Assessment of Food Sources and the Intake of the Colourless Carotenoids Phytoene and Phytofluene in Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruits, Vegetables and Processed Food Samples
2.2. Extraction and Rapid Resolution Liquid Chromatography (RRLC) Analysis of Carotenoids in Foods
2.3. Subjects
2.4. Dietary Carotenoid Intake Assessment
3. Results
3.1. Phytoene and Phytofluene Concentration in Spanish Foods
3.2. Daily Intakes of Phytoene and Phytofluene in Spain
4. Discussion
4.1. Phytoene and Phytofluene Concentrations in Spanish Foods
4.2. Phytoene and Phytofluene Dietary Intakes in Spanish Adult Population
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limón, C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lip. Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandiç, A.I.; Mapelli-Brahm, P.; et al. Carotenoids: Considerations for their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals and Novel Foods in the Context of Sustainability, Circular Economy and Climate Change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef]
- Erdman, J.W.; Smith, J.W.; Kuchan, M.J.; Mohn, E.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.S.; Neuriner, M. Lutein and brain function. Foods 2015, 4, 547–564. [Google Scholar] [CrossRef] [Green Version]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2021, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Stinco, C.M. The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. J. Food Compost. Anal. 2018, 67, 91–103. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids and Vitamin A in Agro-Food, Nutrition, Health and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León, R.; Vila, M.; Hernánz, D.; Vilchez, C. Production of phytoene by herbicide-treated microalgae Dunaliella bardawil in two-phase systems. Biotechno. Bioeng. 2005, 92, 695–701. [Google Scholar] [CrossRef]
- Alquezar, B.; Rodrigo, M.J.; Zacarías, L. Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara Cara. Phytochemistry 2008, 69, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J. Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. J. Exp. Bot. 2003, 54, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Melendez-Martínez, A.J.; Stinco, C.M.; Liu, C.; Wang, X.-D. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem. 2013, 138, 1341–1350. [Google Scholar] [CrossRef]
- Moran, N.E.; Novotny, J.A.; Cichon, M.J.; Riedl, K.M.; Rogers, R.B.; Grainger, E.M.; Schwartz, S.J.; Erdman, J.W., Jr.; Clinton, S.K. Absorption and distribution kinetics of the 13C-labeled tomato carotenoid phytoene in healthy adults. J. Nutr. 2016, 146, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.; Greenfield, J.R.; Harats, D.; Shaish, A.; Samocha-Bonet, D. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J. Nutr. 2020, 150, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Banerjee, O.; Bhattacharjee, A.; Kumari, S.; Ananya, P. Effect of individual and combined supplementation of phytoene, phytofluene, and lycopene against nicotine-induced pancreatic islet cell dysfunction. Toxicol. Environ. Health Sci. 2020, 12, 11–22. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Meléndez-Martínez, A.J. The colourless carotenoids phytoene and phytofluene: Sources, consumption, bioavailability and health effects. Curr. Opin. Food Sci. 2021, 41, 201–209. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P. The undercover colorless carotenoids phytoene and phytofluene: Importance in agro-food and health in the Green Deal era and possibilities for innovation. Trends Food Sci. Technol. 2021, 116, 255–263. [Google Scholar] [CrossRef]
- Biehler, E.; Alkerwi, A.; Hoffmann, L.; Krause, E.; Guillaume, M.; Lair, M.L.; Bohn, T. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. J. Food Compost. Anal. 2012, 25, 56–65. [Google Scholar] [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.G.; Borge, G.I.A.; Kljak, K.; Mandić, A.I.; Mapelli-Brahm, P.; Olmedilla-Alonso, B.; Pintea, A.M.; Ravasco, F.; Saponjac, V.T.; Sereikaité, J.; et al. European Database of Carotenoid Levels in Foods. Factors Affecting Carotenoid Content. Foods 2021, 10, 912. [Google Scholar] [CrossRef]
- Stinco, C.M.; Benítez-González, A.M.; Hernanz, D.; Vicario, I.M.; Meléndez-Martínez, A.J. Development and validation of a rapid resolution liquid chromatography method for the screening of dietary plant isoprenoids: Carotenoids, tocopherols and chlorophylls. J. Chromatogr. A 2014, 1370, 162–170. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.; Bohn, T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. UV/Visible Spectroscopy. In Carotenoids. Volume 1B: Spectroscopy; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1995; pp. 13–62. [Google Scholar]
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Evaluación nutricional de la dieta española II. Micronutrientes. Sobre Datos de la Encuesta Nacional de Ingesta Dietética Española (ENIDE). Madrid. 2011. Available online: http://www.cibr.es/ka/apps/cibr/docs/estudio-enide-1.pdf (accessed on 20 July 2021).
- Santiago, R.E.; de Miguel, B.B.; Vives, C.C.; Alonso, B.O. Software application for the calculation of dietary intake of individual carotenoids and of its contribution to vitamin A intake. Nutr. Hosp. 2013, 28, 823–829. [Google Scholar]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Benítez-González, A.; Stinco, C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys. 2015, 572, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Mapelli-Brahm, P.; Stinco, C.M.; Rodrigo, M.J.; Zacarías, L.; Meléndez-Martínez, A.J. Impact of thermal treatments on the bioaccessibility of phytoene and phytofluene in relation to changes in the microstructure and size of orange juice particles. J. Funct. Foods 2018, 46, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Meléndez-Martínez, A.J.; Fraser, P.D.; Bramley, P.M. Accumulation of health promoting phytochemicals in wild relatives of tomato and their contribution to in vitro antioxidant activity. Phytochemistry 2010, 71, 1104–1114. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Mapelli-Brahm, P.; Hernanz, D.; Stinco, C.M.; Beltrán-Sinchiguano, E.; Meléndez-Martínez, A.J. Study of commercial quality parameters, sugars, phenolics, carotenoids and plastids in different tomato varieties. Food Chem. 2019, 277, 480–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lado, J.; Zacarías, L.; Gurrea, A.; Page, A.; Stead, A.; Rodrigo, M.J.; Cara, C. Exploring the diversity in Citrus fruit colouration to decipher the relationship between plastid ultrastructure and carotenoid composition. Planta 2015, 242, 645–661. [Google Scholar] [CrossRef]
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P. Impact of nitrogen supply limitation on tomato fruit composition. Sci. Hortic. 2020, 264, 109173. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Corell, M.; Stinco, C.M.; Hernanz, D.; Moriana, A.; Meléndez-Martínez, A.J. Effect of regulated deficit irrigation on quality parameters, carotenoids and phenolics of diverse tomato varieties (Solanum lycopersicum L.). Food Res. Int. 2017, 96, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Hernanz, D.; Benítez-González, A.M.; Stinco, C.M.; Meléndez-Martínez, A.J. Antioxidants (carotenoids and phenolics) profile of cherry tomatoes as influenced by deficit irrigation, ripening and cluster. Food Chem. 2018, 240, 870–884. [Google Scholar] [CrossRef] [Green Version]
- Stinco, C.M.; Sentandreu, E.; Mapelli-Brahm, P.; Navarro, J.L.; Vicario, I.M.; Meléndez-Martínez, A.J. Influence of high pressure homogenization and pasteurization on the in vitro bioaccessibility of carotenoids and flavonoids in orange juice. Food Chem. 2020, 331, 127259. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Khachik, F. Carotenoids in Food. In Carotenoids. Vol. 5: Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 2009; pp. 55–57. [Google Scholar]
- Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Assessment of dietary vitamin A intake (retinol, α-carotene, β-carotene, β-cryptoxanthin) and its sources in the National Survey of Dietary Intake in Spain (2009–2010). Int. J. Food Sci. Nutr. 2015, 66, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Estévez-Santiago, R.; Beltrán-de-Miguel, B.; Olmedilla-Alonso, B. Assessment of dietary lutein, zeaxanthin and lycopene intakes and sources in the Spanish Survey of Dietary Intake (2009–2010). Int. J. Food Sci. Nutr. 2016, 67, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.-Y.; Katz, N.B. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med. 2002, 227, 845–851. [Google Scholar] [CrossRef]
- Khachik, F.; Spangler, C.J.; Smith, J.C., Jr.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, quantification and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef]
- Stinco, C.M.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; Hernanz, D.; Vicario, I.M. Simultaneous determination of dietary isoprenoids (carotenoids, chlorophylls and tocopherols) in human faeces by Rapid Resolution Liquid Chromatography. J. Chromatog. A 2019, 1583, 63–72. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; Beltrán-de-Miguel, B.; Samaniego-Aguilar, K.X.; Sánchez-Prieto, M.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Extraction and analysis by HPLC-DAD of carotenoids in human faeces from Spanish adults. Antioxidants 2020, 9, 484. [Google Scholar] [CrossRef]
- Zhang, C.-R.; Dissanayake, A.A.; Nair, M.G. Functional food property of honey locust (Gleditsia triacanthos) flowers. J. Func. Foods 2015, 18, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Gijsbers, L.; van Eekelen, H.D.L.M.; de Haan, L.H.J.; Swier, J.M.; Heijink, N.L.; Kloet, S.K.; Rietjens, I.M.C.M. Induction of peroxisome proliferator-activated receptor γ (PPARγ)-mediated gene expression by tomato (Solanum lycopersicum L.) extracts. J. Agric. Food Chem. 2013, 61, 3419–3427. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Nascimento, A.F.; Wang, Y.; Liu, C.; Mao, Y.; Wang, X.-D. Effect of tomato extract supplementation against high-fat diet-induced hepatic lesions. Hepatobiliary Surg. Nutr. 2013, 2, 198–208. [Google Scholar] [PubMed]
- Shaish, A.; Harari, A.; Kamari, Y.; Soudant, E.; Harats, D.; Ben-Amotz, A. A carotenoid algal preparation containing phytoene and phytofluene inhibited LDL oxidation in vitro. Plant. Foods Human Nutr. 2008, 63, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.; Atzmon, A.; Danilenko, M.; Levy, J.; Sharoni, Y. Lycopene and other carotenoids inhibit estrogenic activity of 17-β-estradiol and genistein in cancer cells. Breast Cancer Res. Treat. 2007, 104, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.K.; Stroud, C.K.; Nakamura, M.T.; Lila, M.A.; Erdman, J.W. Serum testosterone is reduced following short-term phytofluene, lycopene, or tomato powder consumption in F344 rats. J. Nutr. 2006, 136, 2813–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar]

| Name | Spanish Name | Scientific Name | Edible Portion | Content (µg)/100 g Fresh Weight (a) | Food Intake (g/p/day) | Dietary Intake (µg/day) | ||
|---|---|---|---|---|---|---|---|---|
| Phytoene | Phytofluene | Phytoene | Phytofluene | |||||
| Fruits | ||||||||
| Apple | Manzana | Malus domestica | 80 | 41.4 | 0.0 | 0.0 | ||
| Apricot | Albaricoque | Prunus armeniaca | 93 | 2818 | 616 | 1.3 | 33.0 | 7.2 |
| Avocado | Aguacate | Persea americana | 72 | 1 | 0.0 | 0.0 | ||
| Banana | Plátano | Musa × paradisiaca | 60 | 24.3 | 0.0 | 0.0 | ||
| Chesnut | Castaña | Castanea sativa | 82 | 0.2 | 0.0 | 0.0 | ||
| Flat peach | Paraguaya | Prunus persica var. platycarpa | 88 | 81 | 12 | 0.6 | 0.4 | 0.1 |
| Grapefruit | Pomelo | Citrus × paradisi | 68 | 151 | 6 | 0.2 | 0.2 | 0.0 |
| Guava | Guayaba | Psidium guajava | 89 | 454 | 83 | 0.005 | 0.0 | 0.0 |
| Guava (red) | Guayaba (roja) | Psidium guajava | 89 | 187 | 32 | 0.005 | 0.0 | 0.0 |
| Kaki | Kaki | Diospyros kaki | 87 | 0.6 | 0.0 | 0.0 | ||
| Kiwi (green) | Kiwi (verde) | Actinidia deliciosa | 66 | 5.7 | 0.0 | 0.0 | ||
| Kiwi (yellow) | Kiwi (amarillo) | Actinidia deliciosa | 66 | 1.4 | 0.0 | 0.0 | ||
| Lemon | Limón | Citrus × limon | 60 | 0.9 | 0.0 | 0.0 | ||
| Loquat (b) | Níspero | Eriobotrya japonica | 65 | 26 | 0.1 | 0.0 | ||
| Mandarine | Mandarina | Citrus reticulata | 73 | 60 | 51 | 9.8 | 4.3 | 3.7 |
| Mango | Mango | Mangifera indica | 68 | 0.4 | 0.0 | 0.0 | ||
| Melon (cantaloupe) | Melón galo | Cucumis melo var. reticulatus | 55 | 4.8 | 0.0 | 0.0 | ||
| Melon (white) | Melón piel de sapo | Cucumis melo ‘Santa Claus’ | 62 | 4.8 | 0.0 | 0.0 | ||
| Melon (yellow) | Melón (amarillo) | Cucumis melo L. | 60 | 4.8 | 0.0 | 0.0 | ||
| Nectarine | Nectarina | Prunus persica var. nucipersica | 89 | 29 | 6 | 0.04 | 0.0 | 0.0 |
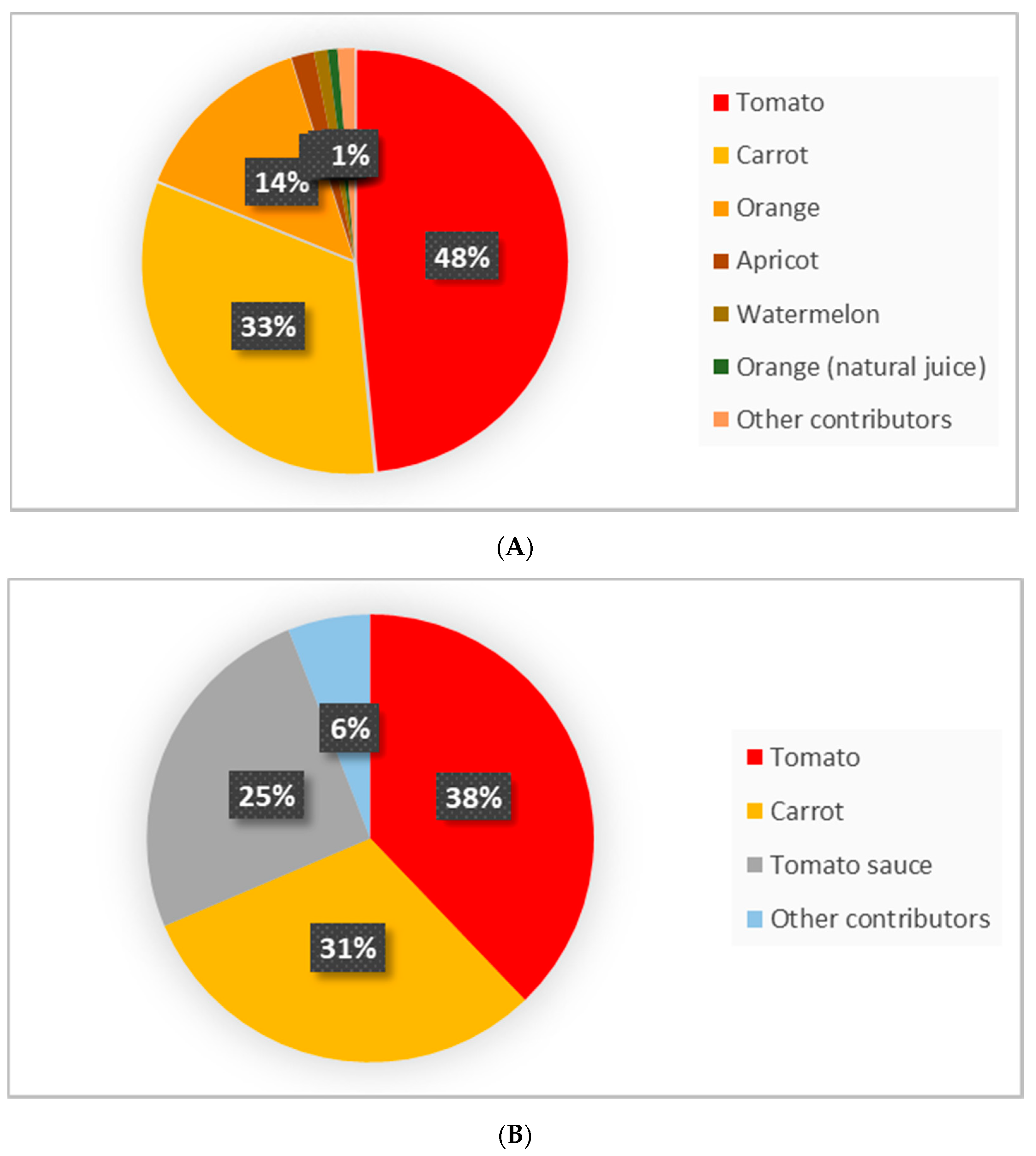
| Orange (b) | Naranja | Citrus × sinensis | 72 | 1065 | 34.6 | 265.6 | ||
| Orange juice (freshly squeeze) | Naranja (zumo natural) | Citrus × sinensis | 100 | 122 | 40 | 11.9 | 14.5 | 4.8 |
| Papaya | Papaya | Carica papaya | 75 | 12 | 10 | 0.9 | 0.1 | 0.1 |
| Peach gelo | Melocotón gelo | Prunus persica | 88 | 3.8 | 0.0 | 0.0 | ||
| Peach (igloo) | Melocotón iglú | Prunus persica | 88 | 3.8 | 0.0 | 0.0 | ||
| Peach (red) | Melocotón (rojo) | Prunus persica | 69 | 99 | 14 | 3.8 | 2.6 | 0.4 |
| Peach (yellow) | Melocotón (amarillo) | Prunus persica | 69 | 26 | 2 | 3.8 | 0.7 | 0.1 |
| Pear (b) | Pera | Pyrus communis | 80 | 28.5 | 18.3 | 4.2 | ||
| Pineapple | Piña | Ananas comosus | 57 | 6.7 | 0.0 | 0.0 | ||
| Plum (green) | Ciruela (verde) | Prunus domestica subsp. domestica | 85 | 1.0 | 0.0 | 0.0 | ||
| Plum (yellow) | Ciruela (amarilla) | Prunus domestica subsp. domestica | 92 | 1.0 | 0.0 | 0.0 | ||
| Quince | Membrillo | Cydonia oblonga | 61 | 116 | 44 | 0.4 | 0.2 | 0.1 |
| Watermelon | Sandía | Citrullus lanatus | 78 | 144 | 55 | 17.0 | 19.0 | 7.3 |
| Subtotal | 209.4 | 344.8 | 23.8 | |||||
| Vegetables | ||||||||
| Artichoke | Alcachofa | Cynara scolymus | 47 | 2.6 | 0.0 | 0.0 | ||
| Asparagus (green) | Espárrago (verde) | Asparagus officinalis | 50 | 1.8 | 0.0 | 0.0 | ||
| Beans (green) | Judías (verdes) | Phaseolus vulgaris var. vulgaris | 93 | 8.3 | 0.0 | 0.0 | ||
| Broccoli | Brécol | Brassica oleracea var. italica | 97 | 0.1 | 0.0 | 0.0 | ||
| Cabbage | Col | Brassica oleracea | 2.3 | 0.0 | 0.0 | |||
| Carrot | Zanahoria | Daucus carota | 85 | 7264 | 1701 | 10.0 | 618.1 | 144.7 |
| Cauliflower | Coliflor | Brassica oleracea var. botrytis | 84 | 2.9 | 0.0 | 0.0 | ||
| Chard | Acelga | Beta vulgaris var. Cicla | 88 | 3.0 | 0.0 | 0.0 | ||
| Corn | Maíz | Zea mays | 100 | 1.8 | 0.0 | 0.0 | ||
| Cucumber | Pepino | Cucumis sativus | 70 | 4.6 | 0.0 | 0.0 | ||
| Eggplant | Berenjena | Solanum melongena | 85 | 3.4 | 0.0 | 0.0 | ||
| Garlic (white) | Ajo (blanco) | Allium sativum | 100 | 2.6 | 0.0 | 0.0 | ||
| Lamb’s lettuce | Canónigo | Valerianella locusta | 100 | 0.3 | 0.0 | 0.0 | ||
| Lettuce (heart) | Lechuga (cogollo) | Lactiva longifolia | 100 | 6.9 | 0.0 | 0.0 | ||
| Lettuce (iceberg) | Lechuga (iceberg) | Lactuca sativa var. Capitata | 88 | 6.9 | 0.0 | 0.0 | ||
| Lettuce (romana) | Lechuga (romana) | Lactuca sativa | 50 | 6.9 | 0.0 | 0.0 | ||
| Mushroom | Champiñón | Agaricus bisporus | 80 | 5.6 | 0.0 | 0.0 | ||
| Peas | Guisante | Pisum sativum | 100 | 3.5 | 0.0 | 0.0 | ||
| Pepper (green) | Pimiento (verde) | Capsicum annuum Group | 95 | 3.4 | 0.0 | 0.0 | ||
| Pepper (orange) | Pimiento (naranja) | Capsicum annuum Group | 87 | 3.4 | 0.0 | 0.0 | ||
| Pepper (red) | Pimiento (rojo) | Capsicum annuum Group | 85 | 3.4 | 0.0 | 0.0 | ||
| Pepper (yellow) | Pimiento (amarillo) | Capsicum annuum Group | 81 | 3.4 | 0.0 | 0.0 | ||
| Potato | Patata | Solanum tuberosum | 74 | 68.4 | 0.0 | 0.0 | ||
| Pumpkin | Calabaza | Cucurbita maxima | 67 | 2.2 | 0.0 | 0.0 | ||
| Spinach | Espinaca | Spinacia oleracea | 76 | 4.4 | 0.0 | 0.0 | ||
| Sweet potato | Batata | Ipomoea batatas | 100 | 0.1 | 0.0 | 0.0 | ||
| Tomato | Tomate | Solanum lycopersicum | 97 | 1697 | 330 | 55.6 | 915.2 | 178.0 |
| Zucchini | Calabacín | Cucurbita pepo | 79 | 6.3 | 0.0 | 0.0 | ||
| Subtotal | 224.1 | 1533.3 | 322.7 | |||||
| Processed Foods | ||||||||
| Orange juice (from concentrate) | Naranja (zumo a partir de concentrado) | 100 | 0 | 0 | 11.9 | 0.0 | 0.0 | |
| Tomato canned | Tomate enlatado | 100 | 1878 | 999 | 0 | 0.0 | 0.0 | |
| Tomato juice | Tomate en zumo | 100 | 1994 | 884 | 0.4 | 7.8 | 3.4 | |
| Tomato sauce (b) | Tomate en salsa | 100 | 1165 | 10.3 | 119.6 | |||
| Ketchup | Kétchup | 100 | 391 | 99 | 1.0 | 4.0 | 1.0 | |
| Subtotal | 23.6 | 11.8 | 124.0 | |||||
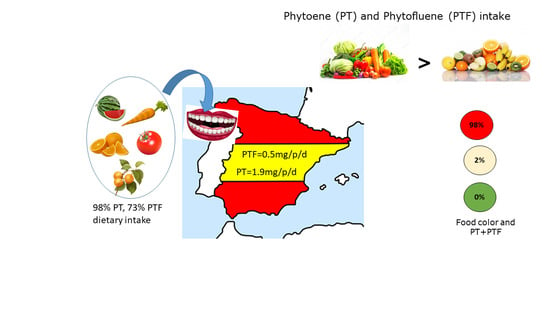
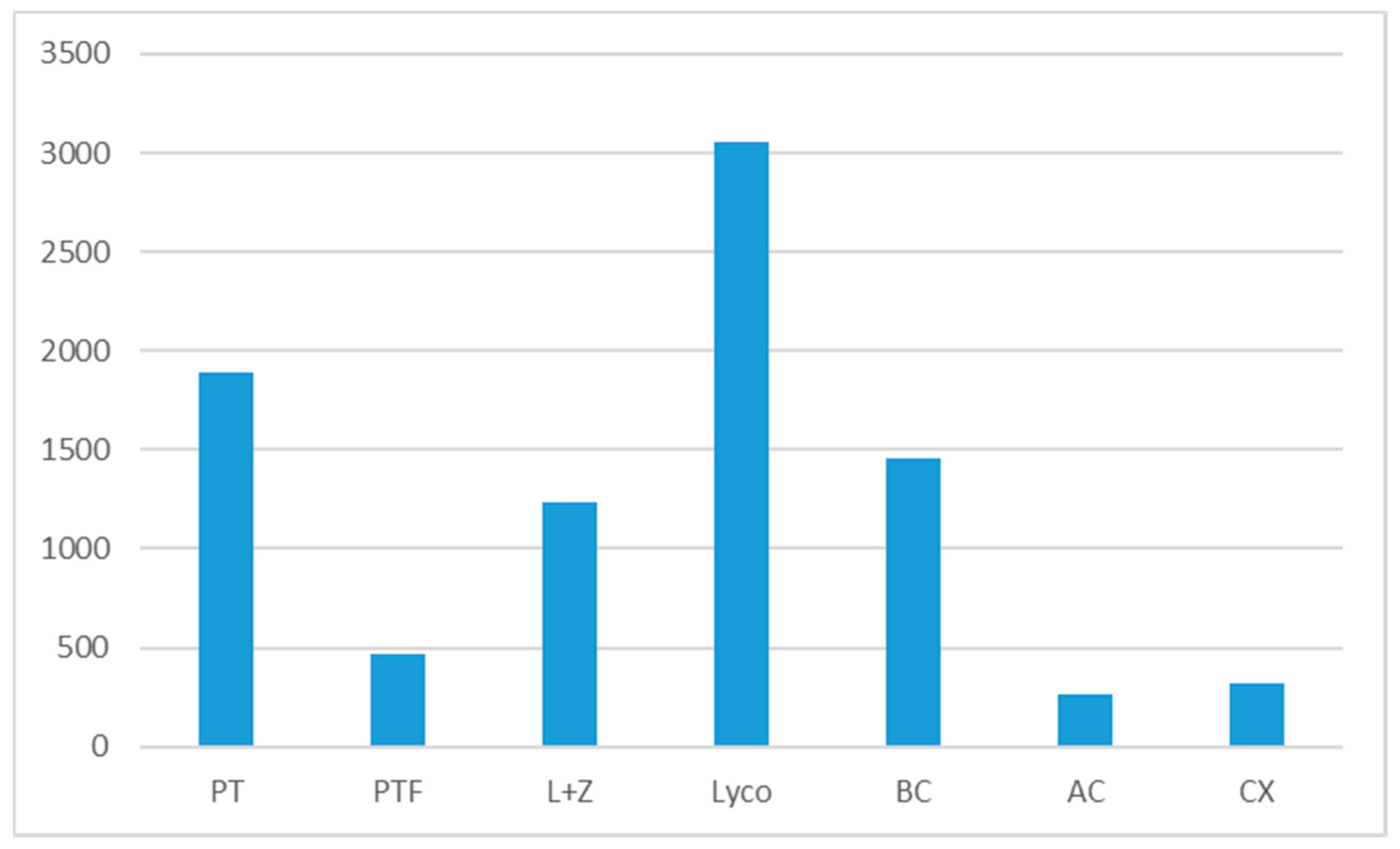
| TOTAL | 457.1 | 1889.9 | 470.5 | |||||
| Food Group | Phytoene | Phytofluene |
|---|---|---|
| Fruit | 330.5 | 18.8 |
| Vegetables | 1533.3 | 322.7 |
| Sauces | 4.0 | 120.7 |
| Non alcoholic beverages (orange juice, tomato juice) | 22.3 | 8.2 |
| Total | 1890 | 470 |
| Food Color | Phytoene | Phytofluene |
|---|---|---|
| Red/orange | 1852.0 | 463.0 |
| Green | 0.0 | 0.0 |
| White-yellowish | 37.9 | 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedilla-Alonso, B.; Benítez-González, A.M.; Estévez-Santiago, R.; Mapelli-Brahm, P.; Stinco, C.M.; Meléndez-Martínez, A.J. Assessment of Food Sources and the Intake of the Colourless Carotenoids Phytoene and Phytofluene in Spain. Nutrients 2021, 13, 4436. https://doi.org/10.3390/nu13124436
Olmedilla-Alonso B, Benítez-González AM, Estévez-Santiago R, Mapelli-Brahm P, Stinco CM, Meléndez-Martínez AJ. Assessment of Food Sources and the Intake of the Colourless Carotenoids Phytoene and Phytofluene in Spain. Nutrients. 2021; 13(12):4436. https://doi.org/10.3390/nu13124436
Chicago/Turabian StyleOlmedilla-Alonso, Begoña, Ana M. Benítez-González, Rocío Estévez-Santiago, Paula Mapelli-Brahm, Carla M. Stinco, and Antonio J. Meléndez-Martínez. 2021. "Assessment of Food Sources and the Intake of the Colourless Carotenoids Phytoene and Phytofluene in Spain" Nutrients 13, no. 12: 4436. https://doi.org/10.3390/nu13124436