Assessment of Intramuscular Fat and Correlation with Body Composition in Patients with Rheumatoid Arthritis and Spondyloarthritis: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Imaging Data
2.3. Body Composition Assessments with DXA
2.4. Muscle and Fat Assessments with pQCT
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlations between pQCT and Total Body Composition (DXA)
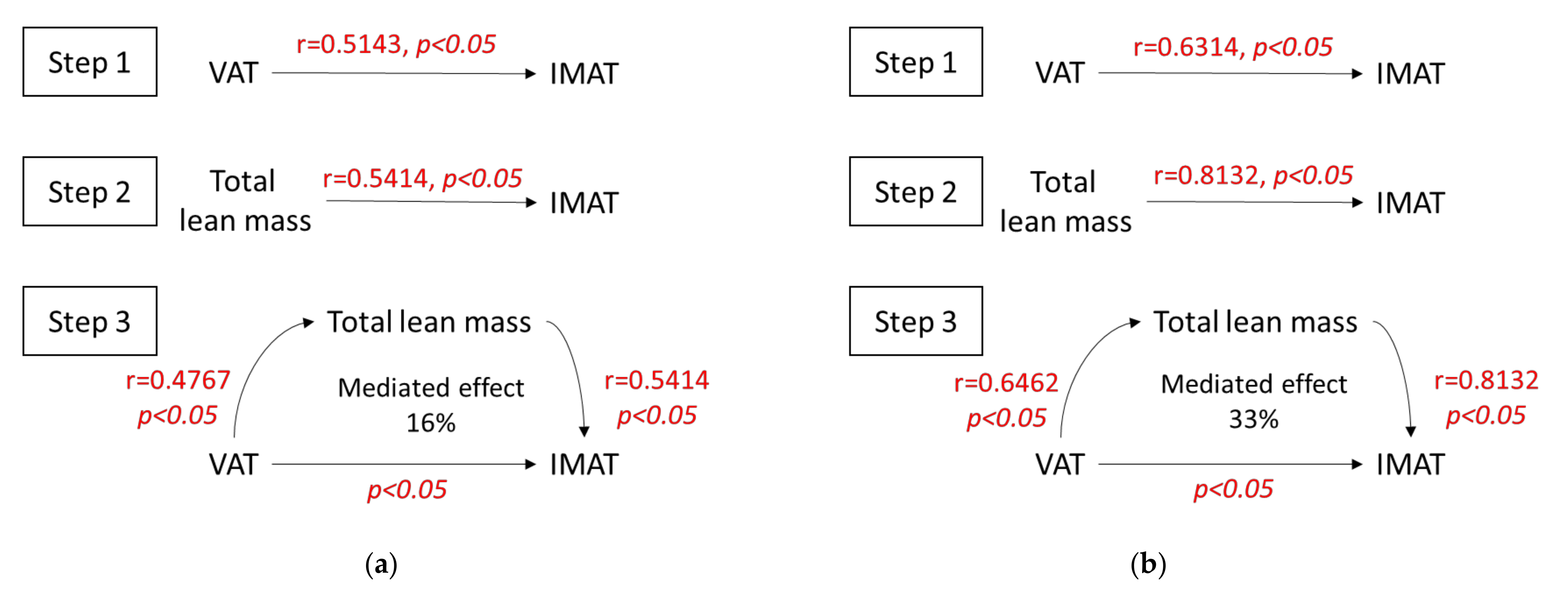
3.3. Mediation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IMAT | Intramuscular adipose tissue |
| CSA | Cross-sectional area |
| FMI | Fat mass index |
| FFMI | Fat-free mass index |
| SMI | Skeletal muscle mass index |
| VAT | Visceral adipose tissue |
| SAT | Subcutaneous adipose tissue (cm2) |
References
- Tournadre, A.; Mathieu, S.; Soubrier, M. Managing cardiovascular risk in patients with inflammatory arthritis: Practical considerations. Ther. Adv. Musculoskelet. Dis. 2016, 8, 180–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tournadre, A.; Pereira, B.; Dutheil, F.; Giraud, C.; Courteix, D.; Sapin, V.; Frayssac, T.; Mathieu, S.; Malochet-Guinamand, S.; Soubrier, M. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J. Cachexia Sarcopenia Muscle 2017, 8, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Ling, S.M.; Ferrucci, L.; Bartlett, S.J.; Andersen, R.E.; Towns, M.; Muller, D.; Fontaine, K.R.; Bathon, J.M. Abnormal Body Composition Phenotypes in Older Rheumatoid Arthritis Patients: Association with Disease Characteristics and Pharmacotherapies. Arthritis Rheum. 2008, 59, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santo, R.C.E.; Fernandes, K.Z.; Lora, P.S.; Filippin, L.I.; Xavier, R.M. Prevalence of Rheumatoid Cachexia in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2018, 9, 816–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlietstra, L.; Meredith-Jones, K.; Stebbings, S.; Abbott, J.H.; Treharne, G.J.; Waters, D.L. Sarcopenic Obesity Is More Prevalent in Osteoarthritis than Rheumatoid Arthritis: Are Different Processes Involved? Rheumatology 2017, 56, 1816–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerekes, G.; Nurmohamed, M.T.; González-Gay, M.A.; Seres, I.; Paragh, G.; Kardos, Z.; Baráth, Z.; Tamási, L.; Soltész, P.; Szekanecz, Z. Rheumatoid Arthritis and Metabolic Syndrome. Nat. Rev. Rheumatol. 2014, 10, 691–696. [Google Scholar] [CrossRef]
- Marcora, S.; Casanova, F.; Williams, E.; Jones, J.; Elamanchi, R.; Lemmey, A. Preliminary evidence for cachexia in patients with well-established ankylosing spondylitis. Rheumatology 2006, 45, 1385–1388. [Google Scholar] [CrossRef] [Green Version]
- Nordén, K.R.; Dagfinrud, H.; Løvstad, A.; Raastad, T. Reduced Appendicular Lean Body Mass, Muscle Strength, and Size of Type II Muscle Fibers in Patients with Spondyloarthritis versus Healthy Controls: A Cross-Sectional Study. Sci. World J. 2016, 2016, 6507692. [Google Scholar] [CrossRef] [Green Version]
- Plasqui, G.; Boonen, A.; Geusens, P.; Kroot, E.J.; Starmans, M.; van der Linden, S. Physical Activity and Body Composition in Patients with Ankylosing Spondylitis. Arthritis Care Res. 2012, 64, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.J. Human body composition: In vivo methods. Physiol. Rev. 2000, 80, 649–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, J.A.; Ng, B.K.; Sommer, M.J.; Heymsfield, S.B. Body Composition by DXA. Bone 2017, 104, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Wang, Z.; Punyanita, M.; Lei, J.; Sinav, A.; Kral, J.G.; Imielinska, C.; Ross, R.; Heymsfield, S.B. Adipose tissue quantification by imaging methods: A proposed classification. Obes. Res. 2003, 11, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blew, R.M.; Lee, V.R.; Bea, J.W.; Hetherington-Rauth, M.C.; Galons, J.-P.; Altbach, M.I.; Lohman, T.G.; Going, S.B. Validation of Peripheral Quantitative Computed Tomography–Derived Thigh Adipose Tissue Subcompartments in Young Girls Using a 3 T MRI Scanner. J. Clin. Densitom. 2018, 21, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.B.; Jing, T.; Heymsfield, S.B. Relationships in Men of Sex Hormones, Insulin, Adiposity, and Risk Factors for Myocardial Infarction. Metabolism 2003, 52, 784–790. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal Study of Muscle Strength, Quality, and Adipose Tissue Infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef]
- Marcus, R.L.; Addison, O.; Dibble, L.E.; Foreman, K.B.; Morrell, G.; Lastayo, P. Intramuscular Adipose Tissue, Sarcopenia, and Mobility Function in Older Individuals. J. Aging Res. 2012, 2012, 629637. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Després, J.-P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Sherk, V.D.; Thiebaud, R.S.; Chen, Z.; Karabulut, M.; Kim, S.J.; Bemben, D.A. Associations between PQCT-Based Fat and Muscle Area and Density and DXA-Based Total and Leg Soft Tissue Mass in Healthy Women and Men. J. Musculoskelet. Neuronal Interact. 2014, 14, 411–417. [Google Scholar] [PubMed]
- Baker, J.F.; Mostoufi-Moab, S.; Long, J.; Zemel, B.; Ibrahim, S.; Taratuta, E.; Leonard, M.B. Intramuscular Fat Accumulation and Associations with Body Composition, Strength, and Physical Functioning in Patients with Rheumatoid Arthritis. Arthritis Care Res. 2018, 70, 1727–1734. [Google Scholar] [CrossRef] [Green Version]
- Khoja, S.S.; Moore, C.G.; Goodpaster, B.H.; Delitto, A.; Piva, S.R. Skeletal Muscle Fat and Its Association with Physical Function in Rheumatoid Arthritis. Arthritis Care Res. 2018, 70, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The Development of Assessment of SpondyloArthritis International Society Classification Criteria for Axial Spondyloarthritis (Part II): Validation and Final Selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Akkoc, N.; Brandt, J.; Chou, C.T.; Dougados, M.; Huang, F.; Gu, J.; Kirazli, Y.; et al. The Assessment of SpondyloArthritis International Society Classification Criteria for Peripheral Spondyloarthritis and for Spondyloarthritis in General. Ann. Rheum. Dis. 2011, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Shaffer, N.C.; Gonzalez-Freire, M.; Shardell, M.D.; Zoli, M.; Studenski, S.A.; Ferrucci, L. Early Body Composition, but Not Body Mass, Is Associated with Future Accelerated Decline in Muscle Quality. J. Cachexia Sarcopenia Muscle 2017, 8, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodpaster, B.H.; Kelley, D.E.; Thaete, F.L.; He, J.; Ross, R. Skeletal Muscle Attenuation Determined by Computed Tomography Is Associated with Skeletal Muscle Lipid Content. J. Appl. Physiol. 2000, 89, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, D.; Trbojevic, T.; Skinner, E.; Clark, R.A.; Levinger, P.; Haines, T.P.; Sanders, K.M.; Ebeling, P.R. Associations of Calf Inter- and Intra-Muscular Adipose Tissue with Cardiometabolic Health and Physical Function in Community-Dwelling Older Adults. J. Musculoskelet. Neuronal Interact. 2015, 15, 350–357. [Google Scholar] [PubMed]
- Blew, R.M.; Lee, V.R.; Farr, J.N.; Schiferl, D.J.; Going, S.B. Standardizing Evaluation of PQCT Image Quality in the Presence of Subject Movement: Qualitative versus Quantitative Assessment. Calcif. Tissue Int. 2014, 94, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Bourdier, P.; Birat, A.; Rochette, E.; Doré, É.; Courteix, D.; Dutheil, F. Muscle function and architecture in children with juvenile idiopathic arthritis. Acta Paediatr. 2021, 110, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Schiferl, D. Movement Analysis, with Additional Image Filtering Dan Schiferl; Bone Diagnostic LLC (BDLLC): Spring Branch, TX, USA, 2017. [Google Scholar]
- Imai, K.; Keele, L.; Tingley, D.; Yamamoto, T. Unpacking the Black Box of Causality: Learning about Causal Mechanisms from Experimental and Observational Studies. Am. Polit. Sci. Rev. 2011, 105, 765–789. [Google Scholar] [CrossRef]
- Shrout, P.E.; Bolger, N. Mediation in Experimental and Nonexperimental Studies: New Procedures and Recommendations. Psychol. Methods 2002, 7, 422–445. [Google Scholar] [CrossRef] [PubMed]
- Vettor, R.; Milan, G.; Franzin, C.; Sanna, M.; De Coppi, P.; Rizzuto, R.; Federspil, G. The Origin of Intermuscular Adipose Tissue and Its Pathophysiological Implications. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E987–E998. [Google Scholar] [CrossRef] [PubMed]
- Butner, K.L.; Creamer, K.W.; Nickols-Richardson, S.M.; Clark, S.F.; Ramp, W.K.; Herbert, W.G. Fat and Muscle Indices Assessed by PQCT: Relationships with Physical Activity and Type 2 Diabetes Risk. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2012, 15, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ducher, G.; Daly, R.M.; Hill, B.; Eser, P.; Naughton, G.A.; Gravenmaker, K.J.; Seibel, M.J.; Javaid, A.; Telford, R.D.; Bass, S.L. Relationship between Indices of Adiposity Obtained by Peripheral Quantitative Computed Tomography and Dual-Energy X-Ray Absorptiometry in Pre-Pubertal Children. Ann. Hum. Biol. 2009, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Schorr, M.; Dichtel, L.E.; Gerweck, A.V.; Valera, R.D.; Torriani, M.; Miller, K.K.; Bredella, M.A. Sex differences in body composition and association with cardiometabolic risk. Biol. Sex. Differ. 2018, 9, 28. [Google Scholar] [CrossRef] [PubMed]
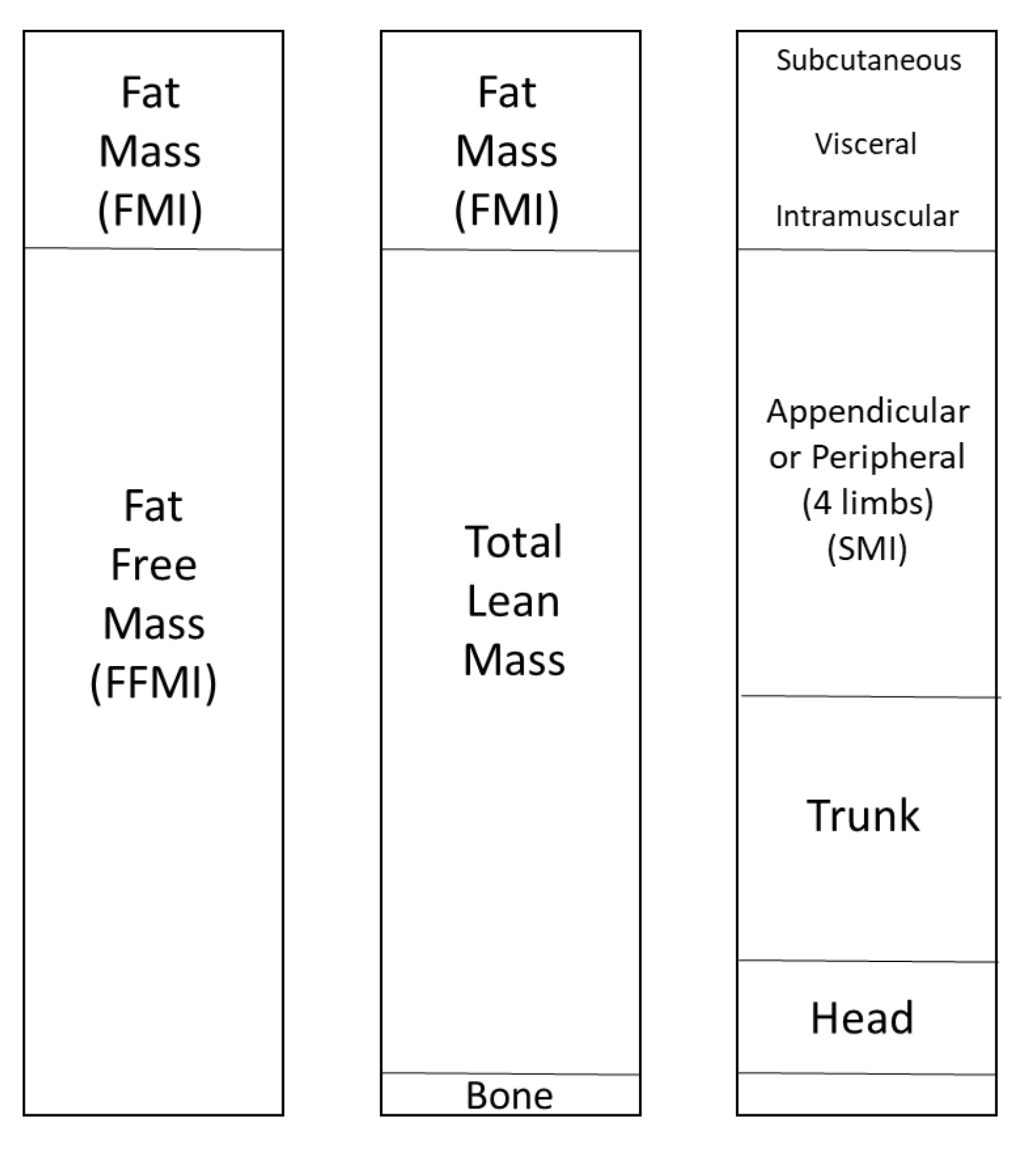
| Characteristic | RA n = 20 | SpA n = 23 |
|---|---|---|
| Age (years) | 58.8 ± 14,2 | 42.9 ± 11.1 |
| Sex (female) | 15 (75) | 13 (56.5) |
| Disease duration (years) | 4.2 ± 6.4 | 4.9 ± 8.0 |
| BMI (kg/m2) | 28.6 ± 7.1 | 25.3 ± 4.6 |
| Rheumatoid factor | 14 (82.3) | NA |
| Anti-CCP | 13 (76.4) | NA |
| Axial SpA | NA | 12 (52.1) |
| Peripheral SpA | NA | 9 (39.1) |
| Radiographic sacroiliitis | NA | 6 (26.0) |
| MRI sacroiliitis | NA | 7 (30.4) |
| HLA B27 | NA | 15 (65.2) |
| DAS28 | 4.09 ± 1.36 | NA |
| DAS28 CRP | 3.79 ± 1.16 | NA |
| BASDAI | NA | 49.7 ± 17.6 |
| BASFI | NA | 41.9 ± 22.6 |
| ASDAS-CRP | NA | 3.1 ± 0.7 |
| RAID | 5.97 ± 1.74 | NA |
| HAQ | 0.897 ± 0.518 | 0.664 ± 0.314 |
| VS (mm/h) | 24.6 ± 17.9 | 18.2 ± 14.4 |
| CRP (mg/L) | 22.3 ± 29.6 | 15.3 ± 19.0 |
| NSAID current use | 3 (16.6) | 14 (70) |
| Corticosteroids current use | 10 (50) | 1 (4.7) |
| Methotrexate current use | 16 (80) | 17 (73.9) |
| Muscle strength (handgrip; kg) | 16.12 ± 14.29 | 28.73 ± 15.66 |
| 6-minute walk test (m) | 423 ± 101 | 457 ± 104 |
| Sedentary time (GPAQ_Q16) (minutes/week) | 2618 ± 1733 | 2142 ± 1528 |
| Parameter | RA n = 20 | SpA n = 23 |
|---|---|---|
| Muscle area (CSA; mm2) | 6166 ± 1541 | 7031 ± 1322 |
| Fat area (mm2) | 3672 ± 2093 | 2402 ± 1297 |
| IMAT (mm2) | 1488 ± 556 | 1760 ± 537 |
| Muscle density (mg/cm3) | 77.01 ± 2.94 | 77.16 ± 3.08 |
| Strength-CSA ratio (kg/mm2) | 0.0024 ± 0.0017 | 0.0039 ± 0.0019 |
| BMI (kg/m2) | 28.6 ± 7.1 | 25.3 ± 4.6 |
| Total fat mass (kg) | 25.13 ± 12.34 | 20.94 ± 7.82 |
| Fat percentage | 31.57 ± 9.54 | 27.22 ± 7.21 |
| FMI (kg/m2) | 10.35 ± 5.75 | 7.23 ± 2.87 |
| Visceral adipose tissue (cm2) | 83.58 ± 61.22 | 86.19 ± 66.46 |
| Subcutaneous adipose tissue (cm2) | 332.3 ± 174.8 | 262.6 ± 121.7 |
| Trunk–peripheral fat ratio | 0.8394 ± 0.2931 | 0.9767 ± 0.2936 |
| Total lean mass (kg) | 52.57 ± 16.43 | 55.99 ± 15.68 |
| FFMI (kg/m2) | 23.04 ± 18.56 | 18.97 ± 3.91 |
| SMI (kg/m2) | 8.26 ± 2.07 | 8.03 ± 1.55 |
| Parameter | Muscle Area (CSA, mm2) | Fat Area (mm2) | IMAT (mm2) | Muscle Density (mg/cm3) | Strength-to-CSA Ratio (kg/mm2) |
|---|---|---|---|---|---|
| BMI (kg/m2) | 0.2632 | 0.4923 * | 0.4757 * | −0.4779 | 0.0214 |
| Total fat mass (kg) | 0.0496 | 0.6767 * | 0.3053 | −0.3474 | −0.0089 |
| Fat percentage | −0.3053 | 0.7068 * | −0.0932 | −0.0737 | −0.2147 |
| FMI (kg/m2) | 0.0165 | 0.6526 * | 0.2556 | −0.2579 | −0.1403 |
| Visceral adipose tissue (cm2) | 0.4571 * | 0.0932 | 0.5143 * | −0.2789 | 0.2201 |
| Subcutaneous adipose tissue (cm2) | 0.1113 | 0.6526 * | 0.3053 | −0.2965 | −0.0857 |
| Trunk-to-peripheral fat ratio | 0.3474 | 0.0511 | 0.4962 * | −0.2684 | −0.0294 |
| Total lean mass (kg) | 0.5940 * | 0.0286 | 0.5414 * | −0.3263 | 0.3826 |
| FFMI (kg/m2) | 0.5534 * | 0.2436 | 0.6827 * | −0.4491 | 0.1477 |
| SMI (kg/m2) | 0.4579 * | 0.2123 | 0.5825 * | −0.5562 * | 0.1901 |
| Muscle strength (handgrip test; kg) | 0.6839 * | −0.5244 * | 0.1713 | 0.2704 | 0.9926 * |
| 6-minute walk test (m) | 0.6539 * | −0.8130 * | 0.1635 | 0.6261 * | 0.5022 |
| Sedentary time (GPAQ_Q16) (minutes/week) | 0.3002 | −0.3671 | 0.0597 | 0.4324 | 0.0308 |
| DAS28 | −0.3642 | 0.5239 * | −0.1466 | −0.1074 | −0.4200 |
| DAS28CRP | −0.2767 | 0.4915 * | −0.0248 | 0.0331 | −0.3718 |
| CRP (mg/L) | −0.0565 | 0.2599 | 0.1469 | −0.3938 | −0.4720 |
| HAQ | −0.2952 | 0.3399 | 0.0698 | −0.3564 | −0.5152 |
| RAID | −0.5750 * | 0.2250 | −0.2786 | −0.1604 | −0.6923 * |
| Parameter | Muscle Area (CSA, mm2) | Fat Area (mm2) | IMAT (mm2) | Muscle density (mg/cm3) | Strength-to-CSA Ratio (kg/mm2) |
|---|---|---|---|---|---|
| BMI (kg/m2) | 0.7509 * | 0.0158 | 0.7632 * | −0.3632 | 0.2588 |
| Total fat mass (kg) | 0.3004 | 0.5089 * | 0.4051 | −0.3696 | 0.2772 |
| Fat percentage | −0.2974 | 0.6314 * | −0.2668 | −0.0069 | −0.3474 |
| FMI (kg/m2) | 0.1443 | −0.0958 | 0.2678 | −0.3043 | −0.1035 |
| Visceral adipose tissue (cm2) | 0.6393 * | 0.0049 | 0.6314 * | −0.5227 * | 0.5544 * |
| Subcutaneous adipose tissue (cm2) | 0.2747 | 0.5524 * | 0.3794 | −0.3577 | 0.1982 |
| Trunk-to-peripheral fat ratio | 0.7688 * | −0.1868 | 0.6957 * | −0.4269 * | 0.5368 * |
| Total lean mass (kg) | 0.8597 * | −0.1621 | 0.8132 * | −0.4249 * | 0.6140 * |
| FFMI (kg/m2) | 0.8491 * | −0.1868 | 0.8211 * | −0.4269 * | 0.3404 |
| SMI (kg/m2) | 0.7509 * | −0.3281 | 0.8386 * | −0.3474 | 0.3824 |
| Muscle strength (handgrip test; kg) | 0.5641 * | −0.2329 | 0.5316* | −0.2750 | 0.9227 * |
| 6-minute walk test (m) | 0.1182 | −0.5013 * | −0.0602 | 0.1889 | 0.1993 |
| Sedentary time (GPAQ_Q16) (minutes/week) | 0.1471 | −0.0940 | 0.1524 | 0.1228 | −0.5529 * |
| BASDAI | −0.2656 | 0.2786 | −0.1690 | −0.0277 | −0.4072 |
| BASFI | −0.2852 | 0.3741 | −0.1462 | −0.3215 | −0.3431 |
| ASDAS CRP | 0.1163 | 0.2327 | 0.4183 | −0.4050 | −0.3586 |
| CRP (mg/l) | 0.7528 * | −0.1104 | 0.7219 * | −0.1366 | 0.1687 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villedon de Naide, M.; Pereira, B.; Courteix, D.; Dutheil, F.; Cassagnes, L.; Boirie, Y.; Soubrier, M.; Tournadre, A. Assessment of Intramuscular Fat and Correlation with Body Composition in Patients with Rheumatoid Arthritis and Spondyloarthritis: A Pilot Study. Nutrients 2021, 13, 4533. https://doi.org/10.3390/nu13124533
Villedon de Naide M, Pereira B, Courteix D, Dutheil F, Cassagnes L, Boirie Y, Soubrier M, Tournadre A. Assessment of Intramuscular Fat and Correlation with Body Composition in Patients with Rheumatoid Arthritis and Spondyloarthritis: A Pilot Study. Nutrients. 2021; 13(12):4533. https://doi.org/10.3390/nu13124533
Chicago/Turabian StyleVilledon de Naide, Marc, Bruno Pereira, Daniel Courteix, Frederic Dutheil, Lucie Cassagnes, Yves Boirie, Martin Soubrier, and Anne Tournadre. 2021. "Assessment of Intramuscular Fat and Correlation with Body Composition in Patients with Rheumatoid Arthritis and Spondyloarthritis: A Pilot Study" Nutrients 13, no. 12: 4533. https://doi.org/10.3390/nu13124533
APA StyleVilledon de Naide, M., Pereira, B., Courteix, D., Dutheil, F., Cassagnes, L., Boirie, Y., Soubrier, M., & Tournadre, A. (2021). Assessment of Intramuscular Fat and Correlation with Body Composition in Patients with Rheumatoid Arthritis and Spondyloarthritis: A Pilot Study. Nutrients, 13(12), 4533. https://doi.org/10.3390/nu13124533








