The Role of the Western Diet and Oral Microbiota in Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Participants
3.2. Differences in Diet Preferences between Patients with PD and Healthy Controls
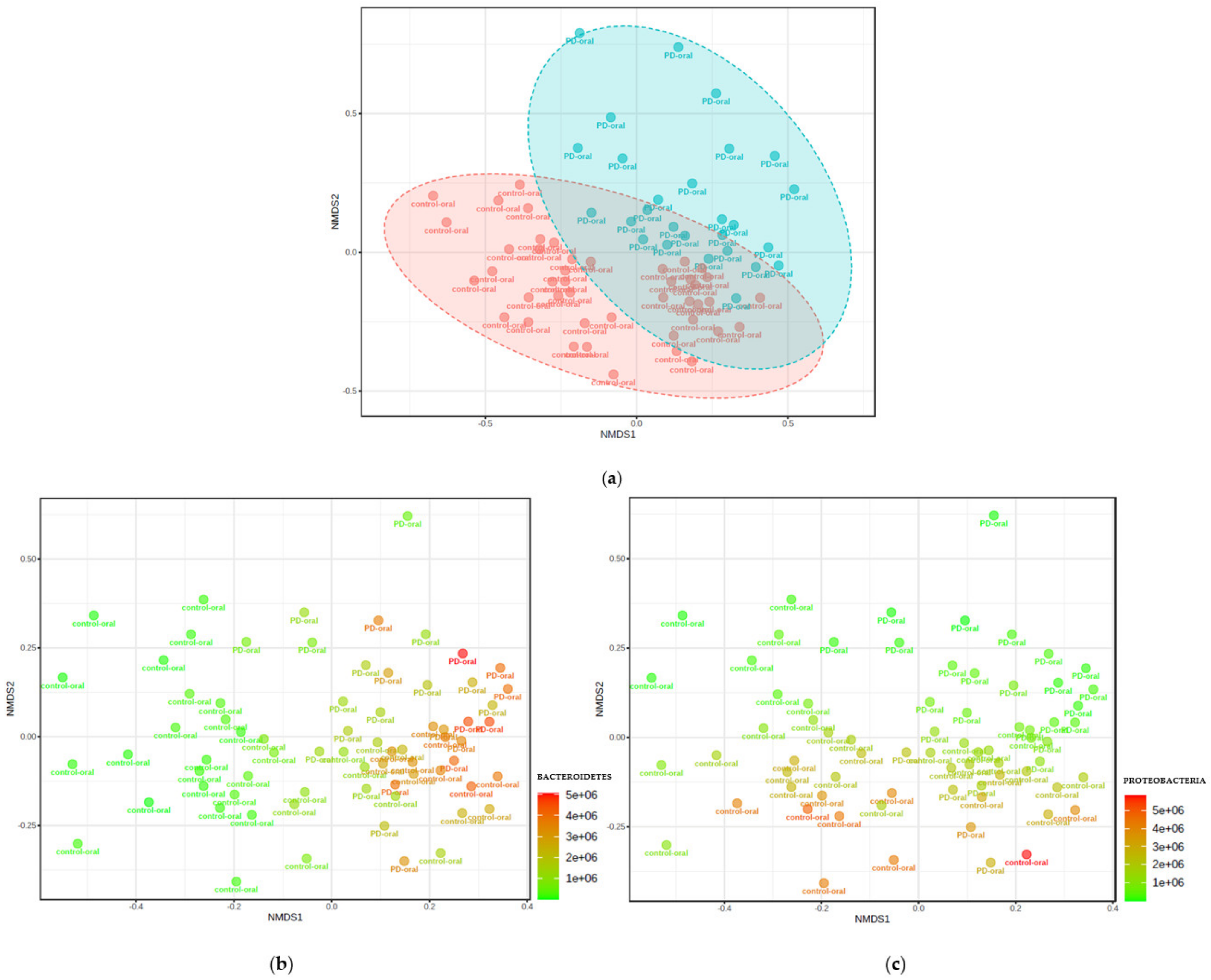
3.3. Microbiota Composition in Patients with PD Group vs. Healthy Controls
3.4. Correlation of Microbiota with Food Preferences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D. Past. Present. Future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef]
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The emerging role ofnutrition in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Perez-Pardo, P.; Kliest, T.; Dodiya, H.B.; Broersen, L.M.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. The gut-brain axis in Parkinson’s disease:Possibilities for food-based therapies. Eur. J. Pharmacol. 2017, 817, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Rassmussen, H.; Piazza, B.R.; Forsyth, C.B.; Keshavarzian, A. Nutrition and gastrointestinal health as modulators of Parkinson’s disease. In Pharma-Nutrition—AAPS Advances in the Pharmaceutical Sciences Series; Folkerts, G., Garssen, J., Eds.; Springer: New York, NY, USA, 2014; pp. 213–242. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of diet and nutritionalsupplements in Parkinson’s disease progression. Oxidative Med. Cell Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [Green Version]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gutmicrobiota in host health and disease. Cell Host Microbe. 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, A.; Günther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.-J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 2018, 172, 162–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myles, I.A. Fast food fever: Reviewing the impacts of the Westerndiet onimmunity. Nutr. J. 2014, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Maraki, M.I.; Yannakoulia, M.; Stamelou, M.; Stefanis, L.; Xiromerisiou, G.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Anastasiou, C.A.; et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov. Disord. 2019, 34, 48–57. [Google Scholar] [CrossRef]
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Bousquet, M.; Calon, F.; Cicchetti, F. Impact of omega-3 fatty acids in Parkinson’s disease. Ageing Res. Rev. 2011, 10, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kamel, F.; Goldman, S.; Umbach, D.M.; Chen, H.; Richardson, G.; Barber, M.R.; Meng, C.; Marras, C.; Korell, M.; Kasten, M.; et al. Dietary fat intake, pesticide use, and Parkinson’s disease. Park. Relat. Disord. 2013, 20, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Mihaila, D.; Donegan, J.; Barns, S.; LaRocca, D.; Du, Q.; Zheng, D.; Vidal, M.; Neville, C.; Uhlig, R.; Middleton, F.A. The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS ONE 2019, 14, e0218252. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.A.B.; Aho, V.T.E.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 38, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Fleury, V.; Zekeridou, A.; Lazarevic, V.; Gaia, N.; Giannopoulou, C.; Genton, L. Oral Dysbiosis and Inflammation in Parkinson’s Disease. J. Parkinsons Dis. 2021, 11, 619–631. [Google Scholar] [CrossRef]
- Rozas, N.; Tribble, G.; Jeter, C. Oral Factors That Impact the Oral Microbiota in Parkinson’s Disease. Microorganisms 2021, 9, 1616. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Good, I.J. The population frequencies of species and the estimation of population parameters. Biometrika 1953, 40, 237–264. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using Microbiome Analyst for comprehensive statistical functional and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Foster, Z.S.; Sharpton, T.J.; Grünwald, N.J. MetacodeR: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2016, 13, e1005404. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.R.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Forsyth, C.B.; Shaikh, M.; Voigt, R.M.; Engen, P.A.; Ramirez, V.; Keshavarzian, A. Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front. Neurol. 2019, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef] [Green Version]
- Castellanos, J.F.-V.; Serrano-Villar, S.; Latorre, A.; Artacho, A.; Ferrús, M.L.; Madrid, N.; Moya, A. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2015, 8, 760–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardone, G.; Compare, D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United Eur. Gastroenterol. J. 2015, 3, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Qu, Y.; Chen, X.; Xu, M.-M. Relationship between high dietary fat intake and Parkinson’s disease risk: A meta-analysis. Neural Regen. Res. 2019, 14, 2156–2163. [Google Scholar] [CrossRef]
- Chen, H.; O’Reilly, E.; McCullough, M.L.; Rodriguez, C.; Schwarzschild, M.A.; Calle, E.E.; Thun, M.J.; Ascherio, A. Consumption of Dairy Products and Risk of Parkinson’s Disease. Am. J. Epidemiol. 2007, 165, 998–1006. [Google Scholar] [CrossRef]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bai, L.; He, J.; Zhong, L.; Duan, X.; Ouyang, L.; Zhu, Y.; Wang, T.; Zhang, Y.; Shi, J. Recent advances in discovery and development of natural products as source for anti-Parkinson’s disease lead compounds. Eur. J. Med. Chem. 2017, 141, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Wang, Y.; Wang, G.; Mao, L.; Zhang, D.; Wang, J. Effects of resveratrol on learning and memory in rats with vascular dementia. Mol. Med. Rep. 2019, 20, 4587–4593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Liu, T.; Dong, S.-Y.; Guo, Y.-J.; Jankovic, J.; Xu, H.; Wu, Y.-C. Rotenone affects p53 transcriptional activity and apoptosis via targeting SIRT1 and H3K9 acetylation in SH-SY5Y cells. J. Neurochem. 2015, 134, 668–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Group | N | M | SD | Min | Max | Q25 | Me | Q75 | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | PD | 59 | 69.34 | 7.07 | 55.00 | 82.00 | 65.00 | 68.00 | 75.00 |
| Controls | 108 | 64.21 | 10.23 | 51.00 | 82.00 | 54.50 | 62.00 | 71.50 | |
| Weight (kg) | PD | 59 | 76.91 | 14.52 | 48.00 | 103.00 | 67.25 | 79.00 | 88.25 |
| Controls | 108 | 72.95 | 13.38 | 47.00 | 118.00 | 65.00 | 70.00 | 80.00 | |
| Height (m) | PD | 59 | 1.70 | 0.11 | 1.50 | 1.86 | 1.62 | 1.70 | 1.78 |
| Controls | 108 | 1.68 | 0.08 | 1.50 | 1.90 | 1.64 | 1.66 | 1.75 | |
| BMI | PD | 59 | 26.33 | 2.92 | 19.23 | 32.30 | 25.14 | 26.53 | 28.16 |
| Controls | 108 | 25.81 | 3.75 | 18.36 | 33.22 | 22.92 | 25.56 | 28.17 |
| Analysis | Result | Reference Values |
|---|---|---|
| Urine color | yellow/dark amber (24/3) | yellow |
| Clarity | clear/cloudy (22/5) | clear |
| Acidity | normal/acidic (17/10) | normal |
| Specific gravity | 1020 ± 0.02 | 1020 ± 0.02 |
| Glucose | negative | negative |
| Ketones | negative | negative |
| Nitrates | negative | negative |
| Bilirubin | negative | negative |
| Urobilinogen | negative | negative |
| Blood | ≤3 red blood cells | ≤3 |
| Red blood cells | ≤ 2 RBCs/hpf | ≤2 |
| White blood cells | ≤2–5 WBCs/hpf | ≤2–5 |
| Protein | ≤150 mg/day | ≤150 mg/day |
| Squamous epithelial cells | negative | negative |
| Casts | negative | negative |
| Crystals | negative | negative |
| Bacteria | none/present (22/5) | none |
| Yeast | none | none |
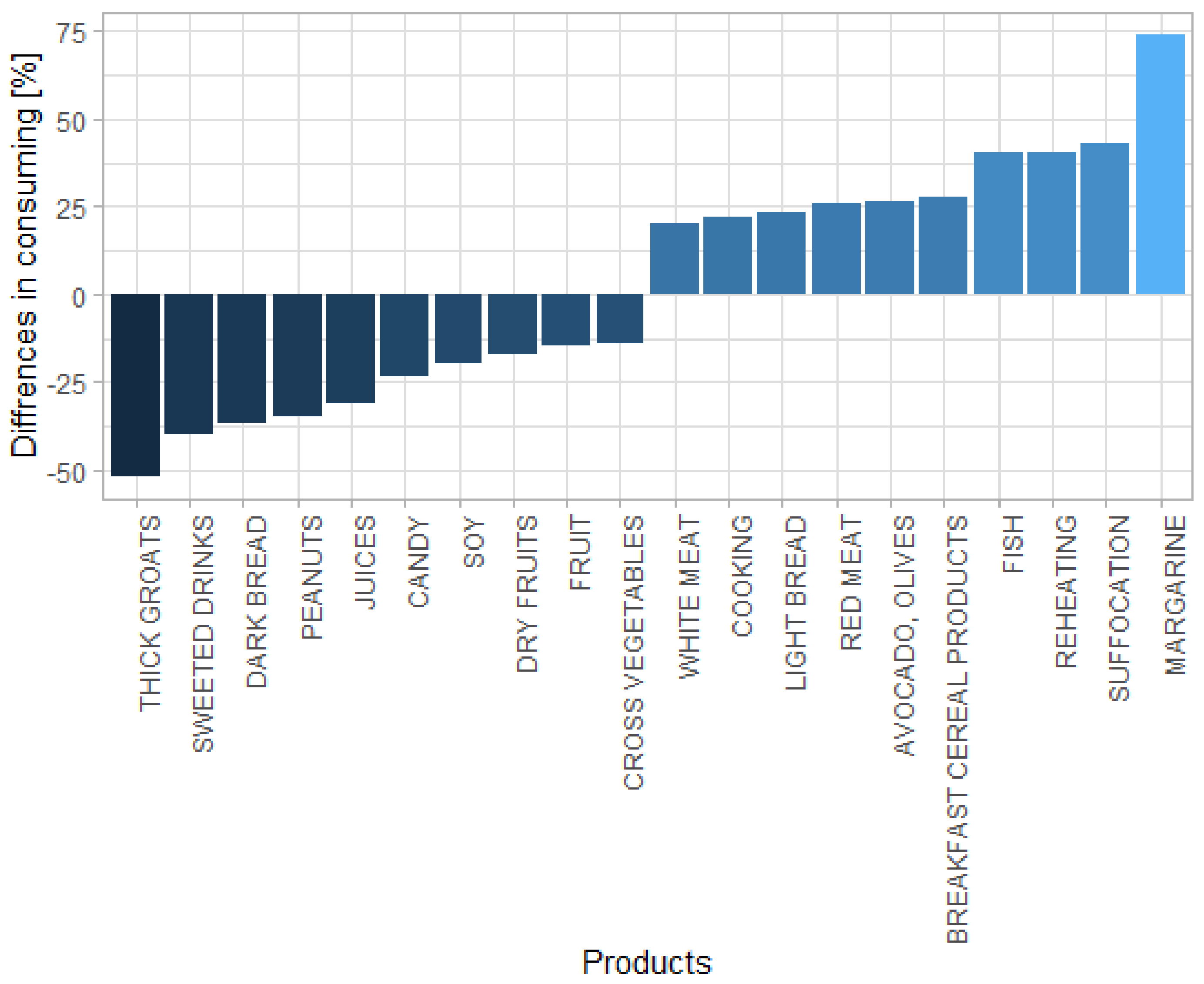
| Product | Differences (%) | p-Value |
|---|---|---|
| Margarine | 74.07 | 0.007 |
| Fish | 40.24 | 0.001 |
| Breakfast cereal products | 27.67 | 0.030 |
| Avocado, olives | 26.74 | 0.001 |
| Red meat | 26.04 | 0.015 |
| Product | Differences * (%) | p-Value |
|---|---|---|
| Thick groats | −51.96 | 0.001 |
| Sweetened drinks | −39.77 | 0.012 |
| Dark bread | −36.89 | 0.001 |
| Peanuts | −35.13 | 0.001 |
| Juices | −31.14 | 0.004 |
| Canned fruits | −23.31 | 0.055 |
| Soy | −19.94 | 0.028 |
| Dry fruits | −17.28 | 0.285 |
| Fresh fruits | −14.58 | 0.008 |
| Cross vegetables | −14.18 | 0.001 |
| Cross Vegetables | Yellow Vegetables | Leaf Vegetables | Parsley | Root Vegetables | Tomatoes | Chicken | Soy | Peanuts | |
|---|---|---|---|---|---|---|---|---|---|
| Prevotella nanceiensis | −0.32 | 0.15 | 0.24 | −0.23 | 0.14 | 0.02 | −0.34 | −0.03 | −0.05 |
| Haemophilus pittmaniae | −0.07 | 0.31 | −0.03 | −0.03 | 0.16 | 0.33 | −0.12 | −0.04 | −0.26 |
| Streptococcus sanguinis | −0.18 | −0.20 | −0.16 | −0.10 | −0.14 | −0.24 | 0.23 | 0.03 | 0.15 |
| Veillonella rogosae | 0.21 | 0.28 | 0.41 | 0.04 | 0.23 | 0.25 | −0.27 | −0.12 | −0.05 |
| Haemophilus parainfluenzae | 0.13 | 0.10 | 0.13 | 0.00 | 0.18 | 0.19 | −0.08 | 0.03 | 0.09 |
| Porphyromonas gingivalis | −0.04 | −0.05 | 0.08 | −0.08 | 0.21 | 0.02 | 0.18 | −0.20 | −0.24 |
| Streptobacillus felis | −0.34 | −0.04 | 0.06 | −0.10 | 0.13 | −0.10 | 0.04 | −0.24 | −0.13 |
| Prevotella salivae | −0.10 | −0.22 | 0.03 | 0.19 | 0.13 | −0.07 | 0.11 | −0.03 | 0.05 |
| Prevotella pallens | −0.12 | −0.07 | 0.10 | −0.02 | 0.17 | −0.19 | −0.14 | −0.03 | 0.19 |
| Megasphaera micronuciformis | 0.10 | 0.20 | 0.22 | 0.21 | 0.30 | −0.01 | −0.05 | 0.08 | 0.06 |
| Prevotella jejuni | −0.05 | −0.30 | 0.23 | 0.28 | 0.18 | −0.23 | 0.08 | 0.09 | 0.32 |
| Prevotella histicola | 0.05 | −0.02 | 0.25 | 0.16 | 0.04 | 0.00 | −0.13 | 0.20 | 0.02 |
| Prevotella melaninogenica | −0.29 | −0.52 | 0.00 | −0.09 | 0.01 | −0.13 | −0.09 | −0.16 | 0.20 |
| Streptococcus sobrinus | 0.01 | 0.22 | 0.06 | −0.09 | −0.13 | −0.07 | −0.27 | −0.10 | −0.10 |
| Fruit | Apricots | Avocado and Olives | Dry Fruits | Fresh Fruits | Canned Fruits | Milk | Eggs | Dark Bread | |
| Prevotella nanceiensis | 0.07 | −0.23 | 0.03 | −0.04 | −0.08 | 0.07 | −0.20 | −0.40 | −0.13 |
| Haemophilus pittmaniae | 0.19 | −0.31 | −0.04 | −0.07 | −0.01 | 0.12 | 0.13 | −0.11 | −0.20 |
| Streptococcus sanguinis | −0.04 | −0.08 | −0.10 | 0.14 | −0.13 | −0.16 | 0.38 | −0.02 | 0.03 |
| Veillonella rogosae | 0.19 | −0.20 | 0.02 | −0.08 | 0.14 | −0.06 | 0.17 | 0.14 | 0.13 |
| Haemophilus parainfluenzae | 0.17 | −0.02 | 0.07 | 0.09 | 0.37 | −0.03 | 0.19 | 0.06 | 0.09 |
| Porphyromonas gingivalis | −0.53 | −0.34 | −0.25 | −0.22 | −0.21 | −0.13 | −0.35 | −0.12 | −0.28 |
| Streptobacillus felis | 0.06 | 0.08 | −0.04 | −0.14 | 0.14 | −0.14 | −0.16 | −0.12 | −0.26 |
| Prevotella salivae | 0.05 | 0.26 | 0.08 | 0.09 | 0.26 | 0.31 | 0.02 | −0.28 | −0.16 |
| Prevotella pallens | −0.04 | 0.03 | 0.19 | 0.10 | −0.08 | 0.15 | −0.08 | −0.43 | −0.05 |
| Megasphaera micronuciformis | 0.00 | 0.26 | 0.24 | 0.06 | 0.04 | 0.06 | −0.02 | −0.45 | −0.03 |
| Prevotella jejuni | 0.09 | 0.09 | 0.21 | 0.29 | −0.03 | 0.13 | 0.19 | −0.22 | 0.10 |
| Prevotella histicola | 0.05 | 0.38 | 0.01 | 0.13 | 0.38 | 0.18 | −0.18 | −0.25 | 0.04 |
| Prevotella melaninogenica | 0.17 | 0.03 | −0.07 | 0.16 | 0.22 | 0.23 | 0.01 | −0.04 | −0.10 |
| Streptococcus sobrinus | −0.26 | 0.03 | 0.11 | −0.21 | −0.36 | −0.42 | −0.10 | −0.07 | 0.26 |
| White Bread | Thick Groats | Breakfast Cereal Products | Butter | Margarine | Plant Oils | Red Meat | White Meat | Fish | |
| Prevotella nanceiensis | 0.10 | −0.20 | 0.25 | −0.17 | 0.15 | −0.22 | −0.28 | −0.18 | −0.15 |
| Haemophilus pittmaniae | 0.18 | −0.19 | 0.34 | 0.04 | −0.02 | 0.02 | 0.05 | −0.31 | −0.14 |
| Streptococcus sanguinis | 0.01 | −0.10 | −0.17 | 0.09 | −0.06 | 0.35 | −0.03 | 0.12 | 0.06 |
| Veillonella rogosae | 0.04 | −0.16 | 0.19 | 0.12 | −0.13 | −0.31 | 0.07 | −0.11 | −0.12 |
| Haemophilus parainfluenzae | −0.02 | 0.03 | 0.22 | −0.04 | −0.01 | −0.24 | −0.18 | −0.20 | −0.09 |
| Porphyromonas gingivalis | 0.21 | −0.12 | 0.16 | 0.16 | −0.19 | 0.19 | 0.20 | −0.25 | −0.12 |
| Streptobacillus felis | −0.26 | −0.20 | −0.11 | −0.35 | 0.31 | −0.23 | −0.33 | −0.03 | −0.16 |
| Prevotella salivae | 0.00 | 0.07 | 0.02 | −0.04 | −0.02 | −0.18 | −0.26 | −0.19 | 0.00 |
| Prevotella pallens | 0.13 | −0.16 | 0.00 | −0.09 | 0.06 | −0.22 | −0.15 | −0.02 | −0.03 |
| Megasphaera micronuciformis | −0.07 | 0.16 | 0.01 | −0.17 | 0.10 | −0.11 | −0.34 | −0.25 | −0.05 |
| Prevotella jejuni | −0.03 | −0.12 | 0.07 | 0.13 | −0.18 | −0.23 | −0.20 | −0.15 | −0.19 |
| Prevotella histicola | −0.17 | 0.33 | 0.02 | −0.32 | 0.27 | −0.08 | −0.44 | −0.13 | −0.17 |
| Prevotella melaninogenica | 0.13 | −0.22 | 0.06 | 0.26 | −0.28 | 0.08 | 0.18 | −0.14 | −0.18 |
| Streptococcus sobrinus | −0.08 | −0.10 | −0.25 | −0.28 | 0.31 | −0.07 | 0.07 | 0.10 | −0.06 |
| Juices | Sweetened Drinks | Coffee | Alcohol | Amount of Water | Frying | Cooking | Baking | Suffocation | |
| Prevotella nanceiensis | −0.19 | 0.03 | −0.47 | −0.26 | −0.06 | −0.36 | 0.03 | −0.20 | 0.04 |
| Haemophilus pittmaniae | 0.05 | 0.30 | −0.13 | 0.03 | −0.17 | −0.12 | 0.00 | −0.46 | −0.19 |
| Streptococcus sanguinis | −0.05 | 0.12 | 0.04 | −0.16 | −0.02 | 0.17 | −0.08 | 0.08 | −0.14 |
| Veillonella rogosae | 0.07 | −0.01 | 0.06 | −0.04 | −0.10 | 0.04 | 0.00 | −0.17 | −0.31 |
| Haemophilus parainfluenzae | −0.21 | −0.18 | −0.30 | −0.21 | −0.03 | −0.37 | 0.03 | −0.51 | −0.65 |
| Porphyromonas gingivalis | 0.16 | −0.02 | 0.09 | 0.01 | −0.24 | 0.17 | 0.02 | −0.09 | 0.11 |
| Streptobacillus felis | 0.00 | −0.22 | −0.28 | −0.13 | 0.03 | −0.17 | −0.29 | 0.15 | 0.06 |
| Prevotella salivae | 0.03 | −0.09 | −0.29 | −0.24 | −0.33 | −0.27 | −0.32 | 0.00 | 0.11 |
| Prevotella pallens | −0.20 | −0.10 | −0.49 | −0.48 | −0.18 | −0.22 | −0.05 | −0.04 | 0.30 |
| Megasphaera micronuciformis | −0.09 | −0.10 | −0.37 | −0.42 | −0.04 | −0.33 | −0.19 | 0.03 | 0.16 |
| Prevotella jejuni | −0.17 | −0.17 | −0.29 | −0.36 | −0.30 | −0.12 | −0.32 | −0.04 | 0.01 |
| Prevotella histicola | 0.02 | −0.21 | −0.23 | −0.25 | −0.03 | −0.31 | −0.14 | 0.02 | 0.02 |
| Prevotella melaninogenica | −0.26 | −0.01 | −0.03 | −0.12 | −0.52 | 0.16 | −0.01 | −0.04 | −0.14 |
| Streptococcus sobrinus | −0.17 | −0.07 | 0.10 | 0.16 | 0.36 | −0.11 | 0.00 | −0.01 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapała, B.; Stefura, T.; Milewicz, T.; Wątor, J.; Piwowar, M.; Wójcik-Pędziwiatr, M.; Doręgowska, M.; Dudek, A.; Jania, Z.; Rudzińska-Bar, M. The Role of the Western Diet and Oral Microbiota in Parkinson’s Disease. Nutrients 2022, 14, 355. https://doi.org/10.3390/nu14020355
Zapała B, Stefura T, Milewicz T, Wątor J, Piwowar M, Wójcik-Pędziwiatr M, Doręgowska M, Dudek A, Jania Z, Rudzińska-Bar M. The Role of the Western Diet and Oral Microbiota in Parkinson’s Disease. Nutrients. 2022; 14(2):355. https://doi.org/10.3390/nu14020355
Chicago/Turabian StyleZapała, Barbara, Tomasz Stefura, Tomasz Milewicz, Julia Wątor, Monika Piwowar, Magdalena Wójcik-Pędziwiatr, Magdalena Doręgowska, Alicja Dudek, Zuzanna Jania, and Monika Rudzińska-Bar. 2022. "The Role of the Western Diet and Oral Microbiota in Parkinson’s Disease" Nutrients 14, no. 2: 355. https://doi.org/10.3390/nu14020355
APA StyleZapała, B., Stefura, T., Milewicz, T., Wątor, J., Piwowar, M., Wójcik-Pędziwiatr, M., Doręgowska, M., Dudek, A., Jania, Z., & Rudzińska-Bar, M. (2022). The Role of the Western Diet and Oral Microbiota in Parkinson’s Disease. Nutrients, 14(2), 355. https://doi.org/10.3390/nu14020355






