Weight Maintenance after Dietary Weight Loss: Systematic Review and Meta-Analysis on the Effectiveness of Behavioural Intensive Intervention
Abstract
1. Introduction
2. Materials and Methods
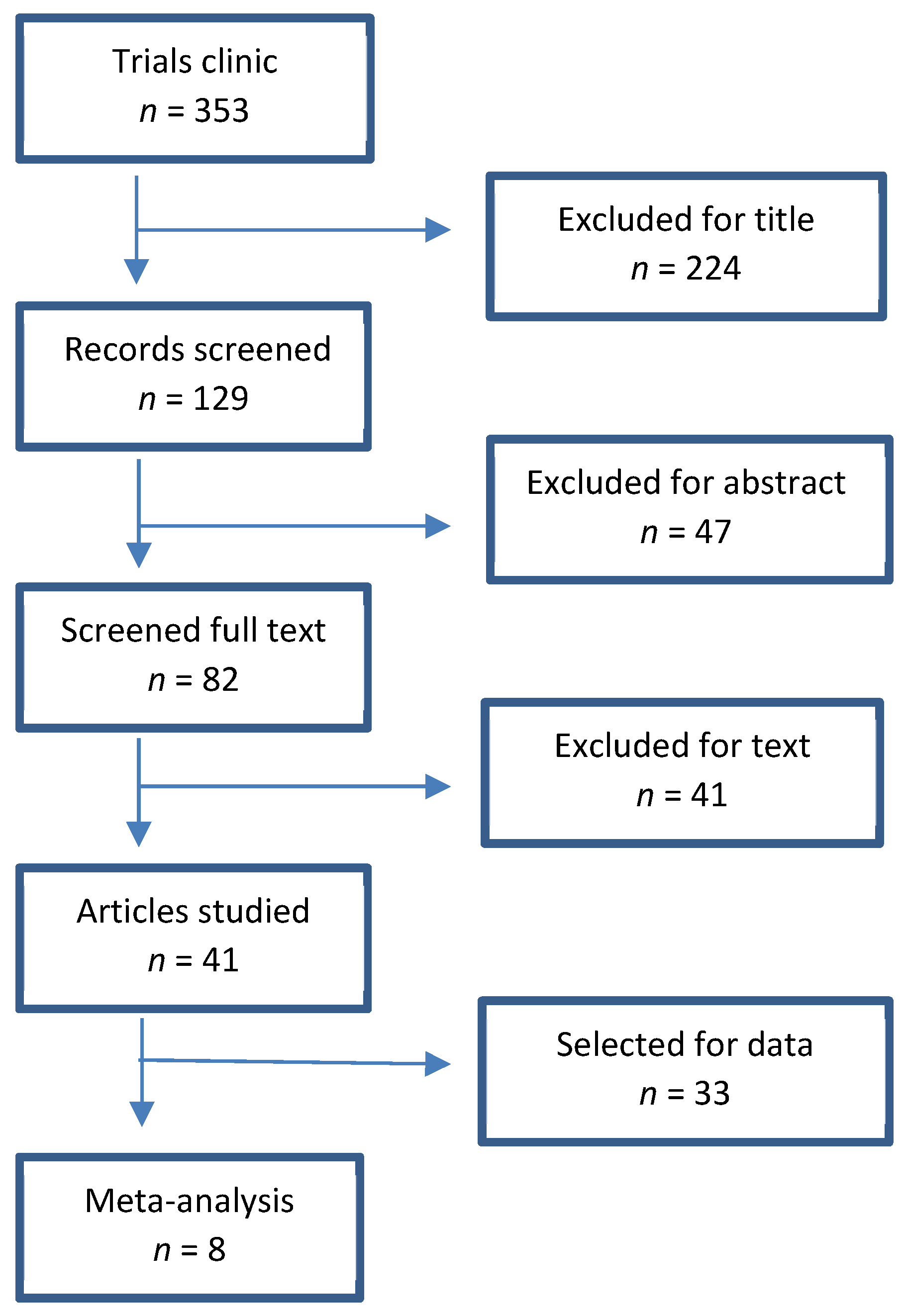
2.1. Research Strategy
2.2. Inclusion Criteria
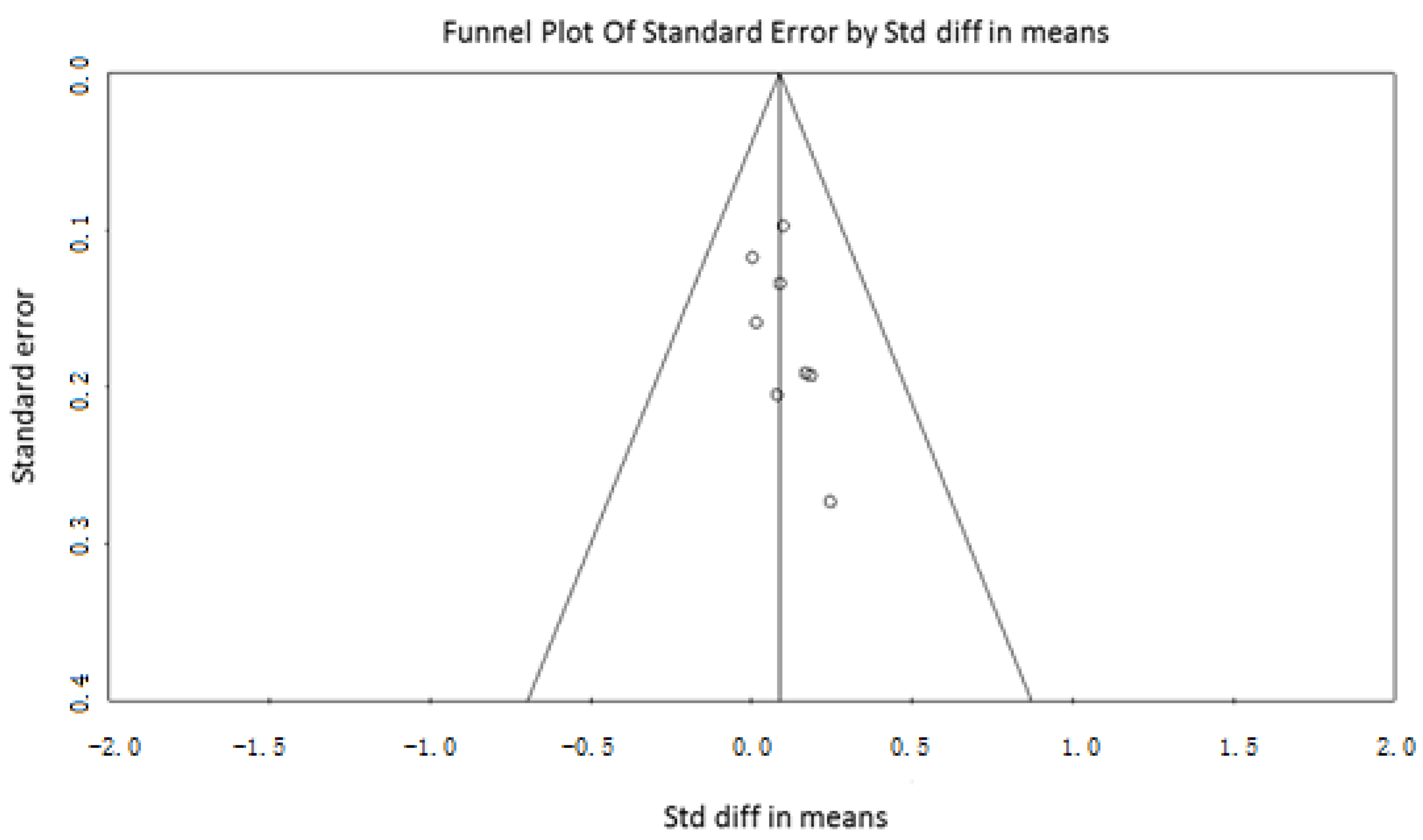
2.3. Data Extraction and Quality Assessment
3. Results
3.1. Characteristics of the Studies Included in the Meta-Analysis
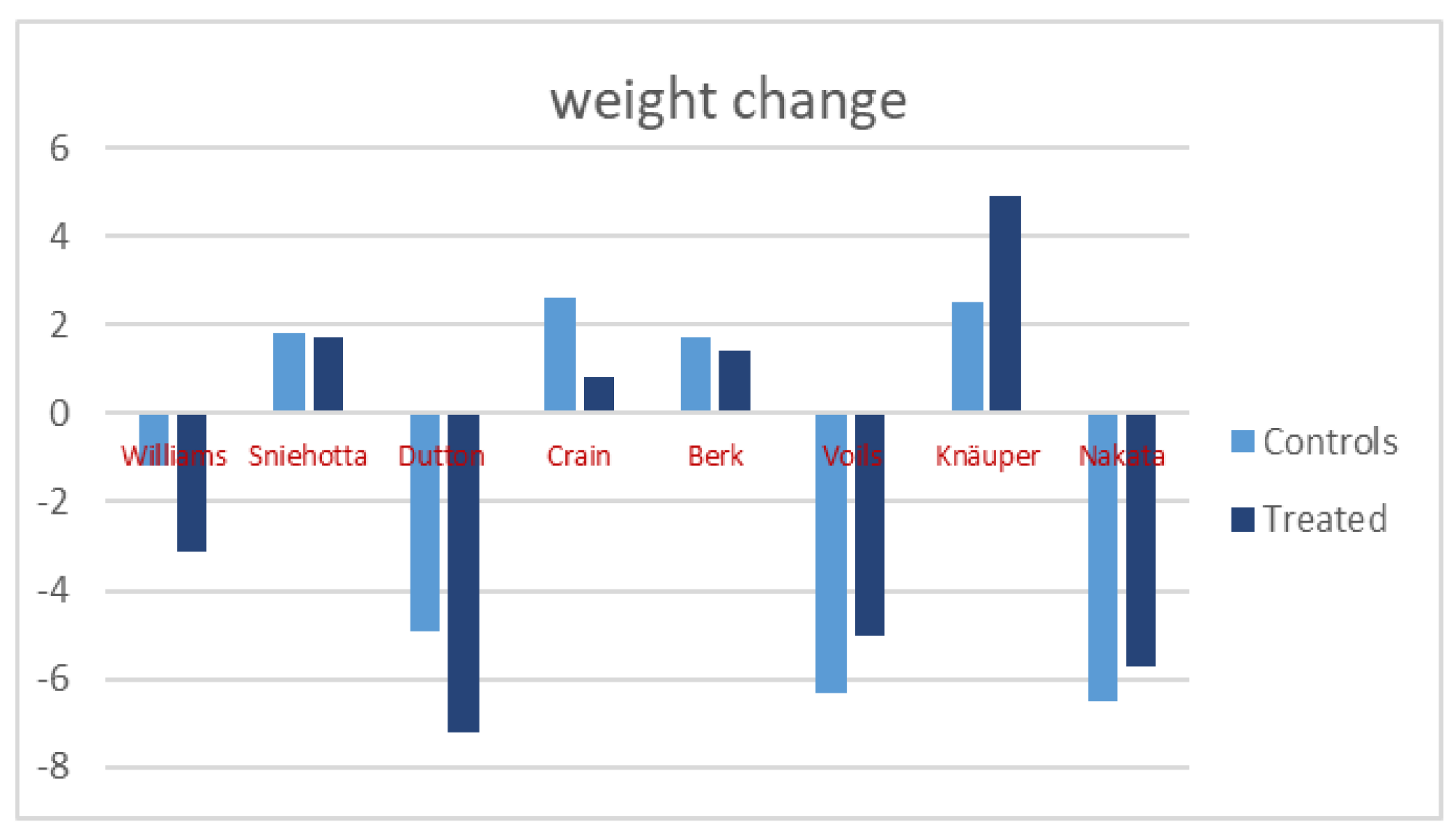
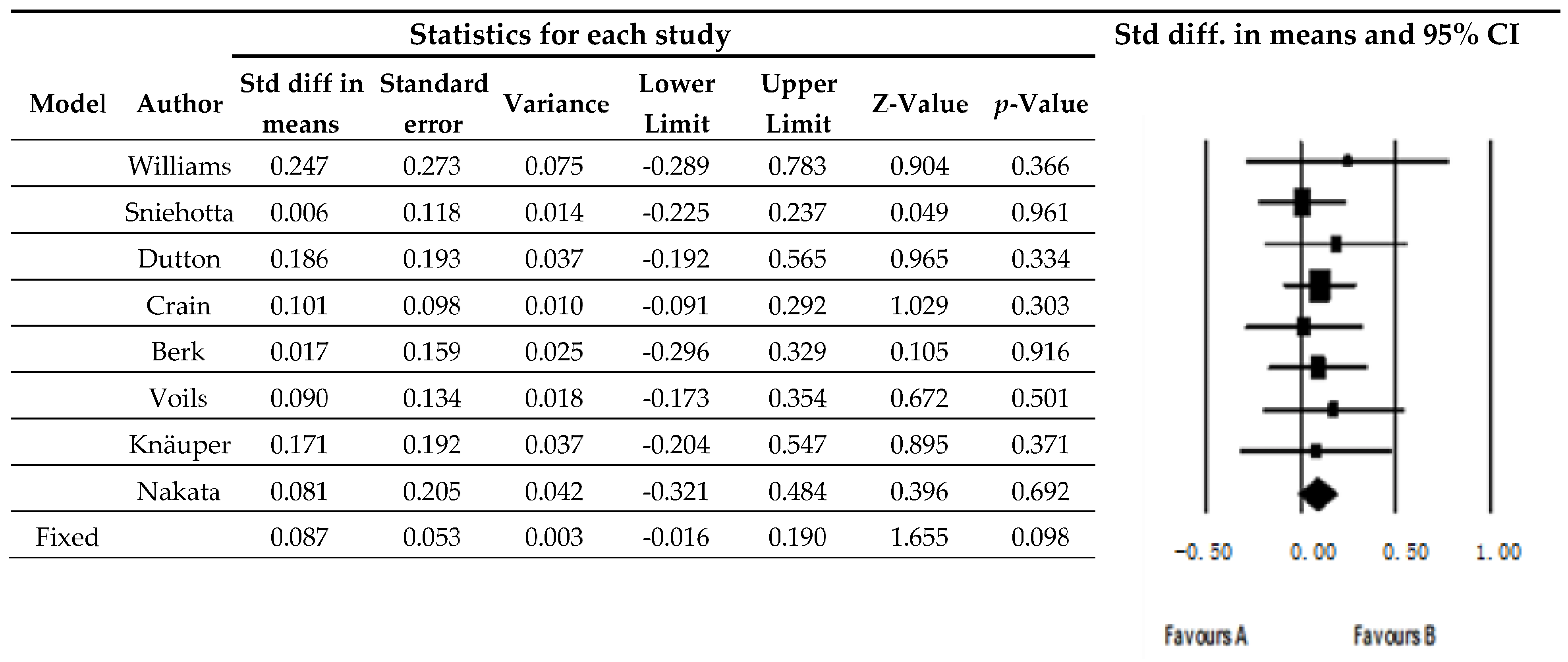
3.2. Weight Variations
4. Discussion
4.1. Behavioural Approach
4.2. Gender Difference
4.3. Physical Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Athayde, R.A.B.; de Oliveira Filho, J.R.B.; Lorenzi Filho, G.; Genta, P.R. Obesity Hypoventilation Syndrome: A Current Review. J. Bras. Pneumol. 2018, 44, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M. Osteoarthritis, Obesity and Type 2 Diabetes: The Weight of Waist Circumference. Ann. Phys. Rehabil. Med. 2016, 59, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk Factors, Complications, and Strategies for Sustainable Long-term Weight Management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Bomberg, E.; Birch, L.; Endenburg, N.; German, A.J.; Neilson, J.; Seligman, H.; Takashima, G.; Day, M.J. The Financial Costs, Behaviour and Psychology of Obesity: A One Health Analysis. J. Comp. Pathol. 2017, 156, 310–325. [Google Scholar] [CrossRef]
- Kim, D.D.; Basu, A. Estimating the Medical Care Costs of Obesity in the United States: Systematic Review, Meta-Analysis, and Empirical Analysis. Value Health 2016, 19, 602–613. [Google Scholar] [CrossRef]
- Pont, S.J.; Puhl, R.; Cook, S.R.; Slusser, W.; Section on obesity. The obesity society Stigma Experienced by Children and Adolescents with Obesity. Pediatrics 2017, 140, e20173034. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, P.; Mahalingam, K. Molecular Genetics of Human Obesity: A Comprehensive Review. Comptes. Rendus. Biol. 2017, 340, 87–108. [Google Scholar] [CrossRef]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota–Gut–Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut Microbiota Markers Associated with Obesity and Overweight in Italian Adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Hypothyroidism and Obesity: An Intriguing Link—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27366725/ (accessed on 7 April 2021).
- Valk, E.S.; Akker, E.L.T.; Savas, M.; Kleinendorst, L.; Visser, J.A.; Van Haelst, M.M.; Sharma, A.M.; Rossum, E.F.C. A Comprehensive Diagnostic Approach to Detect Underlying Causes of Obesity in Adults. Obes. Rev. 2019, 20, 795–804. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Pinna, F.; Sardu, C.; Orrù, W.; Velluzzi, F.; Loviselli, A.; Contu, P.; Carpiniello, B. Psychopathology, Psychosocial Factors and Obesity. Riv. Psichiatr. 2016, 51, 30–36. [Google Scholar]
- Apovian, C.M. Obesity: Definition, Comorbidities, Causes, and Burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Hanson, P.; Weickert, M.O.; Barber, T.M. Obesity: Novel and Unusual Predisposing Factors. Ther. Adv. Endocrinol. 2020, 11, 204201882092201. [Google Scholar] [CrossRef]
- Durrer Schutz, D.; Busetto, L.; Dicker, D.; Farpour-Lambert, N.; Pryke, R.; Toplak, H.; Widmer, D.; Yumuk, V.; Schutz, Y. European Practical and Patient-Centred Guidelines for Adult Obesity Management in Primary Care. Obes. Facts 2019, 12, 40–66. [Google Scholar] [CrossRef]
- Pisanu, S.; Palmas, V.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Boi, F.; Loviselli, A.; et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients 2020, 12, 2707. [Google Scholar] [CrossRef]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived Antioxidants and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef]
- Chin, S.-H.; Kahathuduwa, C.N.; Binks, M. Physical Activity and Obesity: What We Know and What We Need to Know*: Physical Activity and Obesity. Obes. Rev. 2016, 17, 1226–1244. [Google Scholar] [CrossRef]
- Greenway, F.L. Physiological Adaptations to Weight Loss and Factors Favouring Weight Regain. Int. J. Obes. 2015, 39, 1188–1196. [Google Scholar] [CrossRef]
- Thomas, J.G.; Bond, D.S.; Phelan, S.; Hill, J.O.; Wing, R.R. Weight-Loss Maintenance for 10 Years in the National Weight Control Registry. Am. J. Prev. Med. 2014, 46, 17–23. [Google Scholar] [CrossRef]
- Jiandani, D.; Wharton, S.; Rotondi, M.A.; Ardern, C.I.; Kuk, J.L. Predictors of Early Attrition and Successful Weight Loss in Patients Attending an Obesity Management Program. BMC Obes. 2016, 3, 14. [Google Scholar] [CrossRef]
- Goode, R.W.; Ye, L.; Sereika, S.M.; Zheng, Y.; Mattos, M.; Acharya, S.D.; Ewing, L.J.; Danford, C.; Hu, L.; Imes, C.C.; et al. Socio-Demographic, Anthropometric, and Psychosocial Predictors of Attrition across Behavioral Weight-Loss Trials. Eat. Behav. 2016, 20, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bardus, M.; van Beurden, S.B.; Smith, J.R.; Abraham, C. A Review and Content Analysis of Engagement, Functionality, Aesthetics, Information Quality, and Change Techniques in the Most Popular Commercial Apps for Weight Management. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Ramage, S.; Farmer, A.; Apps Eccles, K.; McCargar, L. Healthy Strategies for Successful Weight Loss and Weight Maintenance: A Systematic Review. Appl. Physiol. Nutr. Metab. 2014, 39, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Marchesini Reggiani, G.; Dalle Grave, R.; Centis, E.; Marzocchi, R.; El Ghoch, M. Major Factors for Facilitating Change in Behavioral Strategies to Reduce Obesity. Psychol. Res. Behav. Manag. 2013, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Burrata, L.; Reginato, E.; Ranucci, C.; Pippi, R.; Aiello, C.; Sbroma Tomaro, E.; Perrone, C.; Tirimagni, A.; Russo, A.; De Feo, P.; et al. Stage of Change and Motivation to a Healthier Lifestyle before and after an Intensive Lifestyle Intervention. J. Obes. 2016, 2016, 1–7. [Google Scholar] [CrossRef][Green Version]
- Michaud, A.; Vainik, U.; Garcia-Garcia, I.; Dagher, A. Overlapping Neural Endophenotypes in Addiction and Obesity. Front. Endocrinol. 2017, 8, 127. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Sartirana, M.; Calugi, S. Personalized Cognitive-Behavioural Therapy for Obesity (CBT-OB): Theory, Strategies and Procedures. Biopsychosoc. Med. 2020, 14, 5. [Google Scholar] [CrossRef]
- Marchesini, G.; Montesi, L.; El Ghoch, M.; Brodosi, L.; Calugi, S.; Dalle Grave, R. Long-Term Weight Loss Maintenance for Obesity: A Multidisciplinary Approach. Diabetes Síndrome Metabólico Obes. Obxectivos Ter. 2016, 9, 37–46. [Google Scholar] [CrossRef]
- Turk, M.W.; Yang, K.; Hravnak, M.; Sereika, S.M.; Ewing, L.J.; Burke, L.E. Randomized Clinical Trials of Weight Loss Maintenance: A Review. J. Cardiovasc. Nurs. 2009, 24, 58–80. [Google Scholar] [CrossRef]
- Kheniser, K.; Saxon, D.R.; Kashyap, S.R. Long-Term Weight Loss Strategies for Obesity. J. Clin. Endocrinol. Metab. 2021, 106, 1854–1866. [Google Scholar] [CrossRef]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of Weight-Loss Diets with Different Compositions of Fat, Protein, and Carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Dias, S.; Hoffmann, G. Impact of Long-Term Lifestyle Programmes on Weight Loss and Cardiovascular Risk Factors in Overweight/Obese Participants: A Systematic Review and Network Meta-Analysis. Syst. Rev. 2014, 3, 130. [Google Scholar] [CrossRef]
- Foright, R.M.; Presby, D.M.; Sherk, V.D.; Kahn, D.; Checkley, L.A.; Giles, E.D.; Bergouignan, A.; Higgins, J.A.; Jackman, M.R.; Hill, J.O.; et al. Is Regular Exercise an Effective Strategy for Weight Loss Maintenance? Physiol. Behav. 2018, 188, 86–93. [Google Scholar] [CrossRef]
- Ruban, A.; Stoenchev, K.; Ashrafian, H.; Teare, J. Current Treatments for Obesity. Clin. Med. 2019, 19, 205. [Google Scholar] [CrossRef]
- Deledda, A.; Pintus, S.; Loviselli, A.; Fosci, M.; Fantola, G.; Velluzzi, F. Nutritional Management in Bariatric Surgery Patients. Int. J. Environ. Res. Public Health 2021, 18, 12049. [Google Scholar] [CrossRef]
- Martin, B.R. Complementary Medicine Therapies That May Assist with Weight Loss: A Narrative Review. J. Chiropr. Med. 2019, 18, 115–126. [Google Scholar] [CrossRef]
- Jane, M.; Hagger, M.; Foster, J.; Ho, S.; Kane, R.; Pal, S. Effects of a Weight Management Program Delivered by Social Media on Weight and Metabolic Syndrome Risk Factors in Overweight and Obese Adults: A Randomised Controlled Trial. PLoS ONE 2017, 12, e0178326. [Google Scholar] [CrossRef]
- Paixão, C.; Dias, C.M.; Jorge, R.; Carraça, E.V.; Yannakoulia, M.; Zwaan, M.; Soini, S.; Hill, J.O.; Teixeira, P.J.; Santos, I. Successful Weight Loss Maintenance: A Systematic Review of Weight Control Registries. Obes. Rev. 2020, 21, e13003. [Google Scholar] [CrossRef]
- Doherty, A.J.; Jones, S.P.; Chauhan, U.; Gibson, J.M.E. An Integrative Review of Multicomponent Weight Management Interventions for Adults with Intellectual Disabilities. J. Appl. Res. Intellect Disabil. 2018, 31, 39–51. [Google Scholar] [CrossRef]
- Williams, L.T.; Collins, C.E.; Morgan, P.J.; Hollis, J.L. Maintaining the Outcomes of a Successful Weight Gain Prevention Intervention in Mid-Age Women: Two Year Results from the 40-Something Randomized Control Trial. Nutrients 2019, 11, 1100. [Google Scholar] [CrossRef]
- Sniehotta, F.F.; Evans, E.H.; Sainsbury, K.; Adamson, A.; Batterham, A.; Becker, F.; Brown, H.; Dombrowski, S.U.; Jackson, D.; Howell, D.; et al. Behavioural Intervention for Weight Loss Maintenance versus Standard Weight Advice in Adults with Obesity: A Randomised Controlled Trial in the UK (NULevel Trial). PLoS Med. 2019, 16, e1002793. [Google Scholar] [CrossRef]
- Nakata, Y. Web-Based Intervention to Promote Weight-Loss Maintenance Using an Activity Monitor: A Randomized Controlled Trial. Prev. Med. Rep. 2019, 14, 100839. [Google Scholar] [CrossRef]
- Dutton, G.R.; Gowey, M.A.; Tan, F.; Zhou, D.; Ard, J.; Perri, M.G.; Lewis, C.E. Comparison of an Alternative Schedule of Extended Care Contacts to a Self-Directed Control: A Randomized Trial of Weight Loss Maintenance. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 107. [Google Scholar] [CrossRef]
- Crain, A.L.; Sherwood, N.E.; Martinson, B.C.; Jeffery, R.W. Mediators of Weight Loss Maintenance in the Keep It Off Trial. Ann. Behav. Med. 2018, 52, 9–18. [Google Scholar] [CrossRef]
- Berk, K.A.; Buijks, H.I.M.; Verhoeven, A.J.M.; Mulder, M.T.; Özcan, B.; van ’t Spijker, A.; Timman, R.; Busschbach, J.J.; Sijbrands, E.J. Group Cognitive Behavioural Therapy and Weight Regain after Diet in Type 2 Diabetes: Results from the Randomised Controlled POWER Trial. Diabetologia 2018, 61, 790–799. [Google Scholar] [CrossRef]
- Voils, C.I.; Olsen, M.K.; Gierisch, J.M.; McVay, M.A.; Grubber, J.M.; Gaillard, L.; Bolton, J.; Maciejewski, M.L.; Strawbridge, E.; Yancy, W.S. Maintenance of Weight Loss After Initiation of Nutrition Training: A Randomized Trial. Ann. Intern. Med. 2017, 166, 463. [Google Scholar] [CrossRef]
- McGill CHIP Healthy Weight Program Investigators; Knäuper, B.; Shireen, H.; Carrière, K.; Frayn, M.; Ivanova, E.; Xu, Z.; Lowensteyn, I.; Sadikaj, G.; Luszczynska, A.; et al. The Effects of If-Then Plans on Weight Loss: Results of the 24-Month Follow-up of the McGill CHIP Healthy Weight Program Randomized Controlled Trial. Trials 2020, 21, 40. [Google Scholar] [CrossRef]
- Santos, I.; Sniehotta, F.F.; Marques, M.M.; Carraça, E.V.; Teixeira, P.J. Prevalence of Personal Weight Control Attempts in Adults: A Systematic Review and Meta-Analysis. Obes. Rev. 2017, 18, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.J. Motivation, Self-Determination, and Long-Term Weight Control. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Avenell, A.; Stewart, F.; Archibald, D.; Douglas, F.; Hoddinott, P.; van Teijlingen, E.; Boyers, D. Clinical Effectiveness of Weight Loss and Weight Maintenance Interventions for Men: A Systematic Review of Men-Only Randomized Controlled Trials (The ROMEO Project). Am. J. Mens Health 2017, 11, 1096–1123. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.T.; Wolin, K.Y.; Scharoun-Lee, M.; Ding, E.L.; Warner, E.T.; Bennett, G.G. Does Perception Equal Reality? Weight Misperception in Relation to Weight-Related Attitudes and Behaviors among Overweight and Obese US Adults. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 20. [Google Scholar] [CrossRef]
- Gregory, C.O.; Blanck, H.M.; Gillespie, C.; Maynard, L.M.; Serdula, M.K. Perceived Health Risk of Excess Body Weight among Overweight and Obese Men and Women: Differences by Sex. Prev. Med. 2008, 47, 46–52. [Google Scholar]
- Del Mar Bibiloni, M.; Coll, J.L.; Pich, J.; Pons, A.; Tur, J.A. Body Image Satisfaction and Weight Concerns among a Mediterranean Adult Population. BMC Public Health 2017, 17, 39. [Google Scholar] [CrossRef]
- Tsai, S.A.; Lv, N.; Xiao, L.; Ma, J. Gender Differences in Weight-Related Attitudes and Behaviors Among Overweight and Obese Adults in the United States. Am. J. Mens Health 2016, 10, 389–398. [Google Scholar] [CrossRef]
- Brierley, M.-E.; Brooks, K.R.; Mond, J.; Stevenson, R.J.; Stephen, I.D. The Body and the Beautiful: Health, Attractiveness and Body Composition in Men’s and Women’s Bodies. PLoS ONE 2016, 11, e0156722. [Google Scholar] [CrossRef]
- Poulimeneas, D.; Maraki, M.I.; Karfopoulou, E.; Koutras, Y.; Chrysostomou, S.; Anastasiou, C.A.; Kavouras, S.A.; Yannakoulia, M. Sex-Specific Physical Activity Patterns Differentiate Weight Loss Maintainers from Regainers: The MedWeight Study. J. Phys. Act. Health 2020, 17, 225–229. [Google Scholar] [CrossRef]
- Crane, M.M.; Jeffery, R.W.; Sherwood, N.E. Exploring Gender Differences in a Randomized Trial of Weight Loss Maintenance. Am. J. Mens Health 2017, 11, 369–375. [Google Scholar] [CrossRef]
- Young, M.D.; Callister, R.; Collins, C.E.; Plotnikoff, R.C.; Aguiar, E.J.; Morgan, P.J. Efficacy of a Gender-Tailored Intervention to Prevent Weight Regain in Men over 3 Years: A Weight Loss Maintenance RCT: Weight Loss Maintenance Program for Men. Obesity 2017, 25, 56–65. [Google Scholar] [CrossRef]
- Verboven, K.; Hansen, D. Critical Reappraisal of the Role and Importance of Exercise Intervention in the Treatment of Obesity in Adults. Sports Med. 2021, 51, 379–389. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001512-8. [Google Scholar]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]
- Pisanu, S.; Deledda, A.; Loviselli, A.; Huybrechts, I.; Velluzzi, F. Validity of Accelerometers for the Evaluation of Energy Expenditure in Obese and Overweight Individuals: A Systematic Review. J. Nutr. Metab. 2020, 2020, 2327017. [Google Scholar] [CrossRef]
- Unick, J.L.; Gaussoin, S.A.; Hill, J.O.; Jakicic, J.M.; Bond, D.S.; Hellgren, M.; Johnson, K.C.; Peters, A.L.; Coday, M.; Kitzman, D.W.; et al. Objectively Assessed Physical Activity and Weight Loss Maintenance among Individuals Enrolled in a Lifestyle Intervention: Physical Activity and Weight Loss Maintenance. Obesity 2017, 25, 1903–1909. [Google Scholar] [CrossRef]
- Kerns, J.C.; Guo, J.; Fothergill, E.; Howard, L.; Knuth, N.D.; Brychta, R.; Chen, K.Y.; Skarulis, M.C.; Walter, P.J.; Hall, K.D. Increased Physical Activity Associated with Less Weight Regain Six Years After “The Biggest Loser” Competition: Physical Activity and Weight Regain. Obesity 2017, 25, 1838–1843. [Google Scholar] [CrossRef]
- Eaton, C.B.; Hartman, S.J.; Perzanowski, E.; Pan, G.; Roberts, M.B.; Risica, P.M.; Gans, K.M.; Jakicic, J.M.; Marcus, B.H. A Randomized Clinical Trial of a Tailored Lifestyle Intervention for Obese, Sedentary, Primary Care Patients. Ann. Fam. Med. 2016, 14, 311–319. [Google Scholar] [CrossRef]
- Coleman, K.J.; Caparosa, S.L.; Nichols, J.F.; Fujioka, K.; Koebnick, C.; McCloskey, K.N.; Xiang, A.H.; Ngor, E.W.; Levy, S.S. Understanding the Capacity for Exercise in Post-Bariatric Patients. Obes. Surg. 2017, 27, 51–58. [Google Scholar] [CrossRef]
- Gray, C.M.; Wyke, S.; Zhang, R.; Anderson, A.S.; Barry, S.; Boyer, N.; Brennan, G.; Briggs, A.; Bunn, C.; Donnachie, C.; et al. Long-Term Weight Loss Trajectories Following Participation in a Randomised Controlled Trial of a Weight Management Programme for Men Delivered through Professional Football Clubs: A Longitudinal Cohort Study and Economic Evaluation. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 60. [Google Scholar] [CrossRef]
- Strasser, B.; Fuchs, D. Diet versus Exercise in Weight Loss and Maintenance: Focus on Tryptophan. Int. J. Tryptophan. Res. 2016, 9, IJTR.S33385. [Google Scholar] [CrossRef]
- Dorling, J.; Broom, D.; Burns, S.; Clayton, D.; Deighton, K.; James, L.; King, J.; Miyashita, M.; Thackray, A.; Batterham, R.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef]
- Annesi, J.J.; Unruh-Rewkowski, J.L.; Mareno, N. Replication and Extension of the Weight Loss for Life Community-Based Treatment Protocol. Behav. Med. 2018, 44, 54–61. [Google Scholar] [CrossRef]
- Annesi, J.; Walsh, S. Evaluation of a new causal chain model for predicting embedded psychosocial and behavioral relationships in a community-based obesitytreatment seeking maintained weight loss. Scand. J. Psychol. 2021, 62, 574–585. [Google Scholar] [CrossRef]
| Author | Country | Year | Patients (Females) | Previous Weight Loss | Intervention Group Protocol | Control Group Protocol |
|---|---|---|---|---|---|---|
| Williams | Australia | 2019 | 54 (54) | ** | Nutritional and sport consultations plus a paper support | Paper material |
| Sniehotta | England (Europe) | 2019 | 288 (223) | ** | Portal where patients entered weight, physical activity, and food diary followed by a comment | SMS every 3 months |
| Dutton | USA | 2017 | 108 (103) | * | Group counselling sessions on diet, physical activity, and strategies to manage weight maintenance | Printed material and phone/mail counselling on request |
| Crain | USA | 2018 | 419 (342) | ** | More frequent phone calls encouraging for physical activity monitoring | Less frequent phone calls plus a book |
| Berk | Holland (Europe) | 2018 | 158 (88) | * | Group sessions of cognitive behaviour therapy with decreasing frequency | Usual care for diabetes |
| Voils | USA | 2018 | 222 (34) | * | Group sessions alternated with phone calls for psychological and practical support | No support |
| Knauper | Canada | 2020 | 110 (87) | * | Group sessions for nutritional and physical activity support plus an if-then plan to manage at-risk situations | No if-then plan |
| Nakata | Japan | 2019 | 95 (59) | * | Daily monitoring of weight and physical activity via the Web | No support |
| Author | Number Patients | Age (Mean ± SD) | Weight of Treated (Mean ± SD) | Weight of Controls (Mean ± SD) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Treated | Controls | Treated | Controls | T0 | T12 | T0 | T12 | |
| Williams | 54 | 28 | 26 | 47.3 ± 1.8 | 47.3 ± 1.8 | 68.7 ± 8.9 | 65.6 ± 8.5 | 68.6 ± 6.7 | 67.4 ± 6.7 |
| Sniehotta | 288 | 144 | 144 | 42.0 ± 11.6 | 41.6 ± 11.4 | 85.1 ± 17.5 | 86.8 ± 18.2 | 85.2 ± 15.7 | 87 ± 16.7 |
| Dutton | 108 | 52 | 56 | 52.13 ± 11.75 | 51.18 ± 14.22 | 92.4 ± 13.8 | 85.2 ± 13.2 | 92.7 ± 12.9 | 87.8 ± 11.7 |
| Crain | 419 | 209 | 210 | 46.6 ± 10.9 | 46.3 ± 10.3 | 80.1 ± 15.8 | 80.9 ± 16.7 | 79.4 ± 16.6 | 82 ± 17.9 |
| Berk | 158 | 83 | 75 | 52.3 ± 11.3 | 55.2 ± 9.3 | 96.3 ± 19.3 | 97.7 ± 20 | 96.8 ± 22.5 | 98.5 ± 15.3 |
| Voils | 222 | 110 | 112 | 61.5 ± 8.3 | 62.0 ± 8.3 | 109.3 ± 19.5 | 104.3 ± 13.9 | 112.2 ± 21.7 | 105.9 ± 14.8 |
| Knäuper | 110 | 51 | 59 | 50.22 ± 11.97 | 50.22 ± 11.97 | 80.8 ± 12.7 | 85.7 ± 14.4 | 85.8 ± 12.9 | 88.3 ± 13.4 |
| Nakata | 95 | 47 | 48 | 54.7 ± 6.6 | 57.0 ± 5.7 | 74.7 ± 10.6 | 69 ± 10.9 | 74.2 ± 8.1 | 67.7 ± 8.7 |
| Study Quality-Assessment Tools | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Williams | 2019 | YES | YES | YES | YES | YES | NO | YES | NS | NS | YES | YES | YES | YES | YES |
| Sniehotta | 2019 | YES | YES | YES | YES | YES | YES | YES | YES | YES | NS | YES | NO | YES | YES |
| Dutton | 2017 | YES | YES | YES | NO | NO | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| Crain | 2018 | YES | YES | NO | NO | NO | YES | YES | NS | YES | YES | YES | NO | YES | NS |
| Berk | 2018 | YES | NS | NS | YES | YES | YES | NO | NO | YES | YES | YES | YES | YES | YES |
| Voils | 2018 | YES | YES | NS | NO | NS | YES | YES | NS | NS | YES | YES | YES | YES | NS |
| Knäuper | 2020 | YES | YES | NS | NO | YES | NS | NO | YES | NS | YES | YES | NS | NS | NS |
| Nakata | 2019 | YES | YES | YES | NO | NS | YES | YES | NS | NS | YES | YES | NS | YES | YES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flore, G.; Preti, A.; Carta, M.G.; Deledda, A.; Fosci, M.; Nardi, A.E.; Loviselli, A.; Velluzzi, F. Weight Maintenance after Dietary Weight Loss: Systematic Review and Meta-Analysis on the Effectiveness of Behavioural Intensive Intervention. Nutrients 2022, 14, 1259. https://doi.org/10.3390/nu14061259
Flore G, Preti A, Carta MG, Deledda A, Fosci M, Nardi AE, Loviselli A, Velluzzi F. Weight Maintenance after Dietary Weight Loss: Systematic Review and Meta-Analysis on the Effectiveness of Behavioural Intensive Intervention. Nutrients. 2022; 14(6):1259. https://doi.org/10.3390/nu14061259
Chicago/Turabian StyleFlore, Giovanna, Antonio Preti, Mauro Giovanni Carta, Andrea Deledda, Michele Fosci, Antonio Egidio Nardi, Andrea Loviselli, and Fernanda Velluzzi. 2022. "Weight Maintenance after Dietary Weight Loss: Systematic Review and Meta-Analysis on the Effectiveness of Behavioural Intensive Intervention" Nutrients 14, no. 6: 1259. https://doi.org/10.3390/nu14061259
APA StyleFlore, G., Preti, A., Carta, M. G., Deledda, A., Fosci, M., Nardi, A. E., Loviselli, A., & Velluzzi, F. (2022). Weight Maintenance after Dietary Weight Loss: Systematic Review and Meta-Analysis on the Effectiveness of Behavioural Intensive Intervention. Nutrients, 14(6), 1259. https://doi.org/10.3390/nu14061259








