Kaempferia parviflora Extracts Protect Neural Stem Cells from Amyloid Peptide-Mediated Inflammation in Co-Culture Model with Microglia
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Amyloid Beta Peptides (Aβ42)
2.3. Plant Extraction and Characterization
2.4. Cell Cultures
2.4.1. Monoculture of Neural Stem Cells (NE-4C)
2.4.2. Monoculture of Microglia Cells (BV-2)
2.4.3. Differentiation of NE-4C Neural Stem Cells into Neurons
2.4.4. Co-Culture System between NE-4C Stem Cells and BV-2 Microglial Cells
2.4.5. Co-Culture System between Differentiated Neurons Derived from NE-4C Cells and BV-2 Microglial Cells
2.5. Cytotoxic Effect of Amyloid Beta Peptides and KP Extracts
2.6. Protective Effects of KP Extracts in Both Mono and Co-Culture
2.7. Anti-Inflammatory Activities of KP Extracts on Aβ42-Induced Inflammation in Monoculture
2.8. Anti-Inflammatory Effects of KP Extracts on Aβ42-Induced Inflammation in Co-Culture
2.9. Effects of KP Extracts on Neurogenesis of Aβ42-Induced NE-4C Cells in Monoculture
2.10. Effects of KP Extracts on Neurogenesis in Aβ42-Induced NE-4C Cells in Co-Culture with BV-2 Cells
2.11. Sodium 3′-[1-(Phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-Methoxy-nitro) Benzene Sulfonic Acid Hydrate (XTT) Reduction Assay
2.12. Determination of ATP Levels
2.13. Interleukin-6 (IL-6) Measurement
2.14. Nitric Oxide (NO) Measurement
2.15. Intercellular Reactive Oxygen Species (ROS) Measurement
2.16. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT–qPCR)
2.17. Statistical Analysis
3. Results
3.1. KP Extraction and Phytochemical Analysis
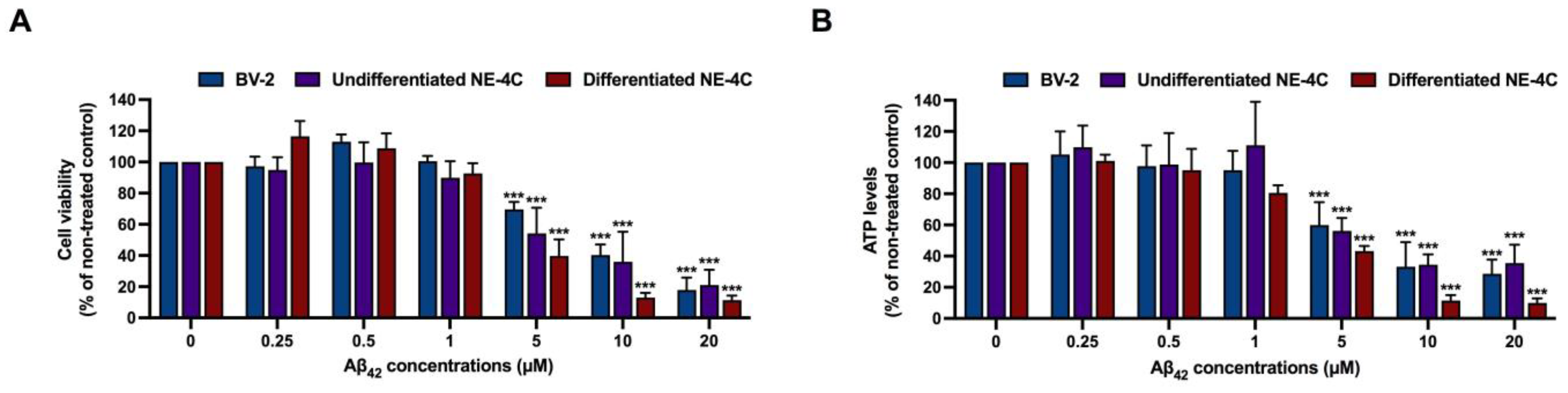
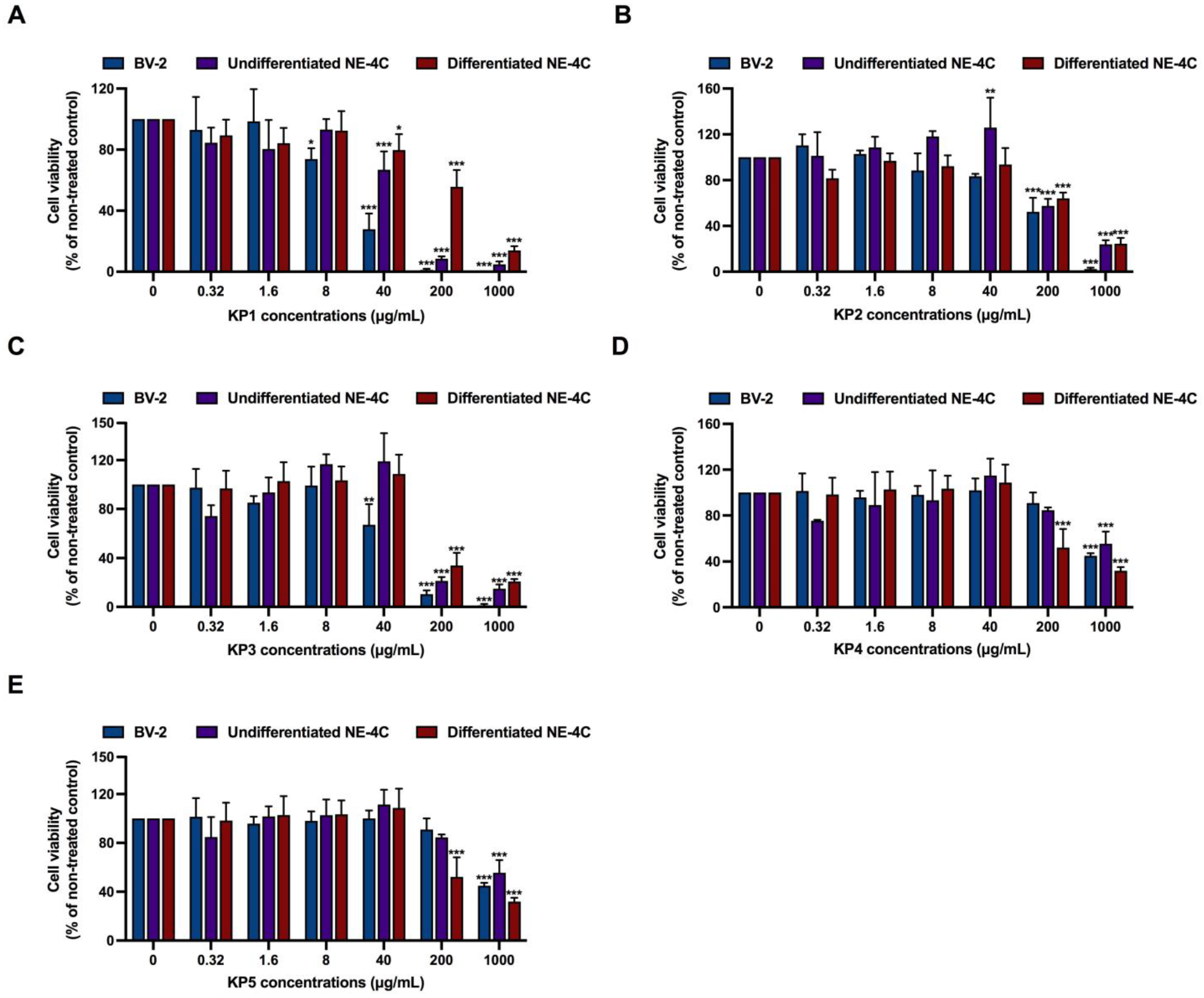
3.2. Cytotoxicity Determination of Aβ42 and K. parviflora Extracts
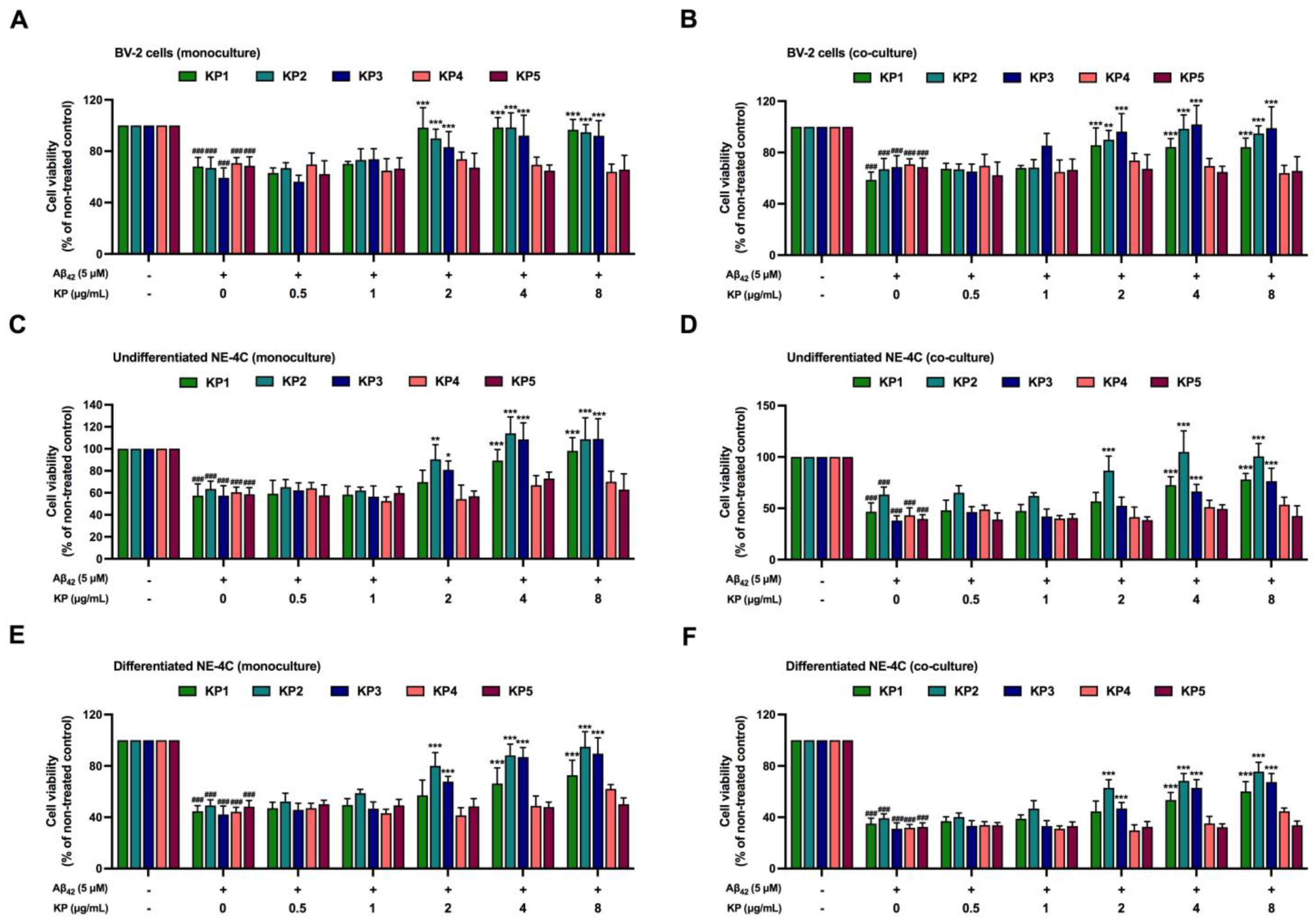
3.3. Protective Effects of K. parviflora Extracts on Aβ42-Mediated Neurotoxicity
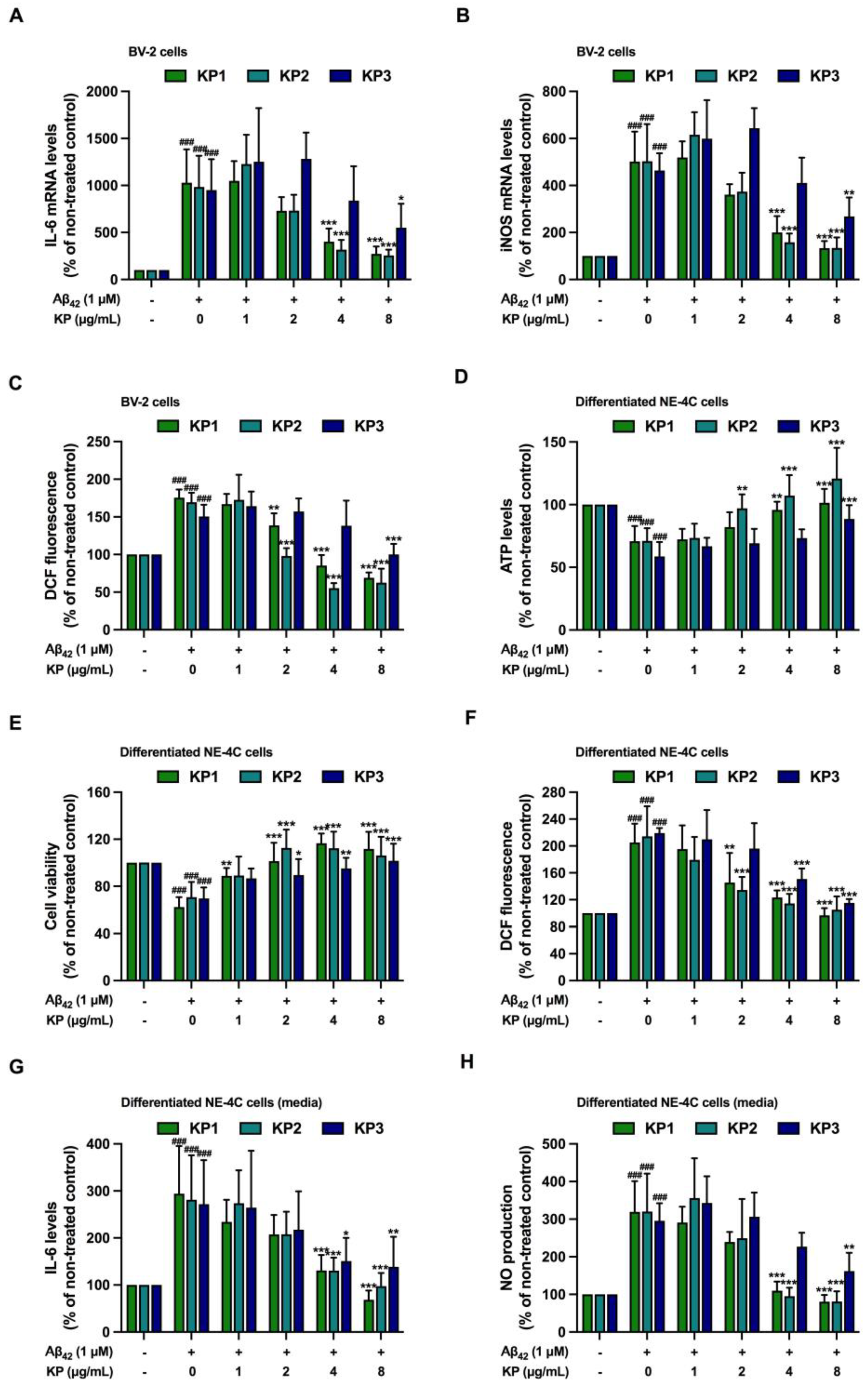
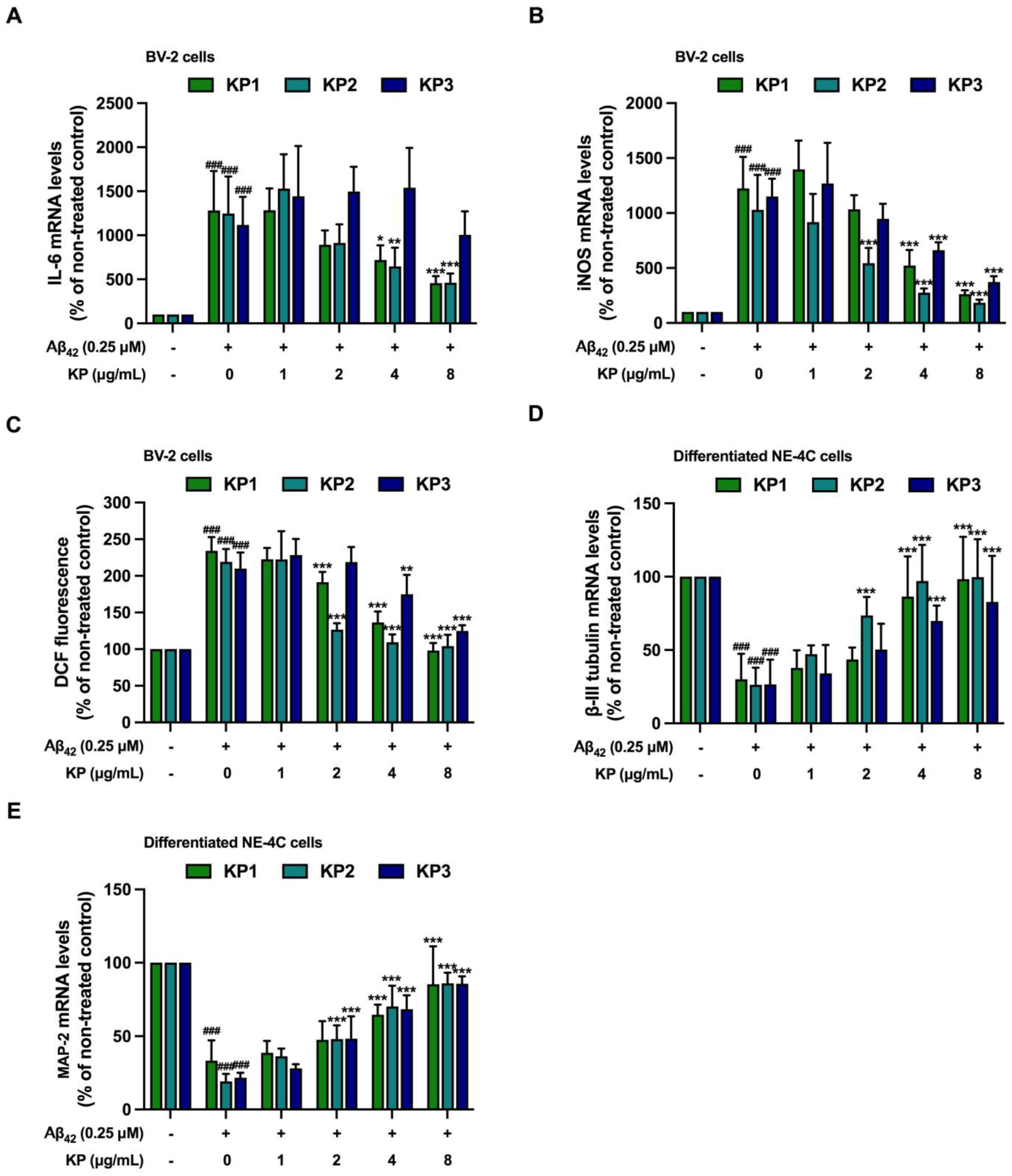
3.4. Suppression of Aβ42-Induced Inflammation and Oxidative Stress by K. parviflora Extracts in BV-2 Cell Monoculture
3.5. Suppression of Aβ42-Induced Inflammation and Oxidative Stress by K. parviflora Extracts in Co-Culture between Differentiated NE-4C and BV-2 Cells
3.6. Suppression of Aβ42-Induced Inflammation and Oxidative Stress by K. parviflora Extracts in Co-Culture between Undifferentiated NE-4C and BV-2 Cells
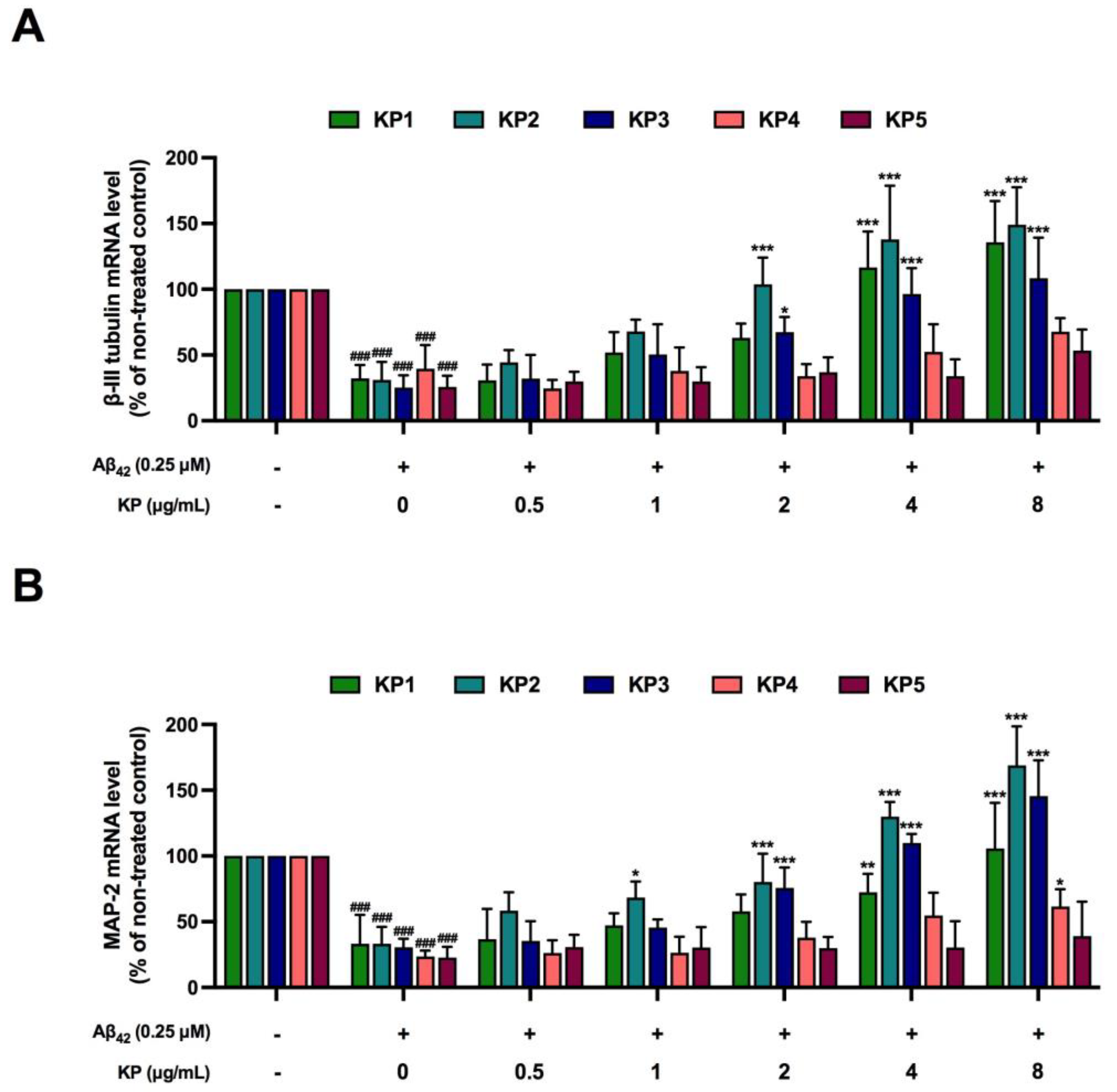
3.7. Protective Effects of K. parviflora Extracts on Neurogenesis of Aβ42-Treated NE-4 Cells in Monoculture
3.8. Protective Effects of K. parviflora Extracts Neurogenesis in Aβ42-Induced Differentiated NE-4C in Co-Culture with BV-2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Hu, J.; Wang, X. Alzheimer’s Disease: From Pathogenesis to Mesenchymal Stem Cell Therapy—Bridging the Missing Link. Front. Cell. Neurosci. 2021, 15, 811852. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Hemonnot, A.L.; Hua, J.; Ulmann, L.; Hirbec, H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front. Aging Neurosci. 2019, 11, 233. [Google Scholar] [CrossRef]
- Mansor, N.I.; Ntimi, C.M.; Abdul-Aziz, N.M.; Ling, K.H.; Adam, A.; Rosli, R.; Hassan, Z.; Nordin, N. Asymptomatic neurotoxicity of amyloid β-peptides (Aβ1-42 and Aβ25-35) on mouse embryonic stem cell-derived neural cells. Bosn. J. Basic Med. Sci. 2021, 21, 98–110. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.-H.; Koh, S.-H.; Hojin, C. Neural Stem Cell Death Mechanisms Induced by Amyloid Beta. Dement. Neurocogn. Disord. 2017, 16, 121. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Mollinari, C.; Garaci, E.; Merlo, D.; Pei, G. Amyloid-β Oligomers-induced Mitochondrial DNA Repair Impairment Contributes to Altered Human Neural Stem Cell Differentiation. Curr. Alzheimer Res. 2019, 16, 934–949. [Google Scholar] [CrossRef]
- Li Puma, D.D.; Piacentini, R.; Grassi, C. Does impairment of adult neurogenesis contribute to pathophysiology of alzheimer’s disease? A still open question. Front. Mol. Neurosci. 2021, 13, 578211. [Google Scholar] [CrossRef]
- Mimica, N.; Presecki, P. Side effects of approved antidementives. Psychiatr. Danub. 2009, 21, 108–113. [Google Scholar]
- Kim, H.J. Regulation of Neural Stem Cell Fate by Natural Products. Biomol. Ther. 2019, 27, 15–24. [Google Scholar] [CrossRef]
- Saud, B.; Malla, R.; Shrestha, K. A Review on the Effect of Plant Extract on Mesenchymal Stem Cell Proliferation and Differentiation. Stem. Cells Int. 2019, 2019, 7513404. [Google Scholar] [CrossRef]
- Jiang, J.; Hai, J.; Liu, W.; Luo, Y.; Chen, K.; Xin, Y.; Pan, J.; Hu, Y.; Gao, Q.; Xiao, F.; et al. Gallic Acid Induces Neural Stem Cell Differentiation into Neurons and Proliferation through the MAPK/ERK Pathway. J. Agric. Food Chem. 2021, 69, 12456–12464. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Zhang, J.; Chung, H.L.; Wu, X.Y.; Liu, B.; Zhao, B.X.; Sze, S.C.; Zhou, P.Z.; Yung, K.K.; Zhang, S.Q. Total Ginsenoside Extract from Panax ginseng Enhances Neural Stem Cell Proliferation and Neuronal Differentiation by Inactivating GSK-3β. Chin. J. Integr. Med. 2022, 28, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Takuathung, M.N.; Potikanond, S.; Sookkhee, S.; Mungkornasawakul, P.; Jearanaikulvanich, T.; Chinda, K.; Wikan, N.; Nimlamool, W. Anti-psoriatic and anti-inflammatory effects of Kaempferia parviflora in keratinocytes and macrophage cells. Biomed Pharm. 2021, 143, 112229. [Google Scholar] [CrossRef] [PubMed]
- Thaklaewphan, P.; Ruttanapattanakul, J.; Monkaew, S.; Buatoom, M.; Sookkhee, S.; Nimlamool, W.; Potikanond, S. Kaempferia parviflora extract inhibits TNF-α-induced release of MCP-1 in ovarian cancer cells through the suppression of NF-κB signaling. Biomed Pharm. 2021, 141, 111911. [Google Scholar] [CrossRef]
- Yoshino, S.; Awa, R.; Miyake, Y.; Fukuhara, I.; Sato, H.; Ashino, T.; Tomita, S.; Kuwahara, H. Daily intake of Kaempferia parviflora extract decreases abdominal fat in overweight and preobese subjects: A randomized, double-blind, placebo-controlled clinical study. Diabetes Metab. Syndr. Obes. 2018, 11, 447–458. [Google Scholar] [CrossRef]
- Kobayashi, H.; Suzuki, R.; Sato, K.; Ogami, T.; Tomozawa, H.; Tsubata, M.; Ichinose, K.; Aburada, M.; Ochiai, W.; Sugiyama, K.; et al. Effect of Kaempferia parviflora extract on knee osteoarthritis. J. Nat. Med. 2018, 72, 136–144. [Google Scholar] [CrossRef]
- Chen, D.; Li, H.; Li, W.; Feng, S.; Deng, D. Kaempferia parviflora and Its Methoxyflavones: Chemistry and Biological Activities. Evid.-Based Complement Altern. Med. 2018, 2018, 4057456. [Google Scholar] [CrossRef]
- Sawasdee, P.; Sabphon, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora . Phytother. Res. 2009, 23, 1792–1794. [Google Scholar] [CrossRef]
- Welbat, J.U.; Chaisawang, P.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Sripanidkulchai, B.; Wigmore, P. Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Ann. Anat. 2016, 206, 7–13. [Google Scholar] [CrossRef]
- Umka, J.; Mustafa, S.; ElBeltagy, M.; Thorpe, A.; Latif, L.; Bennett, G.; Wigmore, P.M. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience 2010, 166, 15–22. [Google Scholar] [CrossRef]
- Mula, M.; Trimble, M.R. Antiepileptic drug-induced cognitive adverse effects: Potential mechanisms and contributing factors. CNS Drugs 2009, 23, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, S.; Awa, R.; Ohto, N.; Miyake, Y.; Kuwahara, H. Toxicological evaluation of standardized Kaempferia parviflora extract: Sub-chronic and mutagenicity studies. Toxicol. Rep. 2019, 6, 544–549. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Wu, D.; Lambert, M.P.; Fernandez, S.J.; Velasco, P.T.; Lacor, P.N.; Bigio, E.H.; Jerecic, J.; Acton, P.J.; Shughrue, P.J.; et al. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol. Aging 2008, 29, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Muñoz, A.M.; Nieto-Escamez, F.A.; Aznar, S.; Colomina, M.T.; Sanchez-Santed, F. Cognitive and histological disturbances after chlorpyrifos exposure and chronic Aβ (1–42) infusions in Wistar rats. Neurotoxicology 2011, 32, 836–844. [Google Scholar] [CrossRef]
- Stine, W.B.; Jungbauer, L.; Yu, C.; LaDu, M.J. Preparing synthetic Aβ in different aggregation states. Methods Mol. Biol. 2011, 670, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Hawiset, T.; Muchimapura, S.; Wattanathorn, J.; Sripanidkulchai, B. Screening Neuropharmacological Activities of Kaempferia parviflora (Krachai dam) in Healthy Adult Male Rats. Am. J. Appl. Sci. 2011, 8, 695–702. [Google Scholar] [CrossRef]
- Plaingam, W.; Sangsuthum, S.; Angkhasirisap, W.; Tencomnao, T. Kaempferia parviflora rhizome extract and Myristica fragrans volatile oil increase the levels of monoamine neurotransmitters and impact the proteomic profiles in the rat hippocampus: Mechanistic insights into their neuroprotective effects. J. Tradit. Complement Med. 2017, 7, 538–552. [Google Scholar] [CrossRef]
- Phochantachinda, S.; Chatchaisak, D.; Temviriyanukul, P.; Chansawang, A.; Pitchakarn, P.; Chantong, B. Ethanolic Fruit Extract of Emblica officinalis Suppresses Neuroinflammation in Microglia and Promotes Neurite Outgrowth in Neuro2a Cells. Evid.-Based Complement. Altern. Med. 2021, 2021, 6405987. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Lertmongkolaksorn, T.; Supasawat, P.; Pitchakarn, P.; Thiyajai, P.; Nusuetrong, P.; Phochantachinda, S.; Chansawhang, A.; Chantong, B. Phikud Navakot extract attenuates lipopolysaccharide-induced inflammatory responses through inhibition of ERK1/2 phosphorylation in a coculture system of microglia and neuronal cells. J. Ethnopharmacol. 2022, 296, 115440. [Google Scholar] [CrossRef]
- Davidson, J.M.; Wong, C.T.; Rai-Bhogal, R.; Li, H.; Crawford, D.A. Prostaglandin E2 elevates calcium in differentiated neuroectodermal stem cells. Mol. Cell. Neurosci. 2016, 74, 71–77. [Google Scholar] [CrossRef]
- Novo, M.; Freire, S.; Al-Soufi, W. Critical aggregation concentration for the formation of early Amyloid-β (1-42) oligomers. Sci. Rep. 2018, 8, 1783. [Google Scholar] [CrossRef]
- Cai, Q.; Li, Y.; Pei, G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflamm. 2017, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cui, J.; Lee, J.C.; Ding, S.; Chalimoniuk, M.; Simonyi, A.; Sun, A.Y.; Gu, Z.; Weisman, G.A.; Wood, W.G.; et al. Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: Protective effect of green tea (-)-epigallocatechin-3-gallate. ASN Neuro 2011, 3, e00050. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Nicol, C.J.B.; Cheng, Y.C.; Yen, C.; Lin, C.H.; Chen, S.J.; Huang, R.N. Nanogold Neuroprotection in Human Neural Stem Cells Against Amyloid-beta-induced Mitochondrial Dysfunction. Neuroscience 2020, 435, 44–57. [Google Scholar] [CrossRef]
- Ng, N.S.; Ooi, L. A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization. Bio Protoc. 2021, 11, e3877. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, C. Microglia activation linking amyloid-β drive tau spatial propagation in Alzheimer’s disease. Front. Neurosci. 2022, 16, 951128. [Google Scholar] [CrossRef]
- van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional neurogenesis in the adult hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef]
- Hashiguchi, A.; San Thawtar, M.; Duangsodsri, T.; Kusano, M.; Watanabe, K.N. Biofunctional properties and plant physiology of Kaempferia spp.: Status and trends. J. Funct. Foods 2022, 92, 105029. [Google Scholar] [CrossRef]
- Tonsomboon, A.; Prasanth, M.I.; Plaingam, W.; Tencomnao, T. Kaempferia parviflora Rhizome Extract Inhibits Glutamate-Induced Toxicity in HT-22 Mouse Hippocampal Neuronal Cells and Extends Longevity in Caenorhabditis elegans. Biology 2021, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Phachonpai, W.; Maharun, S.; Tong-Un, T.; Muchimapura, S.; Wattanathorn, J. The functional effect of kaempferia parviflora on ischemic stroke in rats. Am. J. Agric. Biol. Sci. 2012, 7, 173–179. [Google Scholar]
- Youn, K.; Lee, J.; Ho, C.-T.; Jun, M. Discovery of polymethoxyflavones from black ginger (Kaempferia parviflora) as potential β-secretase (BACE1) inhibitors. J. Funct. Foods 2016, 20, 567–574. [Google Scholar] [CrossRef]
- Seo, S.H.; Lee, Y.C.; Moon, H.I. Acetyl-cholinesterase Inhibitory Activity of Methoxyflavones Isolated from Kaempferia parviflora . Nat. Prod. Commun. 2017, 12, 21–22. [Google Scholar] [CrossRef]
- Azuma, T.; Tanaka, Y.; Kikuzaki, H. Phenolic glycosides from Kaempferia parviflora. Phytochemistry 2008, 69, 2743–2748. [Google Scholar] [CrossRef]
- Okabe, Y.; Shimada, T.; Horikawa, T.; Kinoshita, K.; Koyama, K.; Ichinose, K.; Aburada, M.; Takahashi, K. Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferia parviflora. Phytomedicine 2014, 21, 800–806. [Google Scholar] [CrossRef]
- Horigome, S.; Yoshida, I.; Tsuda, A.; Harada, T.; Yamaguchi, A.; Yamazaki, K.; Inohana, S.; Isagawa, S.; Kibune, N.; Satoyama, T.; et al. Identification and evaluation of anti-inflammatory compounds from Kaempferia parviflora . Biosci. Biotechnol. Biochem. 2014, 78, 851–860. [Google Scholar] [CrossRef]
- Kongdang, P.; Jaitham, R.; Thonghoi, S.; Kuensaen, C.; Pradit, W.; Ongchai, S. Ethanolic extract of Kaempferia parviflora interrupts the mechanisms-associated rheumatoid arthritis in SW982 culture model via p38/STAT1 and STAT3 pathways. Phytomedicine 2019, 59, 152755. [Google Scholar] [CrossRef]
- Sae-Wong, C.; Matsuda, H.; Tewtrakul, S.; Tansakul, P.; Nakamura, S.; Nomura, Y.; Yoshikawa, M. Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J. Ethnopharmacol. 2011, 136, 488–495. [Google Scholar] [CrossRef]
- Toda, K.; Hitoe, S.; Takeda, S.; Shimoda, H. Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon 2016, 2, e00115. [Google Scholar] [CrossRef] [PubMed]
- Bei, D.; An, G. Pharmacokinetics and tissue distribution of 5,7-dimethoxyflavone in mice following single dose oral administration. J. Pharm. Biomed. Anal. 2016, 119, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, bioavailability, tissue distribution, excretion, and metabolite identification of methoxyflavones in Kaempferia parviflora extract in rats. Drug Metab. Dispos. 2012, 40, 2342–2353. [Google Scholar] [CrossRef]
- Horigome, S.; Yoshida, I.; Ito, S.; Inohana, S.; Fushimi, K.; Nagai, T.; Yamaguchi, A.; Fujita, K.; Satoyama, T.; Katsuda, S.I.; et al. Inhibitory effects of Kaempferia parviflora extract on monocyte adhesion and cellular reactive oxygen species production in human umbilical vein endothelial cells. Eur. J. Nutr. 2017, 56, 949–964. [Google Scholar] [CrossRef]
- Varga, B.V.; Hádinger, N.; Gócza, E.; Dulberg, V.; Demeter, K.; Madarász, E.; Herberth, B. Generation of diverse neuronal subtypes in cloned populations of stem-like cells. BMC Dev. Biol. 2008, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hwang, J.K. The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients 2020, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.M.; Lee, S.; Hong, S.; Lee, S.; Jung, K.; Kang, K.S. Protective Effect of Polymethoxyflavones Isolated from Kaempferia parviflora against TNF-α-Induced Human Dermal Fibroblast Damage. Antioxidants 2021, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Jakhar, R.; Paul, S.; Park, Y.R.; Han, J.; Kang, S.C. 3,5,7,3′,4′-Pentamethoxyflavone, a quercetin derivative protects DNA from oxidative challenges: Potential mechanism of action. J. Photochem. Photobiol. B Biol. 2014, 131, 96–103. [Google Scholar] [CrossRef]


| Detected Compounds (mg/g Extract) | Crude Ethanolic Extract (KP1) | KP Fractions | |||
|---|---|---|---|---|---|
| Hexane (KP2) | Chloroform (KP3) | Ethyl Acetate (KP4) | Residue (KP5) | ||
| Caffeic acid | ND | ND | ND | 4.07 ± 0.91 | ND |
| Catechin | ND | ND | ND | ND | 15.6 ± 1.40 |
| Rutin | ND | ND | ND | 13.4 ± 2.80 | ND |
| DMF | 103.27 ± 1.15 | 8.75 ± 0.17 | 132.40 ± 0.01 | 2.90 ± 0.05 | ND |
| PMF | 123.95 ± 38.83 | 2.74 ± 0.34 | 156.64 ± 0.14 | 23.34 ± 0.02 | ND |
| TMF | 517.19 ± 2.10 | 35.19 ± 3.31 | 649.54 ± 6.67 | 76.09 ± 0.26 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temviriyanukul, P.; Chansawhang, A.; Karinchai, J.; Phochantachinda, S.; Buranasinsup, S.; Inthachat, W.; Pitchakarn, P.; Chantong, B. Kaempferia parviflora Extracts Protect Neural Stem Cells from Amyloid Peptide-Mediated Inflammation in Co-Culture Model with Microglia. Nutrients 2023, 15, 1098. https://doi.org/10.3390/nu15051098
Temviriyanukul P, Chansawhang A, Karinchai J, Phochantachinda S, Buranasinsup S, Inthachat W, Pitchakarn P, Chantong B. Kaempferia parviflora Extracts Protect Neural Stem Cells from Amyloid Peptide-Mediated Inflammation in Co-Culture Model with Microglia. Nutrients. 2023; 15(5):1098. https://doi.org/10.3390/nu15051098
Chicago/Turabian StyleTemviriyanukul, Piya, Anchana Chansawhang, Jirarat Karinchai, Sataporn Phochantachinda, Shutipen Buranasinsup, Woorawee Inthachat, Pornsiri Pitchakarn, and Boonrat Chantong. 2023. "Kaempferia parviflora Extracts Protect Neural Stem Cells from Amyloid Peptide-Mediated Inflammation in Co-Culture Model with Microglia" Nutrients 15, no. 5: 1098. https://doi.org/10.3390/nu15051098
APA StyleTemviriyanukul, P., Chansawhang, A., Karinchai, J., Phochantachinda, S., Buranasinsup, S., Inthachat, W., Pitchakarn, P., & Chantong, B. (2023). Kaempferia parviflora Extracts Protect Neural Stem Cells from Amyloid Peptide-Mediated Inflammation in Co-Culture Model with Microglia. Nutrients, 15(5), 1098. https://doi.org/10.3390/nu15051098






