Effect of Black Tea Consumption on Urinary Risk Factors for Kidney Stone Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Study Procedure
2.3. Urinary Parameters
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearle, M.S.; Calhoun, E.A.; Curhan, G.C. Urologic diseases in America project: Urolithiasis. J. Urol. 2005, 173, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Strohmaier, W.L. Economics of stone disease/treatment. Arab. J. Urol. 2012, 10, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, J.B.; Matlaga, B.R. Epidemiology and economics of nephrolithiasis. Investig. Clin. Urol. 2017, 58, 299–306. [Google Scholar] [CrossRef]
- Romero, V.; Akpinar, H.; Assimos, D.G. Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev. Urol. 2010, 12, e86–e96. [Google Scholar]
- Hesse, A.; Brändle, E.; Wilbert, D.; Köhrmann, K.U.; Alken, P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur. Urol. 2003, 44, 709–713. [Google Scholar] [CrossRef]
- Chewcharat, A.; Curhan, G. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis 2021, 49, 27–39. [Google Scholar] [CrossRef]
- Uribarri, J.; Oh, M.S.; Carroll, H.J. The first kidney stone. Ann. Intern. Med. 1989, 111, 1006–1009. [Google Scholar] [CrossRef]
- Tiselius, H.G. Metabolic risk-evaluation and prevention of recurrence in stone disease: Does it make sense? Urolithiasis 2016, 44, 91–100. [Google Scholar] [CrossRef]
- Siener, R.; Hesse, A. Fluid intake and epidemiology of urolithiasis. Eur. J. Clin. Nutr. 2003, 57 (Suppl. 2), S47–S51. [Google Scholar] [CrossRef] [Green Version]
- Borghi, L.; Meschi, T.; Amato, F.; Briganti, A.; Novarini, A.; Giannini, A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J. Urol. 1996, 155, 839–843. [Google Scholar] [CrossRef]
- Siener, R. Nutrition and kidney stone disease. Nutrients 2021, 13, 1917. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Spiegelman, D.; Stampfer, M.J. Prospective study of beverage use and the risk of kidney stones. Am. J. Epidemiol. 1996, 143, 240–247. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Stampfer, M.J. Beverage use and risk for kidney stones in women. Ann. Intern. Med. 1998, 128, 534–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curhan, G.C.; Willett, W.C.; Knight, E.L.; Stampfer, M.J. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch. Intern. Med. 2004, 164, 885–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Dietary factors and the risk of incident kidney stones in men: New insights after 14 years of follow-up. J. Am. Soc. Nephrol. 2004, 15, 3225–3232. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Dietary and lifestyle risk factors associated with incident kidney stones in men and women. J. Urol. 2017, 198, 858–863. [Google Scholar] [CrossRef]
- Lin, B.B.; Lin, M.E.; Huang, R.H.; Hong, Y.K.; Lin, B.L.; He, X.J. Dietary and lifestyle factors for primary prevention of nephrolithiasis: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 267. [Google Scholar] [CrossRef]
- Barghouthy, Y.; Corrales, M.; Doizi, S.; Somani, B.K.; Traxer, O. Tea and coffee consumption and pathophysiology related to kidney stone formation: A systematic review. World J. Urol. 2021, 39, 2417–2426. [Google Scholar] [CrossRef]
- Barghouthy, Y.; Corrales, M.; Doizi, S.; Somani, B.K.; Traxer, O. Tea and coffee consumption and the risk of urinary stones—A systematic review of the epidemiological data. World J. Urol. 2021, 39, 2895–2901. [Google Scholar] [CrossRef]
- Hönow, R.; Gu, K.L.R.; Hesse, A.; Siener, R. Oxalate content of green tea of different origin, quality, preparation and time of harvest. Urol. Res. 2010, 38, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Seidler, A.; Voss, S.; Hesse, A. Oxalate content of beverages. J. Food Compos. Anal. 2017, 63, 184–188. [Google Scholar] [CrossRef]
- McKay, D.W.; Seviour, J.P.; Comerford, A.; Vasdev, S.; Massey, L.K. Herbal tea: An alternative to regular tea for those who form calcium oxalate stones. J. Am. Diet. Assoc. 1995, 95, 360–361. [Google Scholar] [CrossRef]
- Holmes, R.P.; Goodman, H.O.; Assimos, D.G. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 2001, 59, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Siener, R.; Bade, D.J.; Hesse, A.; Hoppe, B. Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J. Transl. Med. 2013, 11, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch, A.M.; Kasidas, G.P.; Rose, G.A. Urine composition in normal subjects after oral ingestion of oxalate-rich foods. Clin. Sci. 1981, 60, 411–418. [Google Scholar] [CrossRef]
- Brinkley, L.J.; Gregory, J.; Pak, C.Y.C. A further study of oxalate bioavailability in foods. J. Urol. 1990, 144, 94–96. [Google Scholar] [CrossRef]
- Siener, R.; Bitterlich, N.; Birwé, H.; Hesse, A. The impact of diet on urinary risk factors for cystine stone formation. Nutrients 2021, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Struwe, F.; Hesse, A. Effect of L-methionine on the risk of phosphate stone formation. Urology 2016, 98, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Werness, P.G.; Brown, C.M.; Smith, L.H.; Finlayson, B. EQUIL2: A basic computer program for the calculation of urinary saturation. J. Urol. 1985, 134, 1242–1244. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. The role of tea in human health: An update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef]
- Cleverdon, R.; Elhalaby, Y.; McAlpine, M.D.; Gittings, W.; Ward, W.E. Total polyphenol content and antioxidant capacity of tea bags: Comparison of black, green, red rooibos, chamomile and peppermint over different steep times. Beverages 2018, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Charrier, M.J.S.; Savage, G.P.; Vanhanen, L. Oxalate content and calcium binding capacity of tea and herbal teas. Asia Pac. J. Clin. Nutr. 2002, 11, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Hönow, R.; Hesse, A. Comparison of extraction methods for the determination of soluble and total oxalate in foods by HPLC-enzyme-reactor. Food Chem. 2002, 78, 511–521. [Google Scholar] [CrossRef]
- Massey, L.K. Tea oxalate. Nutr. Rev. 2000, 58, 88–89. [Google Scholar] [CrossRef]
- Savage, G.P.; Charrier, M.J.S.; Vanhanen, L. Bioavailability of soluble oxalate from tea and the effect of consuming milk with the tea. Eur. J. Clin. Nutr. 2003, 57, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Liebman, M.; Murphy, S. Low oxalate bioavailability from black tea. Nutr. Res. 2007, 27, 273–278. [Google Scholar] [CrossRef]
- Voss, S.; Hesse, A.; Zimmermann, D.J.; Sauerbruch, T.; von Unruh, G.E. Intestinal oxalate absorption is higher in idiopathic calcium oxalate stone formers than in healthy controls: Measurements with the [13C2]oxalate absorption test. J. Urol. 2006, 175, 1711–1715. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Sadeghian, R.; Abbasi, B. The associations between tea and coffee drinking and risk of calcium-oxalate renal stones. Plant Foods Hum. Nutr. 2021, 76, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Von Unruh, G.E.; Voss, S.; Sauerbruch, T.; Hesse, A. Dependence of oxalate absorption on the daily calcium intake. J. Am. Soc. Nephrol. 2004, 15, 1567–1573. [Google Scholar] [CrossRef] [Green Version]
- Zuckerman, J.M.; Assimos, D.G. Hypocitraturia: Pathophysiology and medical management. Rev. Urol. 2009, 11, 134–144. [Google Scholar]
- Zacchia, M.; Preisig, P. Low urinary citrate: An overview. J. Nephrol. 2010, 23, S49–S56. [Google Scholar] [PubMed]
- Hamm, L.L.; Hering-Smith, K.S. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol. Metab. Clin. N. Am. 2002, 31, 885–893. [Google Scholar] [CrossRef]
- Simpson, D.P. Citrate excretion: A window on renal metabolism. Am. J. Physiol. 1983, 244, F223–F234. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Caffeine intake and the risk of kidney stones. Am. J. Clin. Nutr. 2014, 100, 1596–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitt, E.; Pell, D.; Cole, D. Assessing caffeine intake in the United Kingdom diet. Food Chem. 2013, 140, 421–426. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the Safety of Caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef] [Green Version]
- Rieg, T.; Steigele, H.; Schnermann, J.; Richter, K.; Osswald, H.; Vallon, V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J. Pharmacol. Exp. Ther. 2005, 313, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Massey, L.K.; Wise, K.J. Impact of gender and age on urinary water and mineral excretion responses to acute caffeine doses. Nutr. Res. 1992, 12, 605–612. [Google Scholar] [CrossRef]
| Fruit Tea | Black Tea | p-Value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Volume (L/24 h) | 2.477 ± 0.504 | 2.378 ± 0.420 | 0.684 |
| Density (g/cm3) | 1.008 ± 0.002 | 1.008 ± 0.001 | 0.813 |
| pH | 6.27 ± 0.29 | 6.28 ± 0.35 | 0.959 |
| Sodium (mmol/24 h) | 205 ± 47 | 206 ± 22 | 0.959 |
| Potassium (mmol/24 h) | 72 ± 18 | 68 ± 14 | 0.386 |
| Calcium (mmol/24 h) | 5.41 ± 1.81 | 5.40 ± 1.56 | 0.879 |
| Magnesium (mmol/24 h) | 5.11 ± 1.13 | 5.16 ± 0.86 | 0.647 |
| Ammonium (mmol/24 h) | 36.8 ± 6.6 | 38.6 ± 11.0 | 0.575 |
| Chloride (mmol/24 h) | 205 ± 49 | 204 ± 21 | 0.959 |
| Phosphate (mmol/24 h) | 31.1 ± 3.5 | 31.6 ± 5.1 | 0.799 |
| Sulfate (mmol/24 h) | 20.4 ± 4.4 | 17.6 ± 4.3 | 0.169 |
| Creatinine (mmol/24 h) | 18.20 ± 2.02 | 17.32 ± 1.58 | 0.203 |
| Uric acid (mmol/24 h) | 3.58 ± 0.53 | 3.40 ± 0.43 | 0.169 |
| Oxalate (mmol/24 h) | 0.309 ± 0.052 | 0.340 ± 0.032 | 0.139 |
| Citrate (mmol/24 h) | 2.793 ± 0.664 | 3.387 ± 0.789 | 0.002 |
| RS Calcium oxalate | 3.117 ± 1.032 | 3.514 ± 1.249 | 0.386 |
| RS Uric acid | 0.741 ± 0.519 | 0.760 ± 0.692 | 0.799 |
| RS Struvite | 0.065 ± 0.069 | 0.082 ± 0.083 | 0.285 |
| Fruit Tea | Black Tea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interval of Urine Collection (Hours) | Interval of Urine Collection (Hours) | |||||||||||
| 7–10 | 10–13 | 13–16 | 16–19 | 19–22 | 22–7 | 7–10 | 10–13 | 13–16 | 16–19 | 19–22 | 22–7 | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Volume (L/h) | 0.080 ± 0.041 | 0.219 ± 0.108 | 0.154 ± 0.082 | 0.113 ± 0.041 | 0.085 ± 0.036 | 0.058 ± 0.025 | 0.064 ± 0.026 | 0.158 ± 0.079 * | 0.145 ± 0.067 | 0.172 ± 0.056 * | 0.107 ± 0.046 | 0.049 ± 0.016 |
| Density (g/cm3) | 1.012 ± 0.005 | 1.005 ± 0.002 | 1.008 ± 0.005 | 1.007 ± 0.005 | 1.011 ± 0.006 | 1.015 ± 0.005 | 1.013 ± 0.006 | 1.006 ± 0.004 | 1.008 ± 0.004 | 1.007 ± 0.004 | 1.010 ± 0.004 | 1.014 ± 0.004 |
| pH | 6.03 ± 0.47 | 6.47 ± 0.53 | 6.46 ± 0.58 | 6.20 ± 0.40 | 6.08 ± 0.35 | 5.83 ± 0.32 | 5.84 ± 0.25 | 6.63 ± 0.46 | 6.59 ± 0.36 | 6.33 ± 0.70 | 5.98 ± 0.58 | 5.83 ± 0.41 |
| Sodium (mmol/h) | 7.75 ± 2.73 | 12.51 ± 7.82 | 12.72 ± 6.10 | 7.74 ± 2.76 | 8.68 ± 3.98 | 6.34 ± 1.60 | 6.49 ± 3.76 | 11.17 ± 3.75 | 11.88 ± 4.70 | 12.08 ± 3.64 * | 10.63 ± 6.47 | 5.48 ± 1.50 |
| Potassium (mmol/h) | 3.08 ± 1.39 | 4.37 ± 1.96 | 4.87 ±1.49 | 2.45 ± 1.12 | 2.88 ± 1.52 | 2.12 ± 0.64 | 2.99 ± 1.51 | 4.91 ± 1.89 | 4.41 ± 2.45 | 2.83 ± 0.80 | 2.51 ± 1.18 | 1.65 ± 0.49 * |
| Calcium (mmol/h) | 0.270 ± 0.120 | 0.377 ± 0.241 | 0.290 ± 0.139 | 0.243 ± 0.118 | 0.217 ± 0.134 | 0.135 ± 0.053 | 0.259 ± 0.145 | 0.286 ± 0.121 | 0.271 ± 0.124 | 0.333 ± 0.122 * | 0.270 ± 0.122 | 0.127 ± 0.048 |
| Magnesium (mmol/h) | 0.228 ± 0.073 | 0.253 ± 0.121 | 0.212 ± 0.056 | 0.213 ± 0.081 | 0.218 ± 0.115 | 0.194 ± 0.053 | 0.224 ± 0.073 | 0.195 ± 0.06 | 0.208 ± 0.09 | 0.301 ± 0.09 * | 0.249 ± 0.09 | 0.181 ± 0.068 |
| Ammonium (mmol/h) | 1.78 ± 0.53 | 1.81 ± 0.53 | 1.29 ± 0.52 | 1.49 ± 0.54 | 1.30 ± 0.46 | 1.54 ± 0.43 | 1.80 ± 0.60 | 1.39 ± 0.71 | 1.39 ± 0.86 | 2.07 ± 1.02 | 1.76 ± 0.41 * | 1.48 ± 0.66 |
| Chloride (mmol/h) | 8.97 ± 2.69 | 14.40 ± 7.83 | 13.04 ± 5.53 | 7.54 ± 2.42 | 7.87 ± 3.79 | 5.51 ± 1.51 | 8.29 ± 4.24 | 13.55 ± 3.97 | 12.18 ± 5.20 | 10.82 ± 3.43 * | 9.53 ± 5.37 | 4.49 ± 1.33 |
| Phosphate (mmol/h) | 0.87 ± 0.44 | 0.89 ± 0.38 | 1.30 ± 0.53 | 1.28 ± 0.33 | 1.63 ± 0.53 | 1.47 ± 0.21 | 0.81 ± 0.34 | 0.67 ± 0.39 | 1.32 ± 0.50 | 1.75 ± 0.82 | 1.79 ± 0.61 | 1.39 ± 0.41 |
| Sulfate (mmol/h) | 0.77 ± 0.33 | 0.75 ± 0.35 | 0.85 ± 0.27 | 0.89 ± 0.33 | 0.97 ± 0.44 | 0.86 ± 0.15 | 0.71 ± 0.37 | 0.63 ± 0.24 | 0.75 ± 0.33 | 0.95 ± 0.38 | 0.83 ± 0.40 | 0.66 ± 0.21 * |
| Creatinine (mmol/h) | 0.759 ± 0.199 | 0.674 ± 0.163 | 0.752 ± 0.172 | 0.718 ± 0.211 | 0.716 ± 0.278 | 0.815 ± 0.122 | 0.801 ± 0.278 | 0.691 ± 0.296 | 0.669 ± 0.274 | 0.840 ± 0.239 | 0.751 ± 0.191 | 0.673 ± 0.210 * |
| Uric acid (mmol/h) | 0.155 ± 0.066 | 0.181 ± 0.063 | 0.177 ± 0.047 | 0.140 ± 0.039 | 0.133 ± 0.054 | 0.136 ± 0.031 | 0.144 ± 0.058 | 0.165 ± 0.058 | 0.163 ± 0.062 | 0.194 ± 0.064 * | 0.128 ± 0.034 | 0.113 ± 0.038 * |
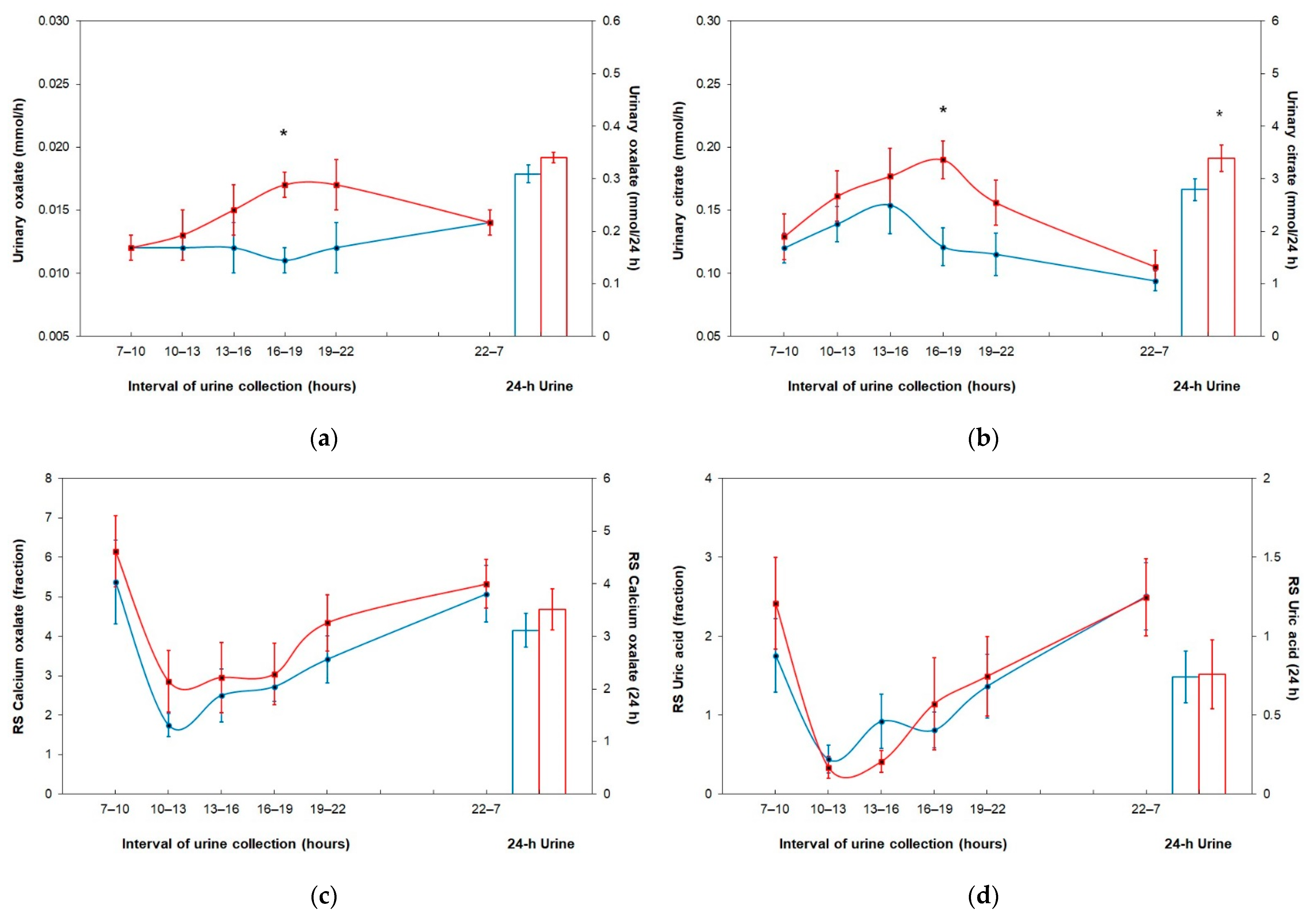
| Oxalate (mmol/h) | 0.012 ± 0.004 | 0.012 ± 0.005 | 0.012 ± 0.005 | 0.011 ± 0.003 | 0.012 ± 0.005 | 0.014 ± 0.004 | 0.012 ± 0.003 | 0.013 ± 0.005 | 0.015 ± 0.006 | 0.017 ± 0.004 * | 0.017 ± 0.005 | 0.014 ± 0.003 |
| Citrate (mmol/h) | 0.012 ± 0.038 | 0.139 ± 0.044 | 0.154 ± 0.073 | 0.121 ± 0.048 | 0.115 ± 0.052 | 0.094 ± 0.026 | 0.129 ± 0.058 | 0.161 ± 0.065 | 0.177 ± 0.071 | 0.190 ± 0.046 * | 0.156 ± 0.056 | 0.105 ± 0.042 |
| RS Calcium oxalate | 5.378 ± 3.340 | 1.747 ± 0.946 | 2.504 ± 2.123 | 2.724 ± 1.165 | 3.419 ± 1.878 | 5.079 ± 2.243 | 6.151 ± 2.842 | 2.860 ± 2.471 | 2.956 ± 2.802 | 3.044 ± 2.467 | 4.345 ± 2.253 | 5.332 ± 1.946 |
| RS Uric acid | 1.757 ± 1.477 | 0.441 ± 0.549 | 0.923 ± 1.095 | 0.813 ± 0.710 | 1.368 ± 1.276 | 2.509 ± 1.339 | 2.419 ± 1.840 | 0.337 ± 0.433 | 0.412 ± 0.448 | 1.144 ± 1.851 | 1.493 ± 1.595 | 2.491 ± 1.543 |
| RS Struvite | 0.064 ± 0.070 | 0.026 ± 0.025 | 0.042 ± 0.038 | 0.095 ± 0.186 | 0.066 ± 0.088 | 0.073 ± 0.068 | 0.048 ± 0.059 | 0.051 ± 0.053 | 0.062 ± 0.061 | 0.056 ± 0.051 | 0.061 ± 0.090 | 0.122 ± 0.221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siener, R.; Hesse, A. Effect of Black Tea Consumption on Urinary Risk Factors for Kidney Stone Formation. Nutrients 2021, 13, 4434. https://doi.org/10.3390/nu13124434
Siener R, Hesse A. Effect of Black Tea Consumption on Urinary Risk Factors for Kidney Stone Formation. Nutrients. 2021; 13(12):4434. https://doi.org/10.3390/nu13124434
Chicago/Turabian StyleSiener, Roswitha, and Albrecht Hesse. 2021. "Effect of Black Tea Consumption on Urinary Risk Factors for Kidney Stone Formation" Nutrients 13, no. 12: 4434. https://doi.org/10.3390/nu13124434
APA StyleSiener, R., & Hesse, A. (2021). Effect of Black Tea Consumption on Urinary Risk Factors for Kidney Stone Formation. Nutrients, 13(12), 4434. https://doi.org/10.3390/nu13124434






