaté, J.; Ros, E. Effects of 2-Year Walnut-Supplemented Diet on Inflammatory Biomarkers. J. Am. Coll. Cardiol. 2020, 76, 2282–2284. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; Uriarte, P.J.L.; Bulló, M.; Ros, E.; Vila, J.J.C.; Salas-Salvadó, J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-L.; Haddad, E.; Rajaram, S.; Shavlik, D.; Sabaté, J. The effect of dietary walnuts compared to fatty fish on eicosanoids, cytokines, soluble endothelial adhesion molecules and lymphocyte subsets: A randomized, controlled crossover trial. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 111–117. [Google Scholar] [CrossRef]
- Canales, A.; Sánchez-Muniz, F.J.; Bastida, S.; Librelotto, J.; Nus, M.; Corella, D.; Guillen, M.; Benedi, J. Effect of walnut-enriched meat on the relationship between VACM, ICAM, and LTB4 levels and PON-1 activity in ApoA4 360 and PON-1 allele carriers at increased cardiovascular risk. Eur. J. Clin. Nutr. 2011, 65, 703–710. [Google Scholar] [CrossRef]
- Lin, L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS ONE 2018, 13, e0204056. [Google Scholar] [CrossRef]
- Orem, A.; Yucesan, F.B.; Orem, C.; Akcan, B.; Kural, B.V.; Alasalvar, C.; Shahidi, F. Hazelnut-Enriched diet improves cardiovascular risk biomarkers beyond a lipid-Lowering effect in hypercholesterolemic subjects. J. Clin. Lipidol. 2013, 7, 123–131. [Google Scholar] [CrossRef]
- Di Renzo, L.; Merra, G.; Botta, R.; Gualtieri, P.; Manzo, A.; Perrone, M.A.; Mazza, M.; Cascapera, S.; De Lorenzo, A. Post-prandial effects of hazelnut-enriched high fat meal on LDL oxidatve status, oxidative and inflammatory gene expression in healthy subjects: A randomized trial. Eur. Rev. Med. Pharm. Sci. 2017, 21, 1610–1626. [Google Scholar]
- Silveira, B.K.S.; da Silva, A.; Hermsdorff, H.H.M.; Bressan, J. Effect of chronic consumption of nuts on oxidative stress: A systematic review of clinical trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 726–737. [Google Scholar] [CrossRef]
- López-Uriarte, P.; Bulló, M.; Casas-Agustench, P.; Babio, N.; Salas-Salvadó, J. Nuts and oxidation: A systematic review. Nutr. Rev. 2009, 67, 497–508. [Google Scholar] [CrossRef]
- Liu, J.-F.; Liu, Y.-H.; Chen, C.-M.; Chang, W.-H.; Chen, C.Y.O. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: A randomized crossover, controlled feeding trial. Eur. J. Nutr. 2013, 52, 927–935. [Google Scholar] [CrossRef]
- Colpo, E.; Vilanova, C.D.D.A.; Reetz, L.G.B.; Duarte, M.M.M.F.; Farias, I.L.G.; Muller, E.I.; Muller, A.L.H.; Flores, E.M.M.; Wagner, R.; da Rocha, J.B.T. A Single Consumption of High Amounts of the Brazil Nuts Improves Lipid Profile of Healthy Volunteers. J. Nutr. Metab. 2013, 2013, 653185. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Stockler-Pinto, M.B.; Mafra, D. Brazil nut consumption modulates Nrf2 expression in hemodialysis patients: A pilot study. Mol. Nutr. Food Res. 2016, 60, 1719–1724. [Google Scholar] [CrossRef]
- Stockler-Pinto, M.B.; Mafra, D.; Moraes, C.; Lobo, J.; Boaventura, G.T.; Farage, N.E.; Silva, W.S.; Cozzolino, S.F.; Malm, O. Brazil Nut (Bertholletia excelsa, H.B.K.) Improves Oxidative Stress and Inflammation Biomarkers in Hemodialysis Patients. Biol. Trace Element Res. 2014, 158, 105–112. [Google Scholar] [CrossRef]
- Haddad, E.H.; Gaban-Chong, N.; Oda, K.; Sabaté, J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 2014, 13, 4. [Google Scholar] [CrossRef]
- López-Uriarte, P.; Nogués, R.; Saez, G.; Bulló, M.; Romeu, M.; Masana, L.; Tormos, C.; Casas-Agustench, P.; Salas-Salvadó, J. Effect of nut consumption on oxidative stress and the endothelial function in metabolic syndrome. Clin. Nutr. 2010, 29, 373–380. [Google Scholar] [CrossRef]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.-Y.O.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute Consumption of Walnuts and Walnut Components Differentially Affect Postprandial Lipemia, Endothelial Function, Oxidative Stress, and Cholesterol Efflux in Humans with Mild Hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef]
- Bayele, H.K.; Debnam, E.S.; Srai, K.S. Nrf2 transcriptional derepression from Keap1 by dietary polyphenols. Biochem. Biophys. Res. Commun. 2016, 469, 521–528. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular crosstalk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. γ-Tocotrienol Inhibits Nuclear Factor-κB Signaling Pathway through Inhibition of Receptor-interacting Protein and TAK1 Leading to Suppression of Antiapoptotic Gene Products and Potentiation of Apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Kaithwas, G. Anti-inflammatory Potential of Alpha-Linolenic Acid Mediated Through Selective COX Inhibition: Computational and Experimental Data. Inflammation 2014, 37, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Ghanavati, M.; Hosseinabadi, S.M.; Parsa, S.A.; Safi, M.; Emamat, H.; Nasrollahzadeh, J. Effect of a nut-enriched low-calorie diet on body weight and selected markers of inflammation in overweight and obese stable coronary artery disease patients: A randomized controlled study. Eur. J. Clin. Nutr. 2021, 75, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Mallard, A.R.; Hollekim-Strand, S.M.; Ingul, C.B.; Coombes, J.S. High day-to-day and diurnal variability of oxidative stress and inflammation biomarkers in people with type 2 diabetes mellitus and healthy individuals. Redox Rep. 2020, 25, 64–69. [Google Scholar] [CrossRef]
- Calle, M.C.; Andersen, C.J. Assessment of Dietary Patterns Represents a Potential, Yet Variable, Measure of Inflammatory Status: A Review and Update. Dis. Markers 2019, 2019, 3102870. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Bulló, M. Beneficial Effect of Pistachio Consumption on Glucose Metabolism, Insulin Resistance, Inflammation, and Related Metabolic Risk Markers: A Randomized Clinical Trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [CrossRef]
- Arpón, A.; Milagro, F.I.; Razquin, C.; Corella, D.; Estruch, R.; Fitó, M.; Marti, A.; Martínez-González, M.A.; Ros, E.; Salas-Salvadó, J.; et al. Impact of Consuming Extra-Virgin Olive Oil or Nuts within a Mediterranean Diet on DNA Methylation in Peripheral White Blood Cells within the PREDIMED-Navarra Randomized Controlled Trial: A Role for Dietary Lipids. Nutrients 2018, 10, 15. [Google Scholar] [CrossRef]
- Creedon, A.C.; Hung, E.S.; Berry, S.E.; Whelan, K. Nuts and their Effect on Gut Microbiota, Gut Function and Symptoms in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 2347. [Google Scholar] [CrossRef]
- Weschenfelder, C.; Gottschall, C.B.A.; Markoski, M.M.; Portal, V.L.; de Quadros, A.S.; Bersch-Ferreira, C.; Marcadenti, A. Effects of supplementing a healthy diet with pecan nuts or extra-virgin olive oil on inflammatory profile of patients with stable coronary artery disease: A randomised clinical trial. Br. J. Nutr. 2022, 127, 862–871. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Chiva-Blanch, G.; Ros, E.; Martinez-Gonzalez, M.A.; Covas, M.-I.; Salas-Salvadó, J.; Fiol, M.; Arós, F.; et al. The Effects of the Mediterranean Diet on Biomarkers of Vascular Wall Inflammation and Plaque Vulnerability in Subjects with High Risk for Cardiovascular Disease. A Randomized Trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef]
- Semmler, G.; Bachmayer, S.; Wernly, S.; Wernly, B.; Niederseer, D.; Huber-Schönauer, U.; Stickel, F.; Aigner, E.; Datz, C. Nut consumption and the prevalence and severity of non-alcoholic fatty liver disease. PLoS ONE 2020, 15, e0244514. [Google Scholar] [CrossRef]
- Li, H.-Y.; Gan, R.-Y.; Shang, A.; Mao, Q.-Q.; Sun, Q.-C.; Wu, D.-T.; Geng, F.; He, X.-Q.; Li, H.-B. Plant-Based Foods and Their Bioactive Compounds on Fatty Liver Disease: Effects, Mechanisms, and Clinical Application. Oxidative Med. Cell. Longev. 2021, 2021, 6621644. [Google Scholar] [CrossRef]
- Kalgaonkar, S.; Almario, R.U.; Gurusinghe, D.; Garamendi, E.M.; Buchan, W.; Kim, K.; Karakas, S.E. Differential effects of walnuts vs. almonds on improving metabolic and endocrine parameters in PCOS. Eur. J. Clin. Nutr. 2010, 65, 386–393. [Google Scholar] [CrossRef]
- Griel, A.E.; Kris-Etherton, P.M.; Hilpert, K.F.; Zhao, G.; West, S.G.; Corwin, R.L. An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr. J. 2007, 6, 2. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Mayr, H.L.; Itsiopoulos, C.; Tierney, A.C.; Ruiz-Canela, M.; Hebert, J.R.; Shivappa, N.; Thomas, C.J. Improvement in dietary inflammatory index score after 6-month dietary intervention is associated with reduction in interleukin-6 in patients with coronary heart disease: The AUSMED heart trial. Nutr. Res. 2018, 55, 108–121. [Google Scholar] [CrossRef]
- Garcia-Arellano, A.; Ramallal, R.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Shivappa, N.; Schröder, H.; Hébert, J.R.; Ros, E.; Gómez-Garcia, E.; et al. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the PREDIMED Study. Nutrients 2015, 7, 4124–4138. [Google Scholar] [CrossRef]
| Reference | Study Country | Sample Size Gender | Follow-Up | Nut Type Amount | Outcome | Inflammatory Biomarkers |
|---|---|---|---|---|---|---|
| Li et al., 2009 [25] | NHS, USA | 6.309 Female | 1989–1990 | Peanuts and mixed nuts Almost never or ≥5 servings/week (Portion size—28 g/day for nuts and 16 g/day for peanut butter) | Incident CVD | TNFR-2, ICAM-1, E-selectin, CRP, and fibrinogen (No changes) |
| Jiang et al., 2006 [27] | MESA, USA | 6.080 Female, Male | Baseline | Mixed nuts, seeds, or peanuts/peanut butter Never/rare or ≥5 times/week (Portion size—no data) | Inflammation biomarkers | ↓CRP, ↓IL-6 and ↓fibrinogen |
| Mantzoros et al., 2006 [26] | NHS, USA | 987 Female | 1989–1990 | Mixed nuts Quintile of nuts intake (Portion size—no data) | Adipocytokine | ↑Adiponectin |
| Bonaccio et al., 2015 [28] | Moli-sani Study, Italy | 19,386 Female, Male | 4.3 years | Walnuts, hazelnuts, almonds, and peanuts Never or ≥8 times/month (Portion size—no data) | Total and specific mortality | ↓CRP, ↓platelet count and ↓neutrophil to lymphocyte ratio |
| Yu et al., 2016 [29] | NHS HPFS, USA | NHS (3654) HPFS (1359) Female, Male | NHS (1989–1990) HPFS (1993–1995) | Peanuts, mixed nuts, and peanut butter Almost never or ≥5 times/week (Portion size—28 g/day) | Inflammatory biomarkers | ↓TNFR-2, ↓CRP and ↓IL-6 |
| Nut Type Reference [#] | CRP | IL-6 | TNF-α | Adhesion Molecules | OxLDL | Antioxidant Enzymes | Oxidized Metabolites |
|---|---|---|---|---|---|---|---|
| Almonds [32,51] | ↓ | ↓ | ↔ | ↔ | ↓ | - | ↔ |
| Brazil nuts [35,51] | ↔ | - | - | - | ↔ | ↑ | ↔ |
| Hazelnuts [36,51,52] | ↔ | - | - | - | - | - | - |
| Pistachios [34,51] | ↔ | - | - | - | ↔ | - | - |
| Walnuts * [33,44,51,52] | ↔ | ↓ | ↓ | ↓ | ↔ | - | ↔ |
| Other ** [30,31,51] | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajaram, S.; Damasceno, N.R.T.; Braga, R.A.M.; Martinez, R.; Kris-Etherton, P.; Sala-Vila, A. Effect of Nuts on Markers of Inflammation and Oxidative Stress: A Narrative Review. Nutrients 2023, 15, 1099. https://doi.org/10.3390/nu15051099
Rajaram S, Damasceno NRT, Braga RAM, Martinez R, Kris-Etherton P, Sala-Vila A. Effect of Nuts on Markers of Inflammation and Oxidative Stress: A Narrative Review. Nutrients. 2023; 15(5):1099. https://doi.org/10.3390/nu15051099
Chicago/Turabian StyleRajaram, Sujatha, Nagila Raquel Teixeira Damasceno, Ribanna Aparecida Marques Braga, Raquel Martinez, Penny Kris-Etherton, and Aleix Sala-Vila. 2023. "Effect of Nuts on Markers of Inflammation and Oxidative Stress: A Narrative Review" Nutrients 15, no. 5: 1099. https://doi.org/10.3390/nu15051099
APA StyleRajaram, S., Damasceno, N. R. T., Braga, R. A. M., Martinez, R., Kris-Etherton, P., & Sala-Vila, A. (2023). Effect of Nuts on Markers of Inflammation and Oxidative Stress: A Narrative Review. Nutrients, 15(5), 1099. https://doi.org/10.3390/nu15051099





