Efficacy of Probiotics in Rheumatoid Arthritis and Spondyloarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
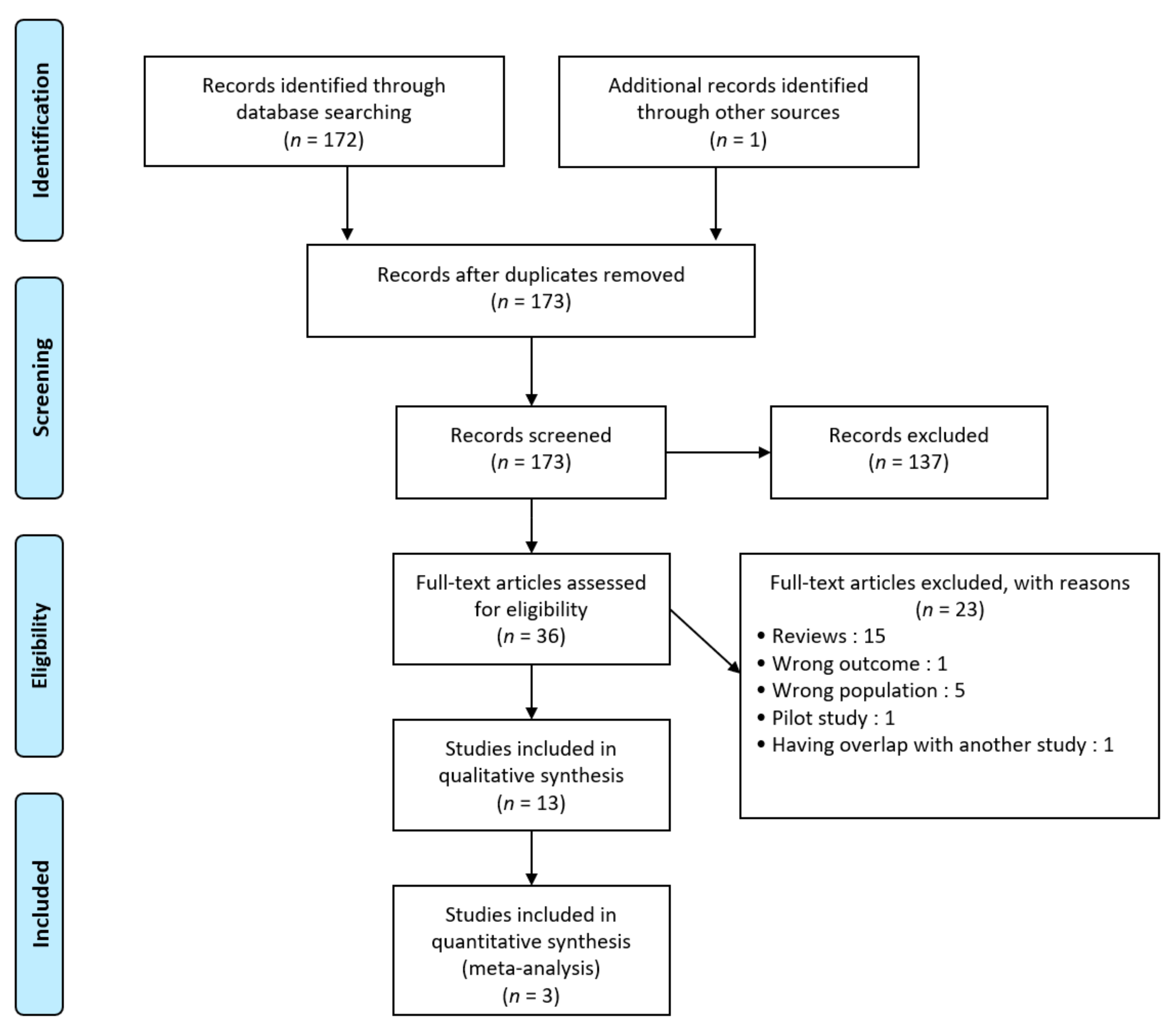
3.1. Study Selection
3.2. Study Characteristics and Search Results
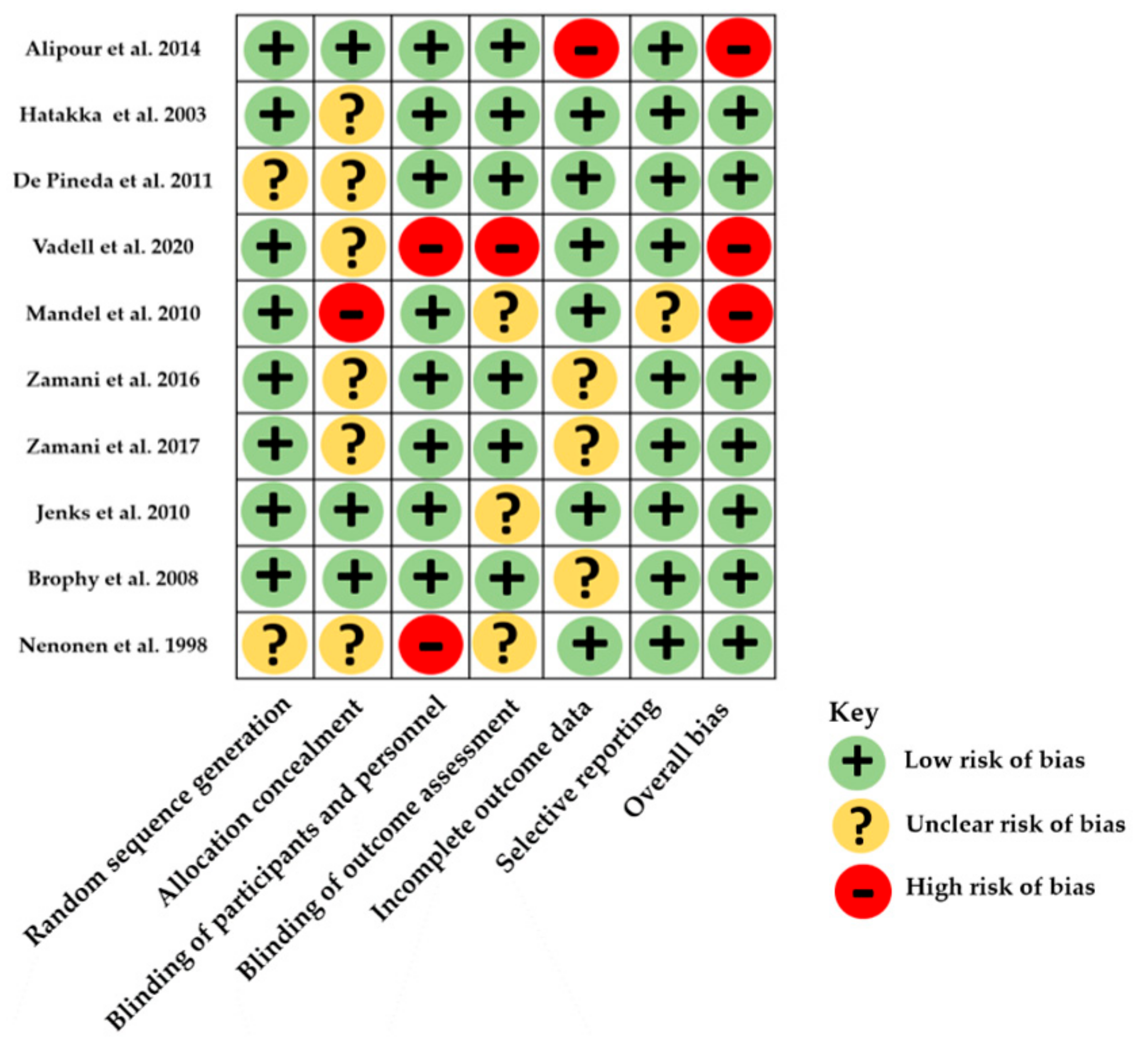
3.3. Risk of Bias within Studies
3.4. Outcomes
3.4.1. Results in Rheumatoid Arthritis
- DAS28
- Inflammatory markers
- TJC
- SJC
- HAQ
3.4.2. Results in Spondyloarthritis
3.5. Tolerance Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization; World Health Organization. Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Acid Bacteria; Report of a Joint FAO/WHO Expert Consultation; FAO Food and Nutrition Paper; World Health Organization: Cordoba, Argentina, 2001; Volume 85, pp. 5–35. [Google Scholar]
- Wang, P. Probiotic bacteria: A viable adjuvant therapy for relieving symptoms of rheumatoid arthritis. Inflammopharmacology 2016, 24, 189–196. [Google Scholar] [CrossRef]
- Diamanti, A.P.; Manuela Rosado, M.; Laganà, B.; D’Amelio, R. Microbiota and Chronic Inflammatory Arthritis: An Interwoven Link. J. Transl. Med. 2016, 14, 233. [Google Scholar] [CrossRef] [Green Version]
- Mielants, H.; De Vos, M.; Cuvelier, C.; Veys, E.M. The Role of Gut Inflammation in the Pathogenesis of Spondyloarthropathies. Acta Clin. Belg. 1996, 51, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Tlaskalová-Hogenová, H.; Štepánková, R.; Hudcovic, T.; Tucková, L.; Cukrowska, B.; Lodinová-Žádnıková, R.; Kozáková, H.; Rossmann, P.; Bártová, J.; Sokol, D. Commensal Bacteria (Normal Microflora), Mucosal Immunity and Chronic Inflammatory and Autoimmune Diseases. Immunol. Lett. 2004, 93, 97–108. [Google Scholar] [CrossRef]
- Sartor, R.B. Review Article: Role of the Enteric Microflora in the Pathogenesis of Intestinal Inflammation and Arthritis. Aliment Pharmacol. Ther. 1997, 11 (Suppl. 3), 17–22; discussion 22–23. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, J.; Lydyard, P.M.; Rook, G.A.W. Rheumatoid arthritis: How well do the theories fit the evidence? Clin. Exp. Immunol. 1993, 92, 1–6. [Google Scholar] [CrossRef]
- Danning, C.L.; Boumpas, D.T. Commonly Used Disease-Modifying Antirheumatic Drugs in the Treatment of Inflammatory Arthritis: An Update on Mechanisms of Action. Clin. Exp. Rheumatol. 1998, 16, 595–604. [Google Scholar] [PubMed]
- Delcenserie, V.; Martel, D.; Lamoureux, M.; Amiot, J.; Boutin, Y.; Roy, D. Immunomodulatory Effects of Probiotics in the Intestinal Tract. Curr. Issues Mol. Biol. 2008, 10, 37–54. [Google Scholar]
- Kano, H.; Kaneko, T.; Kaminogawa, S. Oral Intake of Lactobacillus Delbrueckii Subsp. Bulgaricus OLL1073R-1 Prevents Collagen-Induced Arthritis in Mice. J. Food Prot. 2002, 65, 153–160. [Google Scholar] [CrossRef] [PubMed]
- So, J.-S.; Kwon, H.-K.; Lee, C.-G.; Yi, H.-J.; Park, J.-A.; Lim, S.-Y.; Hwang, K.-C.; Jeon, Y.H.; Im, S.-H. Lactobacillus Casei Suppresses Experimental Arthritis by Down-Regulating T Helper 1 Effector Functions. Mol. Immunol. 2008, 45, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Strowski, M.Z.; Wiedenmann, B. Probiotic Carbohydrates Reduce Intestinal Permeability and Inflammation in Metabolic Diseases. Gut 2009, 58, 1044–1045. [Google Scholar] [CrossRef] [PubMed]
- Daien, C.; Czernichow, S.; Letarouilly, J.G.; Nguyen, Y.; Sanchez, P.; Sigaux, J. Dietary Recommendations of the French Society for Rheumatology for Patients with Chronic Inflammatory Rheumatic Diseases. Jt. Bone Spine 2021, 89, 105319. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 23 November 2021).
- Appendix: Jadad Scale for Reporting Randomized Controlled Trials. In Evidence-Based Obstetric Anesthesia; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 237–238. ISBN 978-0-470-98834-3.
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.-K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic Supplementation Improves Inflammatory Status in Patients with Rheumatoid Arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; Vaghef-Mehrabany, L.; Asghari-Jafarabadi, M.; Nakhjavani, M.R.; Mohtadi-Nia, J. Effects of Lactobacillus Casei Supplementation on Disease Activity and Inflammatory Cytokines in Rheumatoid Arthritis Patients: A Randomized Double-Blind Clinical Trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Martio, J.; Korpela, M.; Herranen, M.; Poussa, T.; Laasanen, T.; Saxelin, M.; Vapaatalo, H.; Moilanen, E.; Korpela, R. Effects of Probiotic Therapy on the Activity and Activation of Mild Rheumatoid Arthritis—A Pilot Study. Scand. J. Rheumatol. 2003, 32, 211–215. [Google Scholar] [CrossRef]
- Vadell, A.K.E.; Bärebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Anti-Inflammatory Diet In Rheumatoid Arthritis (ADIRA)—A Randomized, Controlled Crossover Trial Indicating Effects on Disease Activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef] [Green Version]
- Pineda, M.d.L.A.; Thompson, S.F.; Summers, K.; de Leon, F.; Pope, J.; Reid, G. A Randomized, Double-Blinded, Placebo-Controlled Pilot Study of Probiotics in Active Rheumatoid Arthritis. Med. Sci. Monit. 2011, 17, CR347–CR354. [Google Scholar] [CrossRef] [Green Version]
- Nenonen, M.T.; Helve, T.A. Uncooked, lactobacilli-rich, vegan food and rheumatoid arthritis. Rheumatology 1998, 37, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Mandel, D.R.; Eichas, K.; Holmes, J. Bacillus Coagulans: A Viable Adjunct Therapy for Relieving Symptoms of Rheumatoid Arthritis According to a Randomized, Controlled Trial. BMC Complementary Altern. Med. 2010, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Zamani, B.; Golkar, H.R.; Farshbaf, S.; Emadi-Baygi, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akhavan, R.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and Metabolic Response to Probiotic Supplementation in Patients with Rheumatoid Arthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Rheum. Dis. 2016, 19, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Zamani, B.; Farshbaf, S.; Golkar, H.R.; Bahmani, F.; Asemi, Z. Synbiotic Supplementation and the Effects on Clinical and Metabolic Responses in Patients with Rheumatoid Arthritis: A Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Nutr. 2017, 117, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Jenks, K.; Stebbings, S.; Burton, J.; Schultz, M.; Herbison, P.; Highton, J. Probiotic Therapy for the Treatment of Spondyloarthritis: A Randomized Controlled Trial. J. Rheumatol. 2010, 37, 2118–2125. [Google Scholar] [CrossRef]
- Brophy, S.; Burrows, C.L.; Brooks, C.; Gravenor, M.B.; Siebert, S.; Allen, S.J. Internet-Based Randomised Controlled Trials for the Evaluation of Complementary and Alternative Medicines: Probiotics in Spondyloarthropathy. BMC Musculoskelet. Disord. 2008, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, J.R.; Briggs, A.M.; Whittle, S.; Stephenson, M.D. A Systematic Review of the Effects of Probiotic Administration in Inflammatory Arthritis. Complementary Ther. Clin. Pract. 2020, 40, 101207. [Google Scholar] [CrossRef]
- Aqaeinezhad Rudbane, S.M.; Rahmdel, S.; Abdollahzadeh, S.M.; Zare, M.; Bazrafshan, A.; Mazloomi, S.M. The Efficacy of Probiotic Supplementation in Rheumatoid Arthritis: A Meta-Analysis of Randomized, Controlled Trials. Inflammopharmacology 2018, 26, 67–76. [Google Scholar] [CrossRef]
- Mohammed, A.T.; Khattab, M.; Ahmed, A.M.; Turk, T.; Sakr, N.; Khalil, A.M.; Abdelhalim, M.; Sawaf, B.; Hirayama, K.; Huy, N.T. The Therapeutic Effect of Probiotics on Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Randomized Control Trials. Clin. Rheumatol. 2017, 36, 2697–2707. [Google Scholar] [CrossRef]
- Shukla, A.; Gaur, P.; Aggarwal, A. Effect of Probiotics on Clinical and Immune Parameters in Enthesitis-Related Arthritis Category of Juvenile Idiopathic Arthritis. Clin. Exp. Immunol. 2016, 185, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Dixon, A.; Robertson, K.; Yung, A.; Que, M.; Randall, H.; Wellalagodage, D.; Cox, T.; Robertson, D.; Chi, C.; Sun, J. Efficacy of Probiotics in Patients of Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis. Curr. Hypertens. Rep. 2020, 22, 74. [Google Scholar] [CrossRef]
- Rosenfeldt, V.; Benfeldt, E.; Valerius, N.H.; Paerregaard, A.; Michaelsen, K.F. Effect of Probiotics on Gastrointestinal Symptoms and Small Intestinal Permeability in Children with Atopic Dermatitis. J. Pediatr. 2004, 145, 612–616. [Google Scholar] [CrossRef]
- Berg, R.D. Bacterial Translocation from the Intestines. Jikken Dobutsu 1985, 34, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baharav, E.; Mor, F.; Halpern, M.; Weinberger, A. Lactobacillus GG Bacteria Ameliorate Arthritis in Lewis Rats. J. Nutr. 2004, 134, 1964–1969. [Google Scholar] [CrossRef]
- Malin, M.; Verronen, P.; Korhonen, H.; Syväoja, E.L.; Salminen, S.; Mykkänen, H.; Arvilommi, H.; Eerola, E.; Isolauri, E. Dietary Therapy with Lactobacillus GG, Bovine Colostrum or Bovine Immune Colostrum in Patients with Juvenile Chronic Arthritis: Evaluation of Effect on Gut Defence Mechanisms. Inflammopharmacology 1997, 5, 219–236. [Google Scholar] [CrossRef]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential Uses of Probiotics in Clinical Practice. Clin. Microbiol. Rev. 2003, 16, 658–672. [Google Scholar] [CrossRef] [Green Version]
- Nowak, B.; Ciszek-Lenda, M.; Sróttek, M.; Gamian, A.; Kontny, E.; Górska-Frączek, S.; Marcinkiewicz, J. Lactobacillus Rhamnosus Exopolysaccharide Ameliorates Arthritis Induced by the Systemic Injection of Collagen and Lipopolysaccharide in DBA/1 Mice. Arch. Immunol. Ther. Exp. 2012, 60, 211–220. [Google Scholar] [CrossRef]
- Brusca, S.B.; Abramson, S.B.; Scher, J.U. Microbiome and Mucosal Inflammation as Extra-Articular Triggers for Rheumatoid Arthritis and Autoimmunity. Curr. Opin. Rheumatol. 2014, 26, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An Expansion of Rare Lineage Intestinal Microbes Characterizes Rheumatoid Arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Country | Inclusion Criteria | Groups | Age (Years) Mean (SD) | Disease Duration (Years) Mean (SD) | RF + N (%) | ACPA + N (%) | Activity Score Mean (SD) | Current Medication | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| csDMARDs N (%) | bDMARDs N (%) | Oral CS N (%) | NSAIDs N (%) | |||||||||
| Rheumatoid arthritis: n = 8 | ||||||||||||
| Alipour et al., 2014 [18] | Iran | Women, ACR 1987, for at least 1 year, DAS-28 CRP < 5.1, 20–80 years, no NSAIDs or bDMARDs, oral CS < 10 mg/day | Probiotics | 41.14 (12.65) | 5.25 (3.75, 10.0) a | NR | NR | DAS-CRP 2.56 (1.01) | HCQ: 18 (81.8) | 0 | 21 (95.5) | NR |
| Placebo | 44.29 (9.77) | 4.75 (3.0, 9.0) a | 2.31 (0.90) | MTX: 20 (83.3) | 0 | 23 (95.8) | NR | |||||
| Hatakka et al., 2003 [19] | Finland | ACR 1987, 18–64 years, for at least 1 year, no DMARDs, oral CS < 7.5 mg/day | Probiotics | 50 (10) | 8.3 (7.3) | 5 (62.5) | NR | NR | 0 | 0 | 6 (75) | 6 (75) |
| Placebo | 53 (7) | 11.0 (8.2) | 7 (53.8) | 0 | 0 | 8 (62) | 10 (77) | |||||
| Vadell et al., 2020 [20] | Sweden | 18–70 years, for at least 2 years, DAS-28 ESR ≥ 2.6 | Probiotics mixed with diet rich in fatty acids and fibers | 61 (12) b | 20.0 (9.5) b | 34 (72) c | DAS-ESR 3.8 (0.9) | MTX: 31 (66) b | 16 (34) b | 12 (26) b | 24 (51) b | |
| Typical Swedish diet | 3.6 (0.8) | |||||||||||
| Pineda et al., 2011 [21] | Canada | ACR criteria, 18–80 years, SJC and TJC ≥ 4, no intra-articular CS ≤ 1 month before | Probiotics | 63.8 (7.5) | 19 (12.4) | NR | NR | DAS-CRP 4.18 (1.05) | MTX: 11 (73) | NR | 4 (26) | NR |
| Placebo | 59.1 (9.1) | 13.7 (8.4) | 4.83 (0.91) | MTX: 11 (78) | 3 (21) | NR | ||||||
| Nenonen et al., 1998 [22] | Finland | SJC > 3 or TJC > 5, ESR > 20 mm/h or CRP > 10 mg/L | Probiotics with uncooked vegan diet | 49.1 (7.1) | 12.6 (10.3) | 15 (79) c | DAS-CRP 3.26 | MTX: 10 (52.6) | NA | 10 (52.6) | 16 (84.2) | |
| Normal diet | 55.6 (10.8) | 16.1 (13.6) | 14 (70%) c | 3.44 | MTX: 5 (25) | 9 (45) | 18 (90) | |||||
| Mandel et al., 2010 [23] | USA | 18–80 years, for at least 1 year, oral CS < 10 mg/day, four or more among: MS ≥ 1 h, STS in ≥ 3 joint areas, swelling of IPP or MCP or wrist joints, rheumatoid nodules, FR+, erosions | Probiotics | NR | NR | NR | NR | NR | 18 (78) d 17 (77) d | NR | 2 (9.1) | |
| Placebo | 3 (13.6) | |||||||||||
| Zamani et al., 2016 [24] | Iran | ACR 1987, 25–70 years, for at least 6 months, DAS-28 CRP > 3.2, no bDMARDs | Probiotics | 52.2 (12.2) | 7.0 (5.7) | NR | NR | DAS-CRP 4.0 (0.7) | MTX: 29 (96.7) | 0 | 27 (90.0) | NR |
| Placebo | 50.6 (13.1) | 7.0 (6.7) | 4.1 (0.7) | MTX: 29 (96.7) | 0 | 28 (93.3) | ||||||
| Zamani et al., 2017 [25] | Iran | ACR 1987, 25–70 years, for at least 6 months, DAS-28 CRP > 3.2, no bDMARDs | Probiotics | 49.3 (11.0) | 7.7 (6.1) | NR | NR | DAS-CRP 4.2 (0.7) | MTX: 26 (96.3) | 0 | 24 (88.9) | NR |
| Placebo | 49.5 (12.9) | 7.5 (6.4) | 3.5 (0.8) | MTX: 26 (96.3) | 0 | 25 (92.6) | ||||||
| Spondyloarthritis: n = 2 | ||||||||||||
| Jenks et al., 2010 [26] | New Zealand | ESSG criteria, more than 18 years, BASDAI ≥ 3, BASFI ≥ 3, MASES ≥ 3, TJC or SJC ≥ 2 | Probiotics | 45.5 (15) | 9.8 (13) | NR | NR | BASDAI 4.2 (2.2) | MTX: 2 (6) | NR | 0 | 24 (75) |
| Placebo | 41.1 (10) | 7.9 (7) | 4.5 (2.0) | MTX: 3 (10) | 2 (7) | 24 (77) | ||||||
| Brophy et al., 2008 [27] | UK | X-ray or MRI sacro-ilitis, more than 18 years | Probiotics | 44.8 (12.1) | 20.3 (13.2) | NR | NR | NR | 5 (7.9) d | 0 | 53 (85.5) | |
| Placebo | 42.7 (12.7) | 20.3 (13.4) | 8 (11.9) d | 2 (3.0) | 44 (66.7) | |||||||
| Study | Disease | Probiotic Strains | Other Intervention | Design | Population | Intervention | Control | Outcome | Outcome Measurement | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | N | Type | N | ||||||||
| Lactobacillus: n = 5 | |||||||||||
| Alipour et al., 2014 [18] | RA | L. casei 01 | No | Double-blind RCT | 46 | 108 CFU (capsule) daily for 8 weeks | 22 | Placebo | 24 | DAS-28 CRP, SJC, TJC, GH score, hs-CRP, moderate EULAR response | 8 weeks |
| Hatakka et al., 2003 [19] | RA | L.rhamnosus GG, ATCC 53103 | No | Double-blind RCT | 21 | ≥5 × 109 CFU (capsule) twice daily for 1 year | 8 | Placebo | 13 | SJC, TJC, HAQ score, ESR, CRP, VAS activity | 1 year |
| Pineda et al., 2011 [21] | RA | L.rhamnosus GR1 and L.reuteri RC-14 | No | Double-blind RCT | 29 | 2 × 109 CFU (capsule), each twice daily for 3 months | 15 | Placebo | 14 | ACR20 response, DAS-28 CRP, SJC, TJC, MS, HAQ score, ESR, CRP, VAS pain, VAS fatigue | 3 months |
| Nenonen et al., 1998 [22] | RA | L. plantarum and L. brevis | Uncooked vegan diet | Single-blind RCT | 39 | Daily “living food” diet in packed form containing fermented wheat drink rich in Lactobacilli | 19 | Normal diet | 20 | DAS-28 ESR, CRP, ESR, TJC, SJC, HAQ, MS, VAS pain | 3 months |
| Vadell et al., 2020 [20] | RA | L.plantarum 299 v | Anti-inflammatory diet (rich in fatty acids and fibers): fish, vegetables, cereals | Single-blind crossover RCT | 50 | One shot 5 days a week for 10 weeks | 26 a | Typical Swedish diet | 24 a | DAS-28 CRP, DAS-28 ESR, SJC, TJC, ESR, GH score | 10 weeks |
| Bacillus: n = 1 | |||||||||||
| Mandel et al., 2010 [23] | RA | Bacillus coagulans | No | Double-blind RCT | 45 | 2 × 109 CFU (capsule) daily for 2 months | 23 | Placebo | 22 | ACR20 response, SJC, TJC, HAQ score, VAS pain, VAS activity, ESR, CRP | 2 months |
| Mix of different probiotics types: n = 4 | |||||||||||
| Zamani et al., 2016 [24] | RA | L. acidophilus, L.casei and Bifidobacterium bifidum | No | Double-blind RCT | 60 | 2 × 109 CFU/g (capsule) each strain, daily for 2 months | 30 | Placebo | 30 | DAS-28 CRP, SJC, TJC, hs-CRP, VAS pain | 2 months |
| Zamani et al., 2017 [25] | RA | L. acidophilus, L. casei and Bifidobacterium bifidum | Prebiotic inulin 800 mg | Double-blind RCT | 54 | 2 × 109 CFU/g (capsule) each strain, daily for 2 months | 27 | Placebo | 27 | DAS-28 CRP, SJC, TJC, hs-CRP, VAS pain | 2 months |
| Jenks et al., 2010 [26] | SpA | Streptococcus salivarius K12, Bifidobacterium lactis LAFTI B94 and L. acidophilus LAFTI L10 | No | Double-blind RCT | 63 | 108 CFU/g, 4 × 108 CFU/g, and 4 × 108 CFU/g (powder, about 0.8 g in total twice daily) for 3 months | 32 | Placebo | 31 | BASFI10 response, BASDAI, ASAS20, VAS pain, fatigue, ASQoL, SJC, TJC, CRP | 3 months |
| Brophy et al., 2008 [27] | SpA | L. salivarius CUL61, L. paracasei CUL08, Bifidobacterium infantis CUL34 and Bifidobacterium bifidum CUL20 | No | Double-blind RCT | 134 | 6.25 × 109 CFU, 1.25 × 109 CFU, 1.25 × 109 CFU and 1.25 × 109 CFU (capsule) daily for 3 months | 65 | Placebo | 69 | VAS activity, global well-being, bowel symptoms | 3 months |
| Study | Outcome | Intervention | Control | Mean Difference between Groups * | p-Value (Intervention vs. Controls) | |
|---|---|---|---|---|---|---|
| Baseline Versus End of Treatment | Baseline Versus End of Treatment | |||||
| DAS28 | ||||||
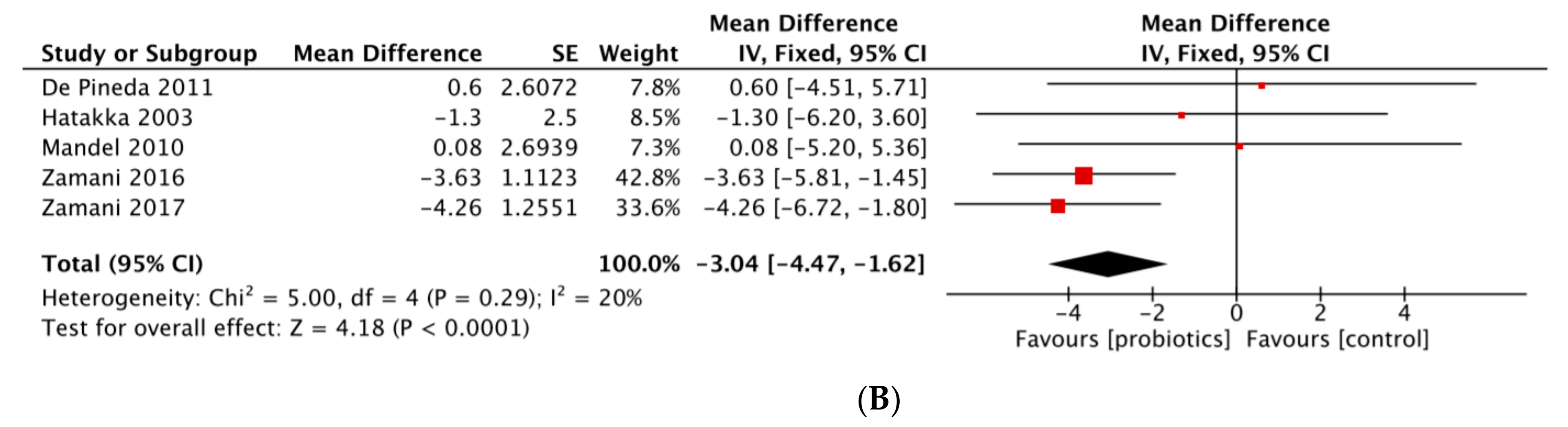
| In favor probiotic intervention | Alipour et al., 2014 [18] | DAS28-CRP | 2.56 (1.01) vs. 2.07 (0.82) | 2.31 (0.90) vs. 2.23 (0.86) | −0.31 (−0.61; −0.02) | p = 0.039 |
| Zamani et al., 2016 [24] | DAS28-CRP | 4.0 (0.7) vs. 3.7 (0.7) | 4.1 (0.7) vs. 4.0 (0.7) | −0.2 | p = 0.01 | |
| Zamani et al., 2017 [25] | DAS28-CRP | 4.2 (0.7) vs. 2.6 (0.7) | 3.5 (0.8) vs. 3.2 (1.1) | −1.3 | p < 0.001 | |
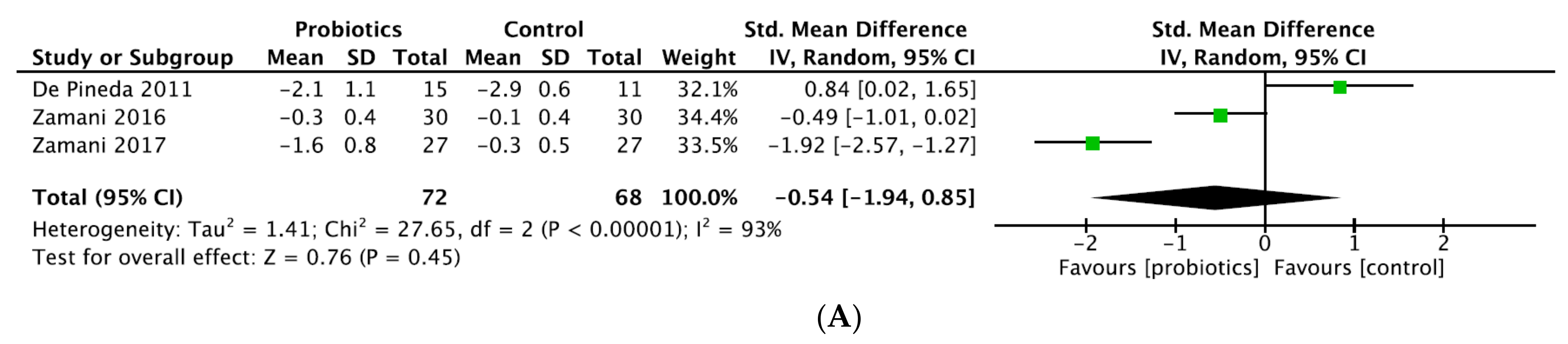
| No significant result | Pineda et al., 2011 [21] | DAS28-CRP | −2.1 (1.1) a | −2.9 (0.6) a | 0.8 | p = 0.77 |
| Vadell et al., 2020 [20] | DAS28-CRP | −0.455 (−0.698; −0.212) b | −0.222 (−0.461; 0.017) b | −0.233 (−0.569; 0.103) | p = 0.169 | |
| DAS28-ESR | −0.369 (−0.628; −0.111) b | −0.080 (−0.335; 0.174) b | −0.289 (−0.652; 0.075) | p = 0.116 | ||
| Nenonen et al., 1998 [22] | DAS28-ESR | 3.26 vs. 3.01 | 3.44 vs. 3.46 | −0.23 | p = 0.7 | |
| Inflammatory markers | ||||||
| In favor probiotic intervention | Alipour et al., 2014 [18] | hs-CRP (mg/L) | 3.10 (1.32; 18.01) vs. 2.80 (0.95; 15.95) c | 2.30 (1.23; 7.99) vs. 3.50 (0.89; 10.38) c | −2.03 (−3.51; −0.54) | p = 0.009 |
| Zamani et al., 2016 [24] | hs-CRP (mg/L) | 7.27 (6.24) vs. 6.61 (6.03) | 6.02 (5.78) vs. 9.09 (7.46) | −3.73 | p < 0.001 | |
| Zamani et al., 2017 [25] | hs-CRP (mg/L) | 6.0·0 (4.8) vs. 4.6 (2.7) | 5.6 (5.1) vs. 8.5 (6.8) | −4.3 | p = 0.001 | |
| No significant result | Hatakka et al., 2003 [19] | CRP (mg/L) | 1.6 (4.6) vs. 2.6 (3.3) | 5.1 (5.7) vs. 7.4 (8.7) | −1.3 (−6.2; 3.6) | p = 0.582 |
| ESR (mm/h) | 17.3 (14.7) vs. 20.7 (17.3) | 18.2 (15.9) vs. 17.9 (14.4) | 3.6 (−0.7; 7.9) | p = 0.095 | ||
| Mandel et al., 2010 [23] | CRP (mg/L) | NR | NR | 0.008 (−0.52. 0.53) | p = 0.98 | |
| ESR (mm/h) | NR | NR | −0.054 (−0.49. 0.38) | p = 0.80 | ||
| Pineda et al., 2011 [21] | CRP (mg/L) | 1.8 (8.4) a | 1.2 (4.8) a | 0.6 | p = 0.75 | |
| ESR (mm/h) | −4.0 (9.8) a | 0.27 (6.8) a | −4.27 | p = 0.76 | ||
| Vadell et al., 2020 [20] | ESR (mm/h) | −0.051 (−0.347; 0.245) b | 0.210 (−0.081; 0.501) b | −0.261 (−0.661; 0.138) | p = 0.194 | |
| Nenonen et al., 1998 [22] | CRP (mg/L) | NR | NR | NR | p = NS | |
| ESR (mm/h) | ||||||
| TJC | ||||||
| In favor probiotic intervention | Alipour et al., 2014 [18] | TJC | 0.0 (0.0; 2.25) vs. 0.0 (0.0; 1.0) c | 0.0 (0.0; 2.75) vs. 0.0 (0.0; 2.75) c | −0.72 (−1.19; −0.25) | p = 0.003 |
| No significant result | Hatakka et al., 2003 [19] | TJC | 3.7 (2.5) vs. 2.5 (1.7) | 3.0 (3.3) vs. 2.6 (2.4) | −0.3 (−2.2; 1.7) | p = 0.784 |
| Mandel et al., 2010 [23] | TJC | NR | NR | −0.074 (−0.81. 0.66) | p = 0.84 | |
| Pineda et al., 2011 [21] | TJC | 0.2 (5.5) a | −0.55 (7.1) a | 1.05 | p = 0.43 | |
| Zamani et al., 2016 [24] | TJC | 5.2 (2.8) vs. 4.8 (2.6) | 5.2 (2.5) vs. 4.7 (2.4) | 0 | p = 0.1 | |
| Vadell et al., 2020 [20] | TJC | 33.2 (16.1; 56.2) b | 27.1 (12.7; 48.7) b | 6.1 (−15.2; 27.3) | p = 0.572 | |
| Nenonen et al., 1998 [22] | TJC | NR | NR | NR | p = NS | |
| SJC | ||||||
| In favor probiotic intervention | Alipour et al., 2014 [18] | SJC | 0.0 (0.0; 2.0) vs. 0.0 (0.0; 1.0) c | 1.0 (0.0; 1.75) vs. 1.0 (0.0; 1.75) c | −0.351 (−0.58; −0.13) | p = 0.003 |
| No significant result | Hatakka et al., 2003 [19] | SJC | 4.5 (5.5) vs. 2.1 (1.7) | 2.5 (3.0) vs. 2.2 (3.1) | −1.1 (−3.0; 0.9) | p = 0.265 |
| Mandel et al., 2010 [23] | SJC | NR | NR | 0.011 (−0.62. 0.64) | p = 0.97 | |
| Pineda et al., 2011 [21] | SJC | −0.4 (3.3) a | −1.0 (3.6) a | 0.6 | p = 0.47 | |
| Zamani et al., 2016 [24] | SJC | 5.5 (3.0) vs. 5.1 (3.1) | 5.8 (2.7) vs. 5.8 (2.8) | −0.37 | p = 0.16 | |
| Vadell et al., 2020 [20] | SJC | 48.6 (23.8; 74.1) b | 37.3 (16.2; 64.5) b | 11.4 (−14.4; 37.2) | p = 0.383 | |
| Nenonen et al., 1998 [22] | SJC | NR | NR | NR | p = NS | |
| Meta-Analysis | Mohammed et al., 2017 [30] | Rudbane et al., 2018 [29] | Lowe et al., 2020 [28] |
|---|---|---|---|
| Method of results analysis | Comparison of Pre/post value variation | Comparison of Pre/post value variation | Comparison of final values |
| Methodological quality according to AMSTAR2 tool | Critically low | Critically low | Critically low |
| DAS28 CRP | |||
| Studies included | Pineda et al., 2011 [21] Alipour et al., 2014 [18] Zamani et al., 2016 [24] | Alipour et al., 2014 [18] Zamani et al., 2016 [24] | Pineda et al., 2011 [21] Alipour et al., 2014 [18] Zamani et al., 2016 [24] Zamani et al., 2017 [25] |
| Total sample size | 132 | 106 | NR |
| Results | SMD = 0.023 (−0.584 to 0.631) p = 0.94 I2 = 73, p = 0.025 | SMD = −0.58 (−0.97 to −0.19) p = NR I2 = 0.0, p = 0.634 | SMD = −0.28 (−0.5 to −0.05) p = 0.016 I2 = NR |
| CRP | |||
| Studies included | 5 (NR) | Hatakka et al., 2003 [19] Pineda et al., 2011 [21] Alipour et al., 2014 [18] Zamani et al., 2016 [24] | Hatakka et al., 2003 [19] Pineda et al., 2011 [21] Alipour et al., 2014 [18] Zamani et al., 2016 [24] Zamani et al., 2017 [25] Jenks et al., 2010 [26] Shukla et al., 2016 [31] |
| Total sample size | 191 | 132 | NR |
| Results (mg/L) | SMD = −2.660 (−6.144 to 0.823) p = 0.134 I2 = 82.3, p < 0.001 | SMD = −0.27 (−0.77 to 0.23) p = NS I2 = 55.3, p = 0.082 | SMD = −2.34 (−4.26 to −0.41) p = 0.017 I2 = 52, p = 0.049 |
| ESR | |||
| Studies included | 4 (NR) | Hatakka et al., 2003 [19] Pineda et al., 2011 [21] | - |
| Total sample size | 129 | 47 | - |
| Results (mm/h) | SMD = 1.861 (−4.481 to 8.202) p = 0.565 I2 = 66.0, p = 0.032 | SMD = −0.17 (−0.76 to 0.42) p = NS I2 = 31.5, p = 0.0227 | - |
| TJC | |||
| Studies included | Hatakka et al., 2003 [19] Mandel et al., 2010 [23] Pineda et al., 2011 [21] Zamani et al., 2016 [24] Shukla et al., 2016 [31] | Hatakka et al., 2003 [19] Pineda et al., 2011 [21] Alipour et al., 2014 [18] Zamani et al., 2016 [24] | - |
| Total sample size | 191 | 153 | - |
| Results | SMD = 0.379 (−0.578 to 1.336) p = 0.437 I2 = 71.5, p = 0.007 | SMD = −0.21 (−0.53 to 0.11) p = NS I2 = 10.1, p = 0.342 | - |
| SJC | |||
| Studies included | Hatakka et al., 2003 [19] Mandel et al., 2010 [23] Pineda et al., 2011 [21] Zamani et al., 2016 [24] Shukla et al., 2016 [31] | Hatakka et al., 2003 [19] Pineda et al., 2011 [21] Alipour et al., 2014 [18] Zamani et al., 2016 [24] | - |
| Total sample size | 191 | 153 | - |
| Results | SMD = 0.171 (−0.391 to 0.733) p = 0.551 I2 = 53.9, p = 0.07 | SMD = −0.30 (0.62 to 0.02) p = NS I2 = 0.0, p = 0.462 | - |
| VAS pain | |||
| Studies included | - | - | Pineda et al., 2011 [21] Zamani et al., 2016 [24] Zamani et al., 2017 [25] Jenks et al., 2010 [26] |
| Total sample size | - | - | NR |
| Results | - | - | SMD = −8.97 (−15.38 to −2.56) p = 0.006 I2 = 41, p = 0.167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, P.; Letarouilly, J.-G.; Nguyen, Y.; Sigaux, J.; Barnetche, T.; Czernichow, S.; Flipo, R.-M.; Sellam, J.; Daïen, C. Efficacy of Probiotics in Rheumatoid Arthritis and Spondyloarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 354. https://doi.org/10.3390/nu14020354
Sanchez P, Letarouilly J-G, Nguyen Y, Sigaux J, Barnetche T, Czernichow S, Flipo R-M, Sellam J, Daïen C. Efficacy of Probiotics in Rheumatoid Arthritis and Spondyloarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022; 14(2):354. https://doi.org/10.3390/nu14020354
Chicago/Turabian StyleSanchez, Pauline, Jean-Guillaume Letarouilly, Yann Nguyen, Johanna Sigaux, Thomas Barnetche, Sébastien Czernichow, René-Marc Flipo, Jérémie Sellam, and Claire Daïen. 2022. "Efficacy of Probiotics in Rheumatoid Arthritis and Spondyloarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 14, no. 2: 354. https://doi.org/10.3390/nu14020354






