Effect of Postoperative Coffee Consumption on Postoperative Ileus after Abdominal Surgery: An Updated Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Protocol
2.2. Inclusion Criteria
2.3. Search Method
2.4. Data Collection and Analysis
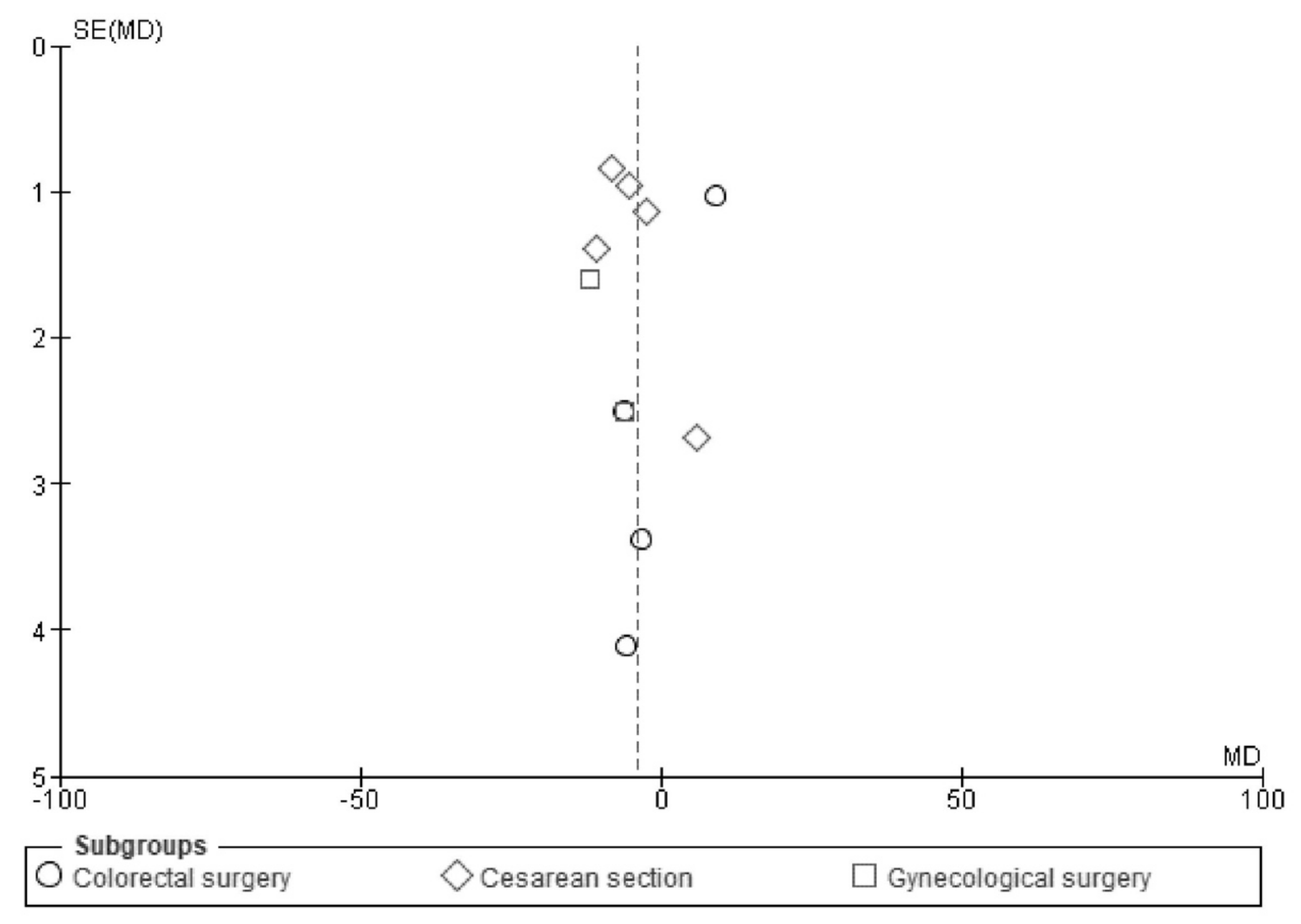
2.5. Assessment of Heterogeneity and Reporting Bias
2.6. Additional Analysis
3. Results
3.1. Primary Outcomes
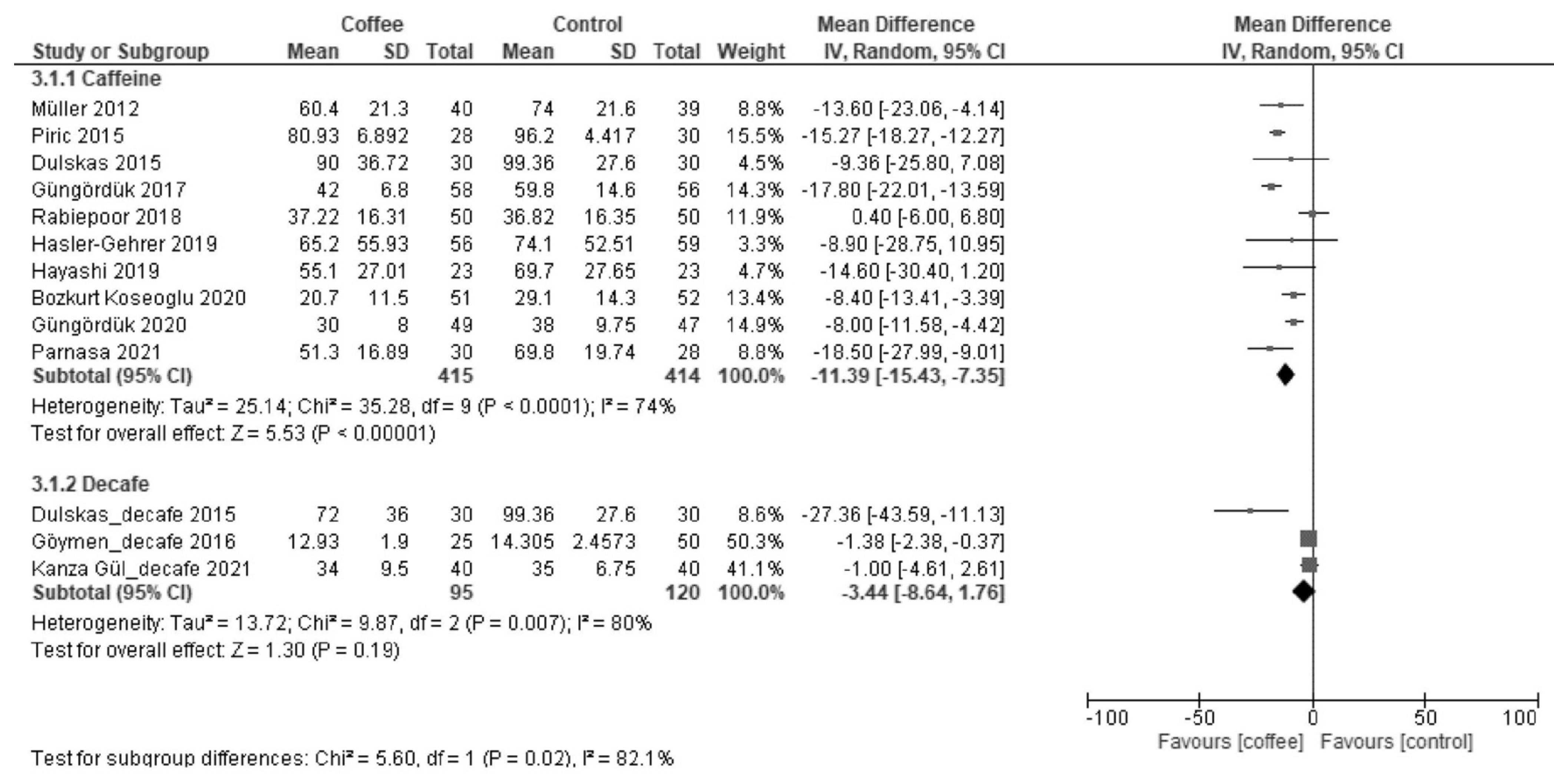
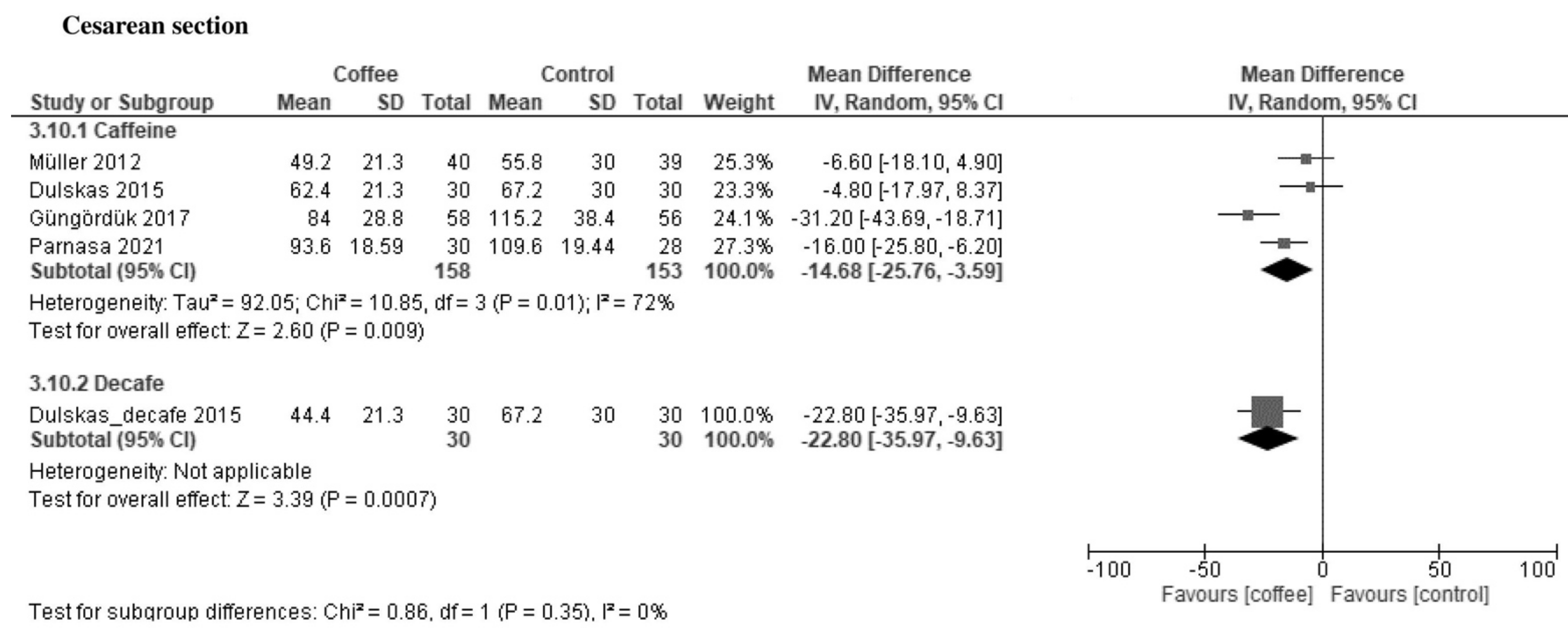
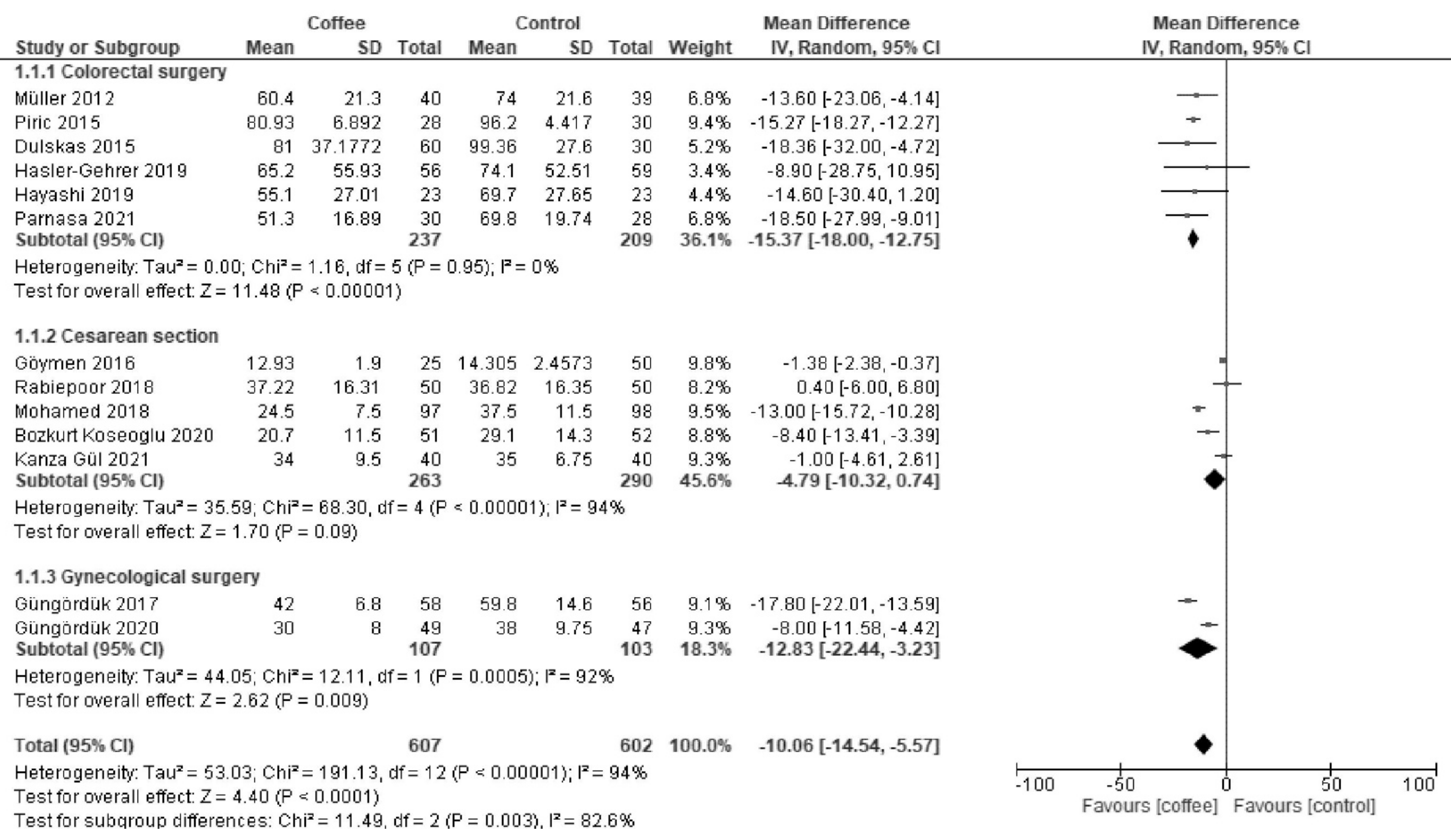
3.1.1. Time to First Defecation (Hours)
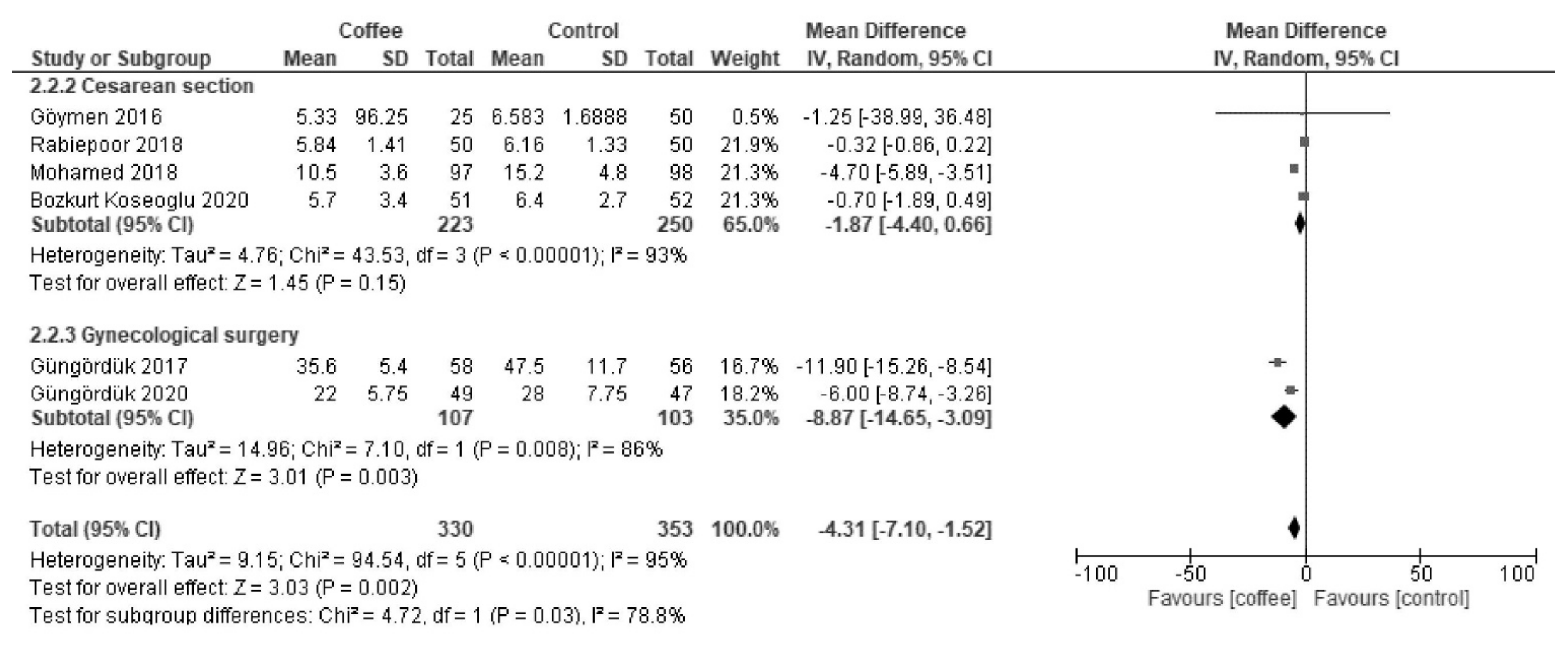
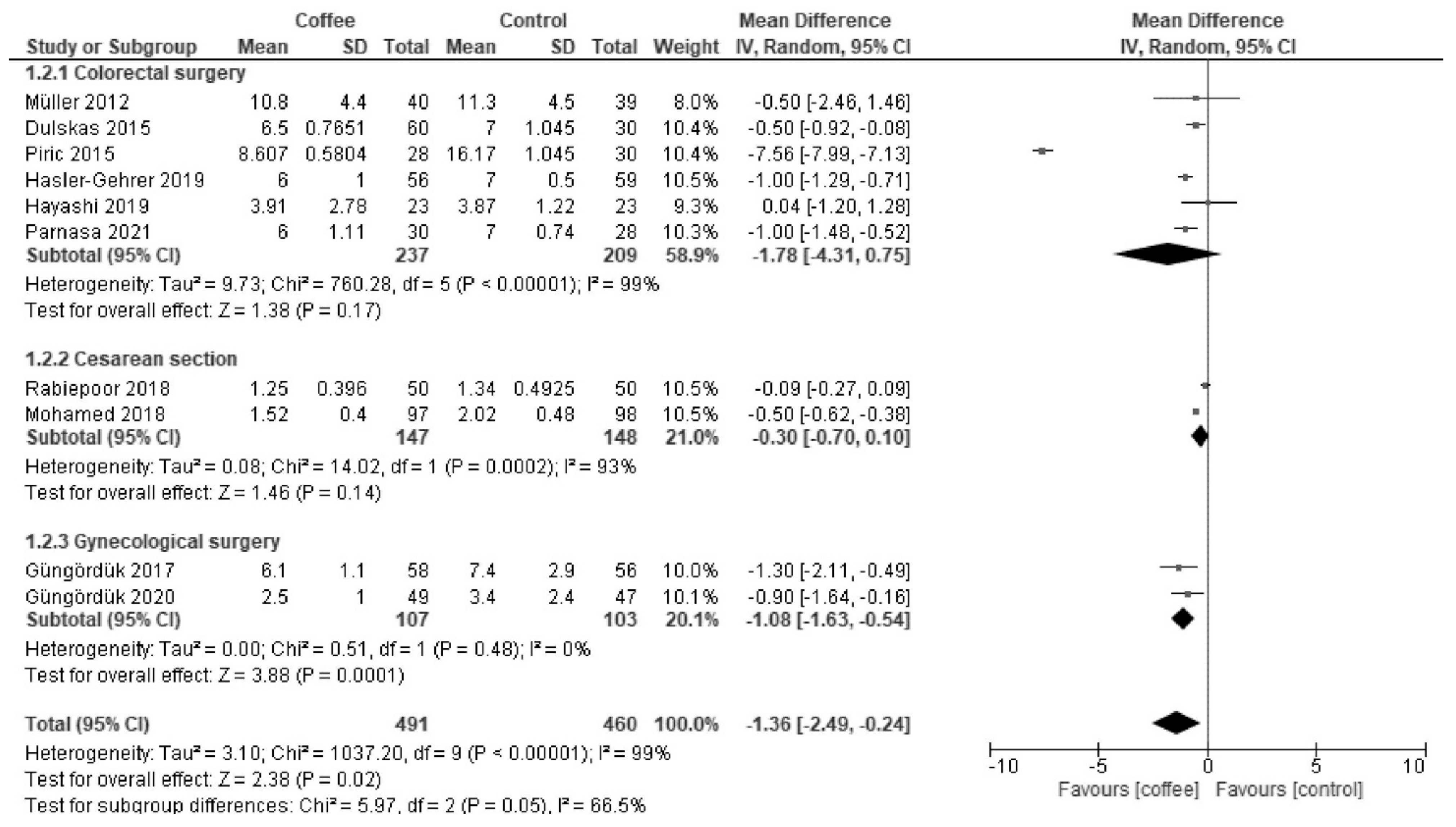
3.1.2. LOS (Days)
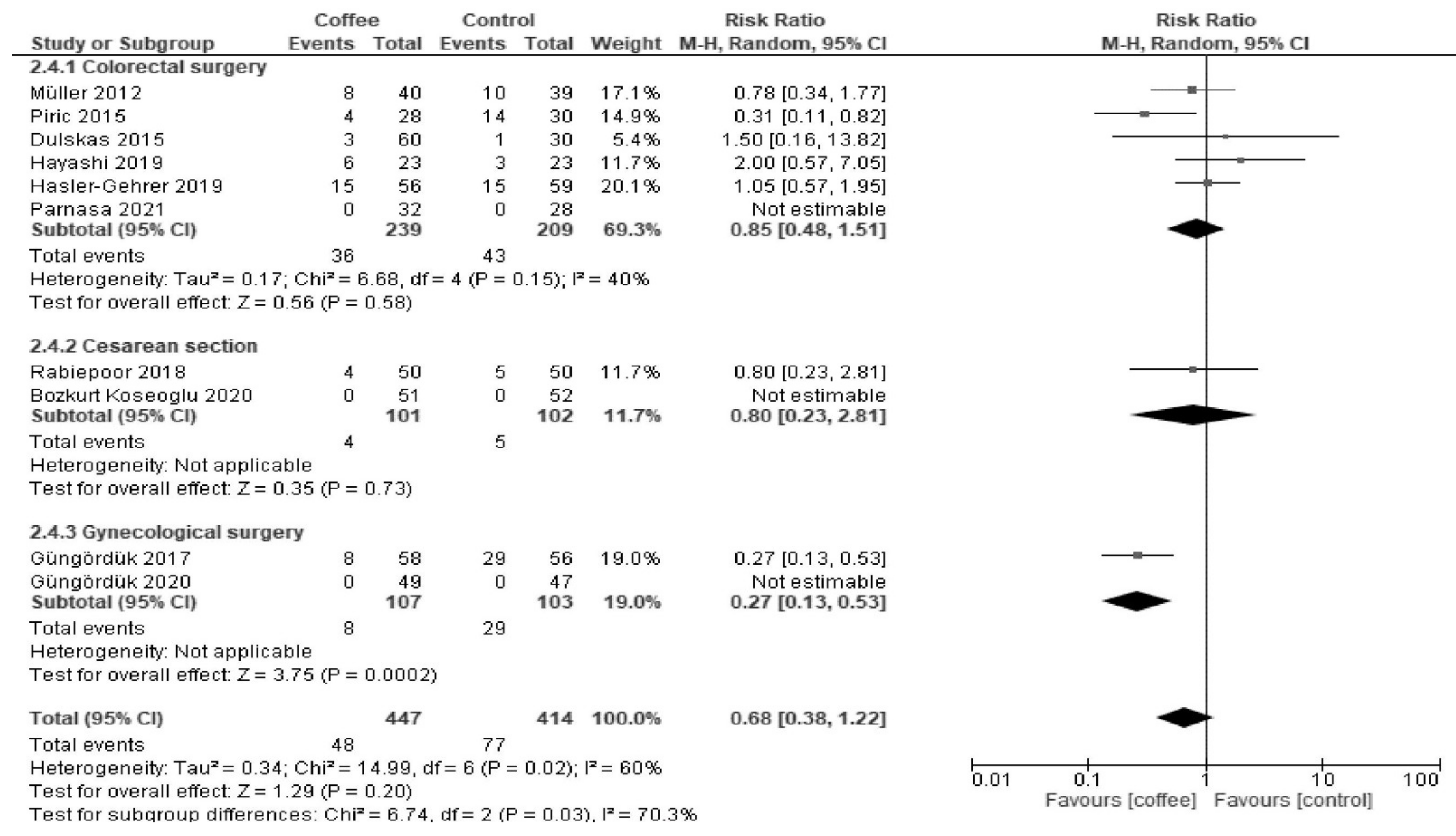
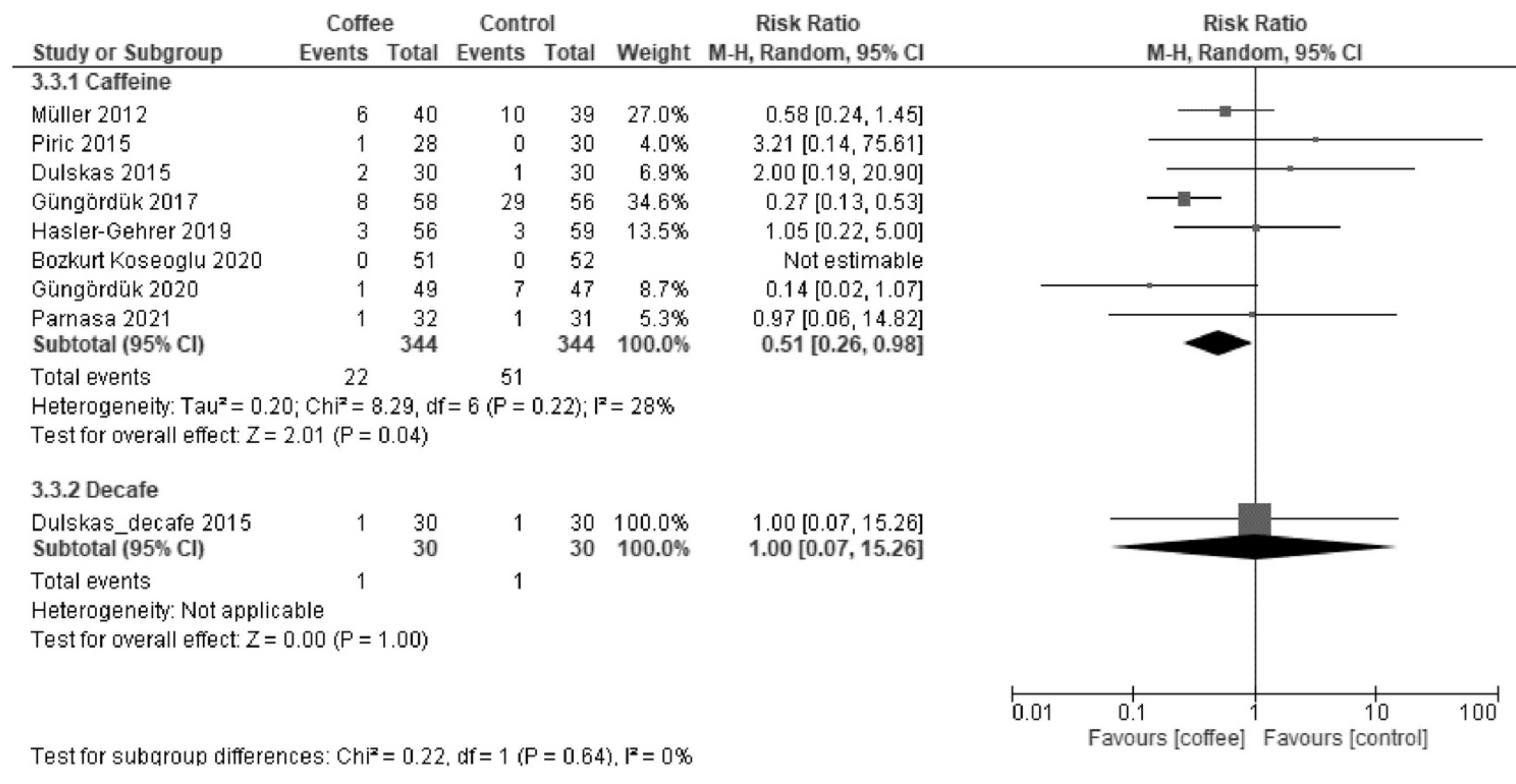
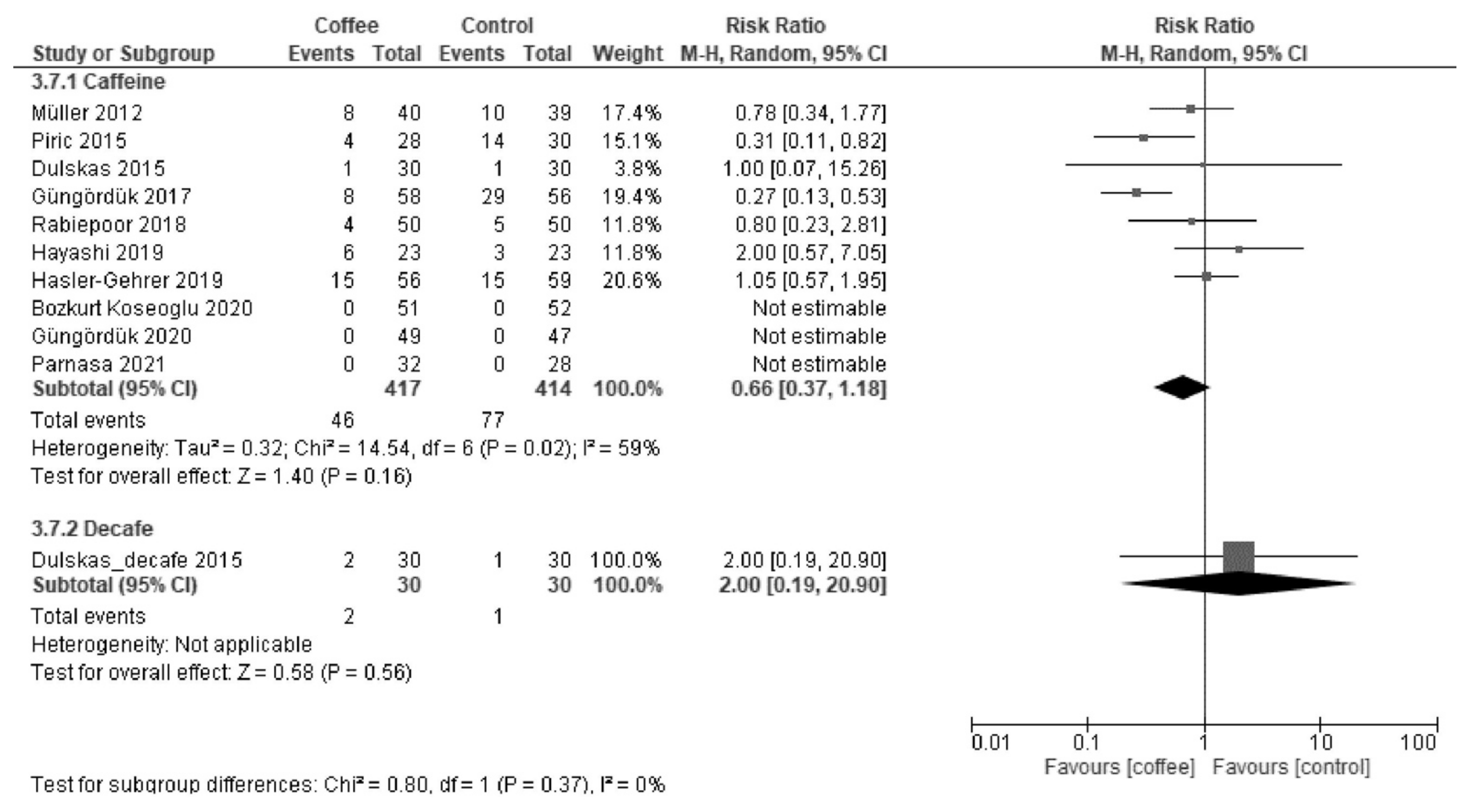
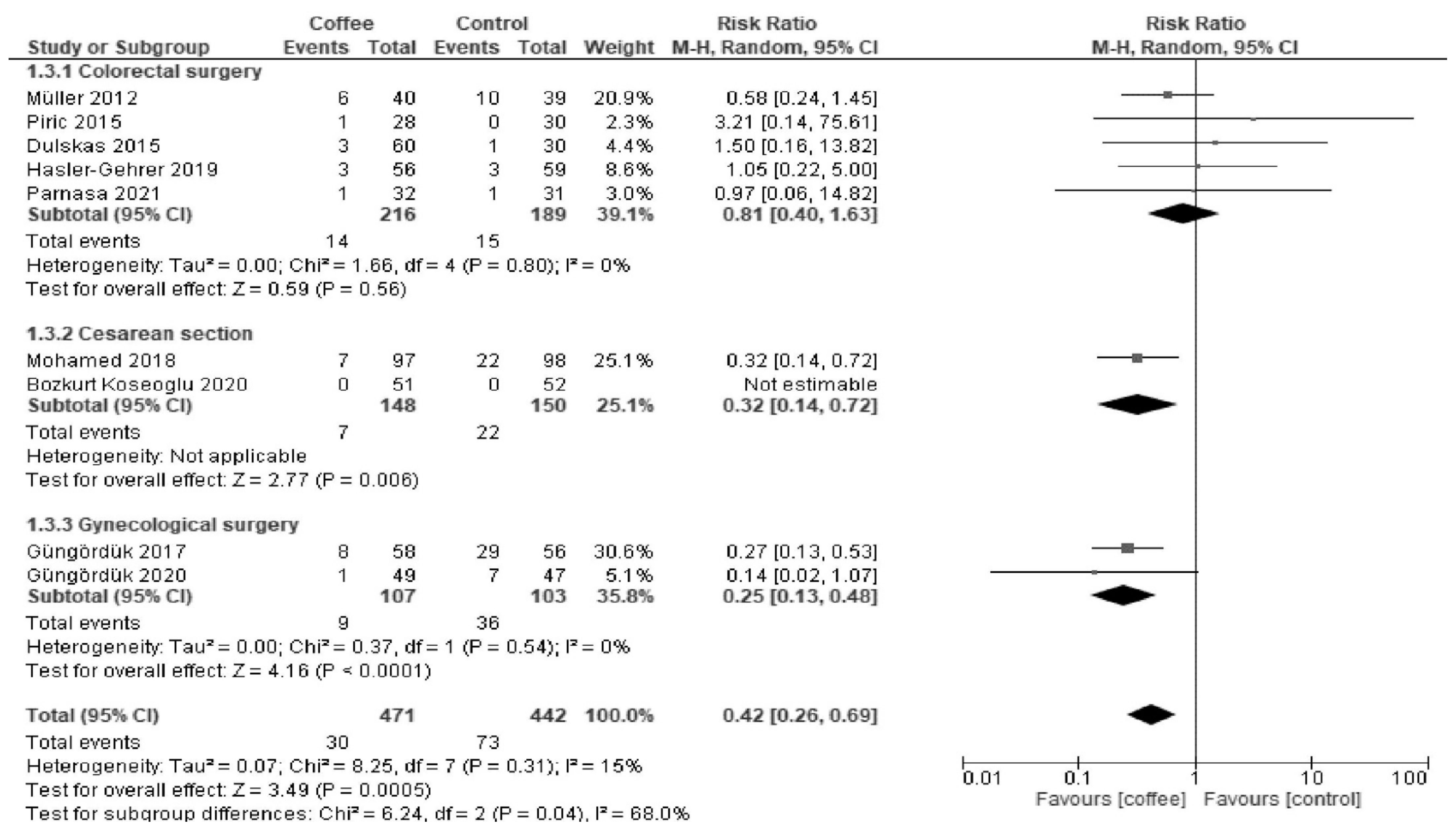
3.1.3. POI
3.2. Secondary Outcomes
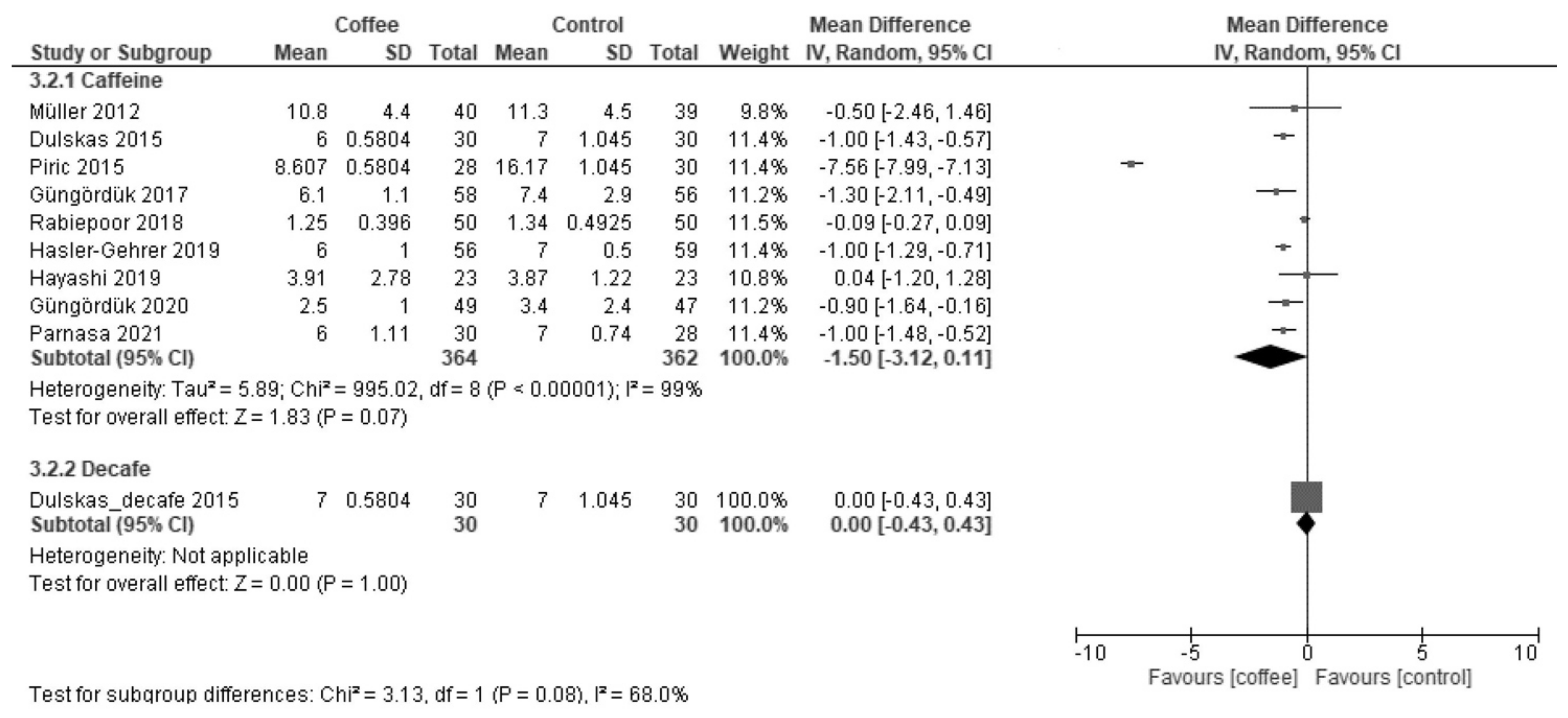
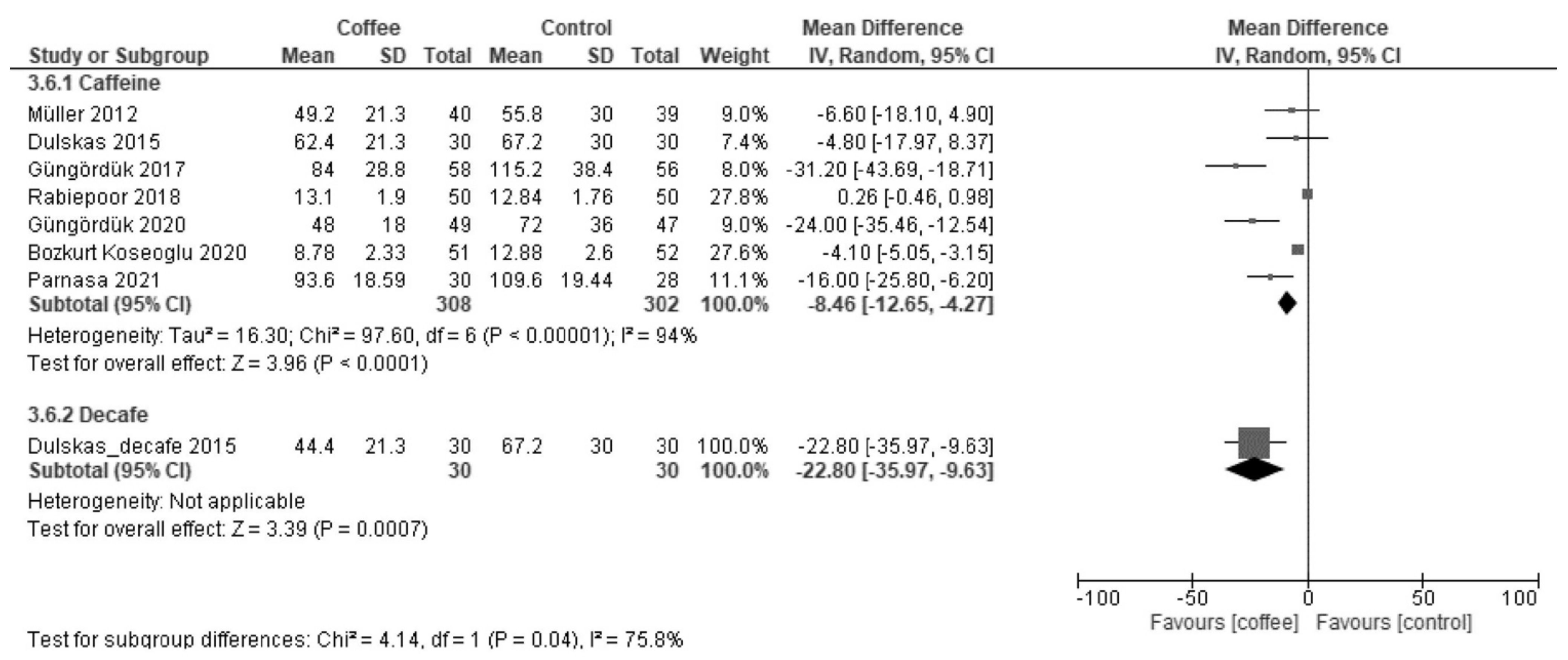
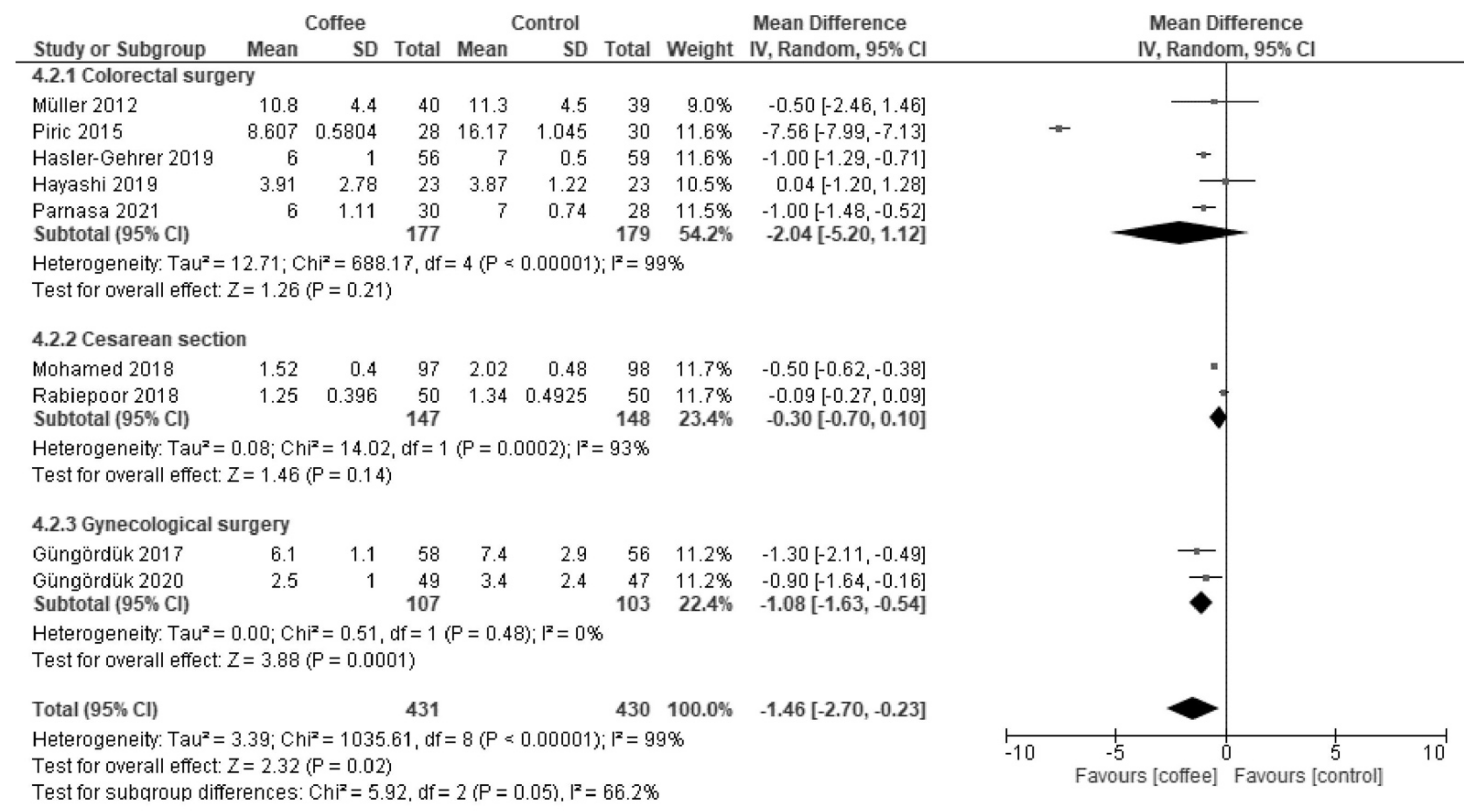
3.2.1. Time to First Flatus (Hours)
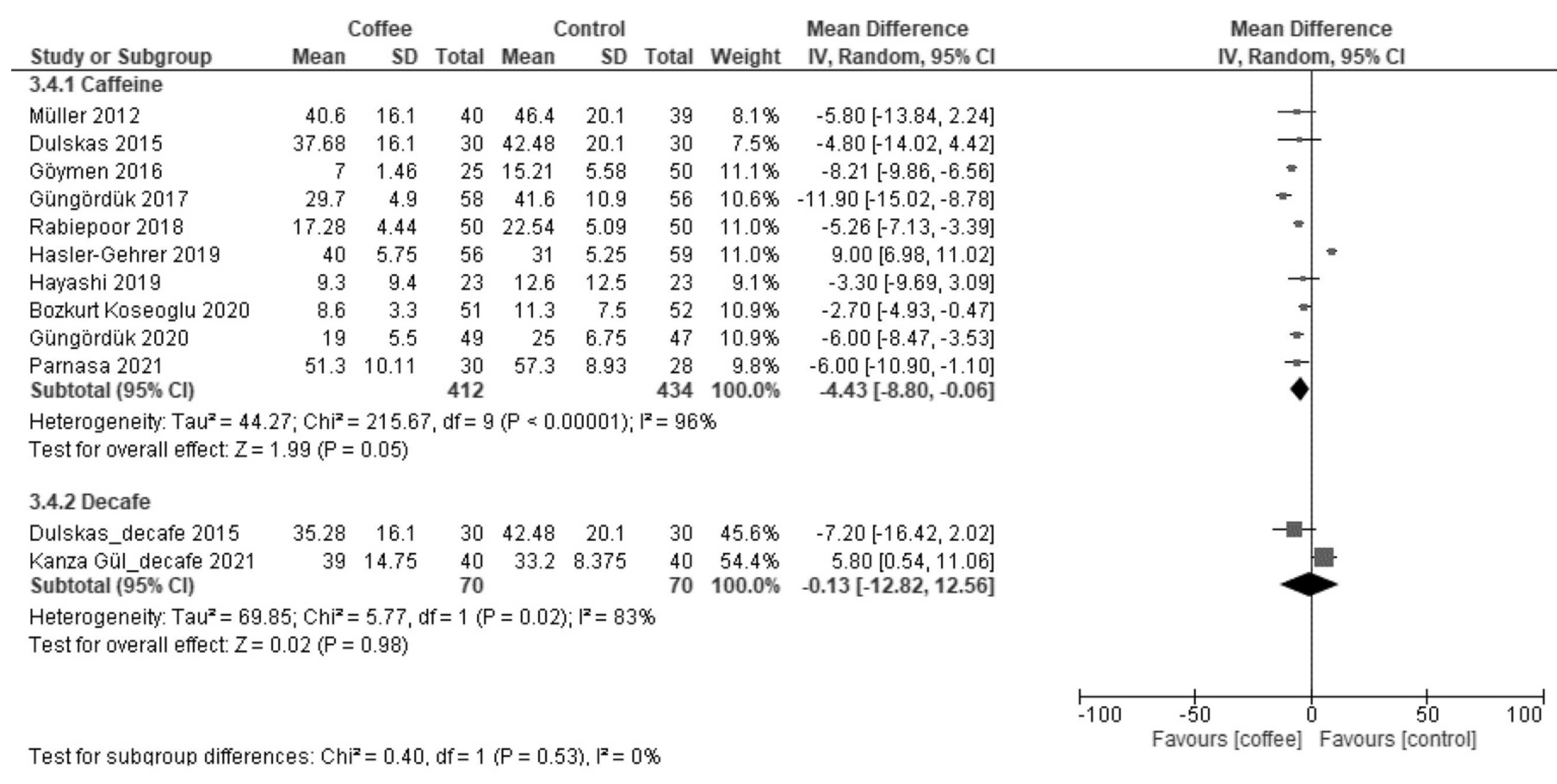
3.2.2. Time to First Bowel Sound (Hours)
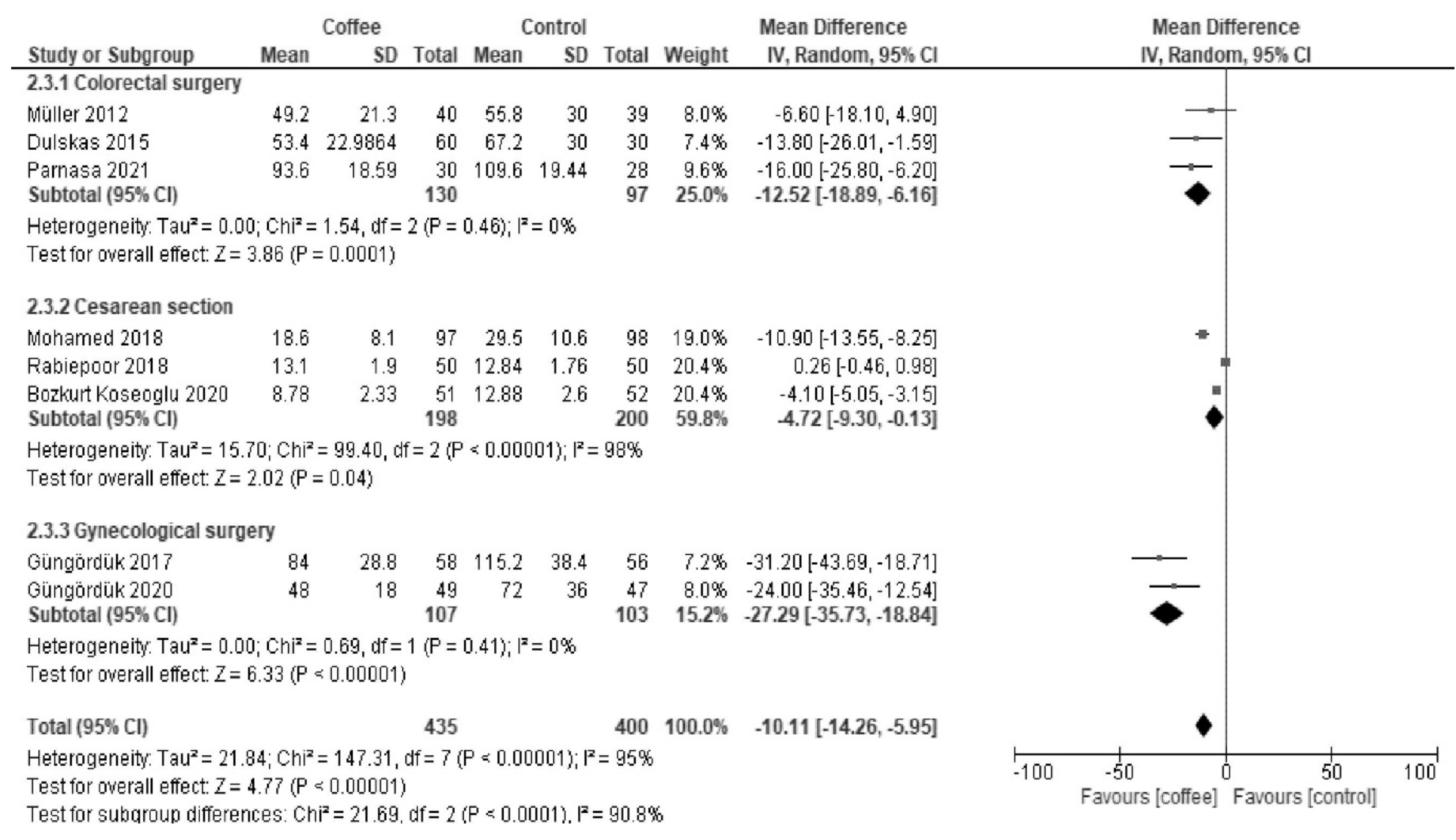
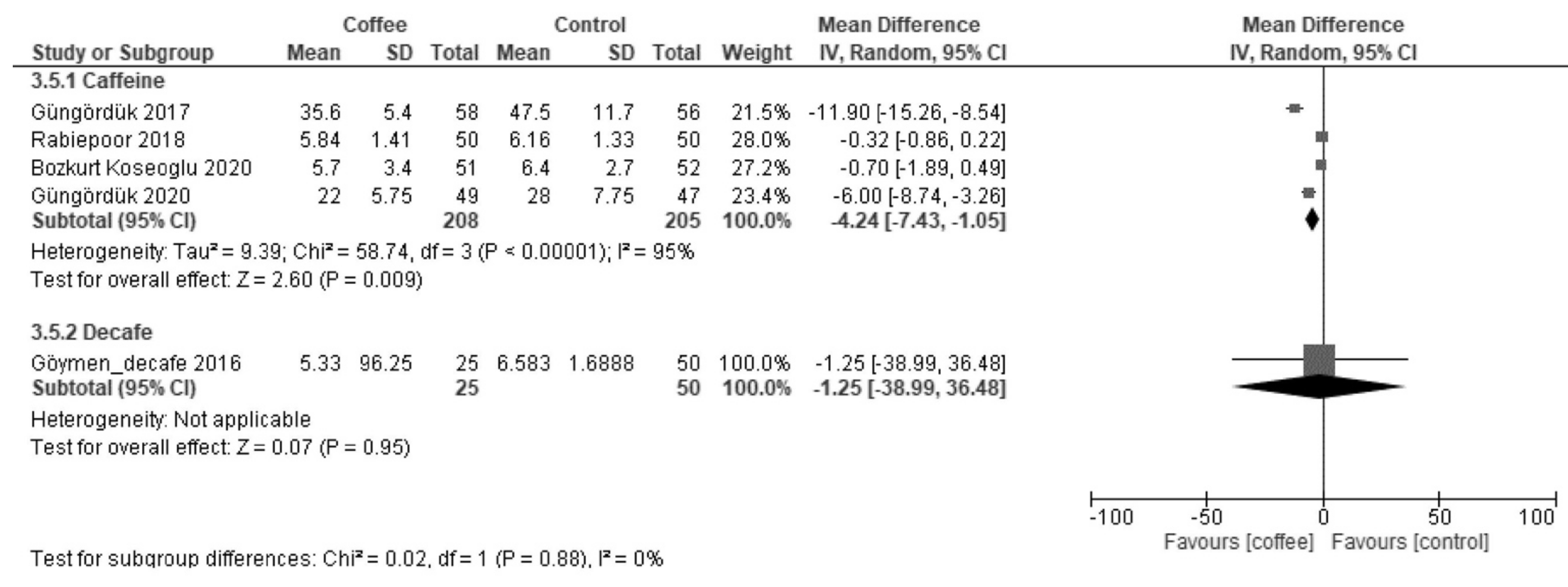
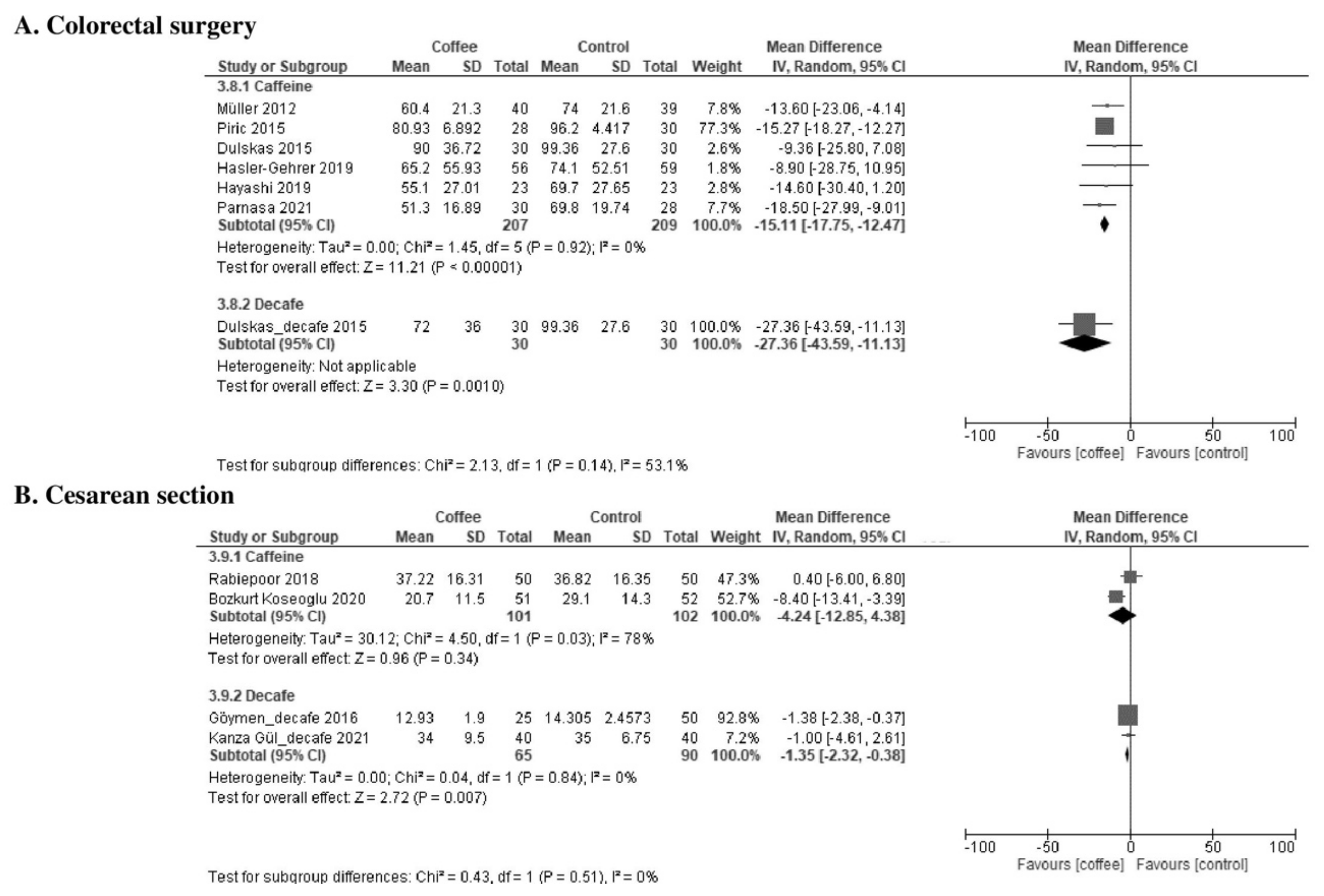
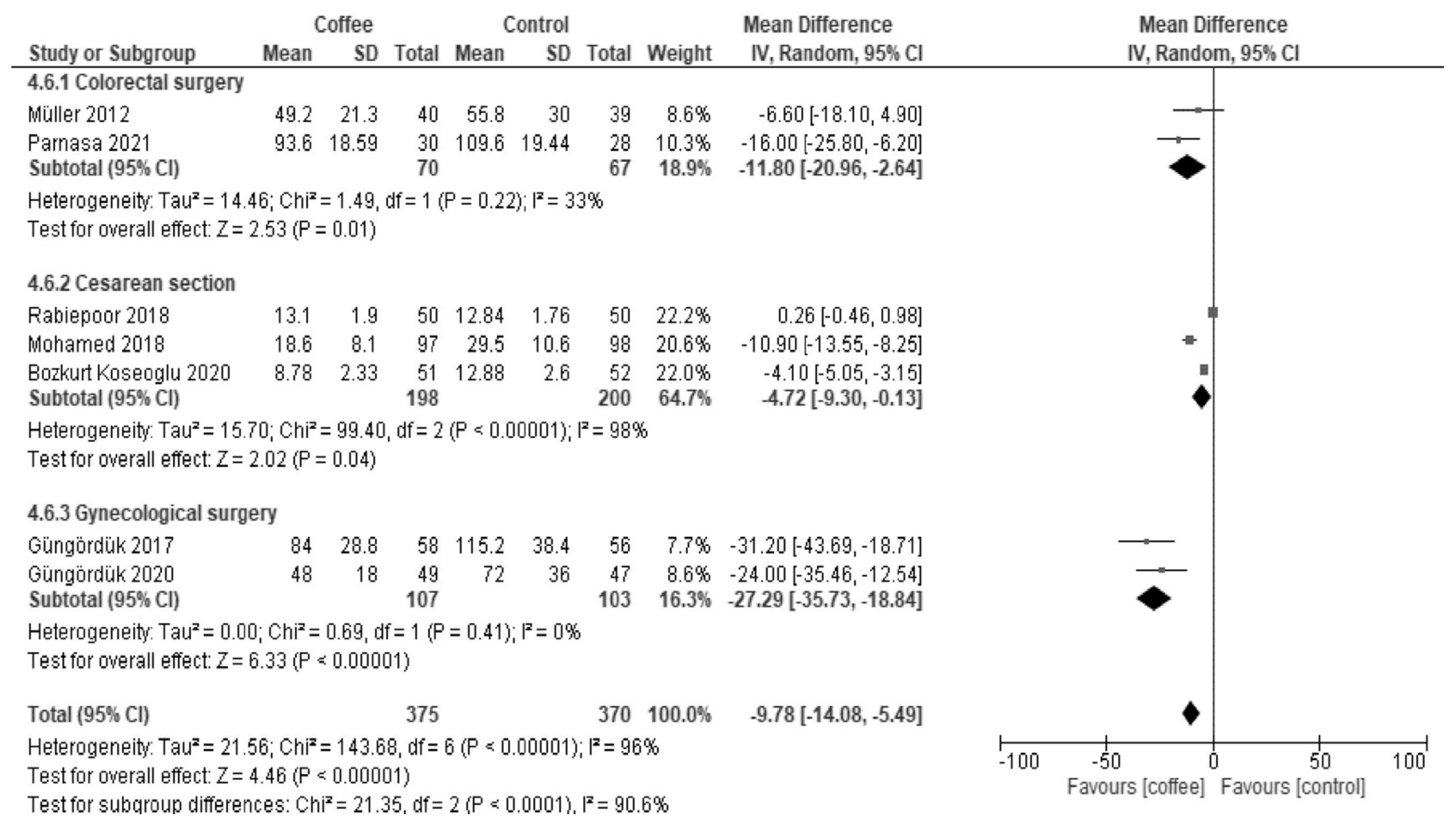
3.2.3. Time to Tolerance of Solid Food (Hours)
3.2.4. Complications/Adverse Events
3.3. Additional Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section and Topic | Item | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | 1 |
| ABSTRACT | |||
| Abstract | 2 | See PRISMA 2020 for the Abstract checklist. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 1 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 2 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 2 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 2 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | Appendix B and Appendix C |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved; whether they worked independently; and if applicable, details of automation tools used in the process. | 2, 3 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report; whether they worked independently; any processes for obtaining or confirming data from study investigators; and if applicable, details of automation tools used in the process. | 2, 3 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | 2, 3 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | 2, 3 | |
| Study risk-of-bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used; how many reviewers assessed each study and whether they worked independently; and if applicable, details of automation tools used in the process. | 2, 3 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | 2, 3 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | 2, 3 |
| 13b | Describe any methods required to prepare the data for presentation; synthesis, such as handling of missing summary statistics; or conversions. | 2, 3 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | 2, 3 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | 2, 3 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | 2, 3 | |
| 13f | Describe any sensitivity analyses conducted to assess the robustness of the synthesized results. | 2, 3 | |
| Reporting bias assessment | 14 | Describe any methods used to assess the risk of bias due to missing results in a synthesis (arising from reporting biases). | 3 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | 3 |
| RESULTS | |||
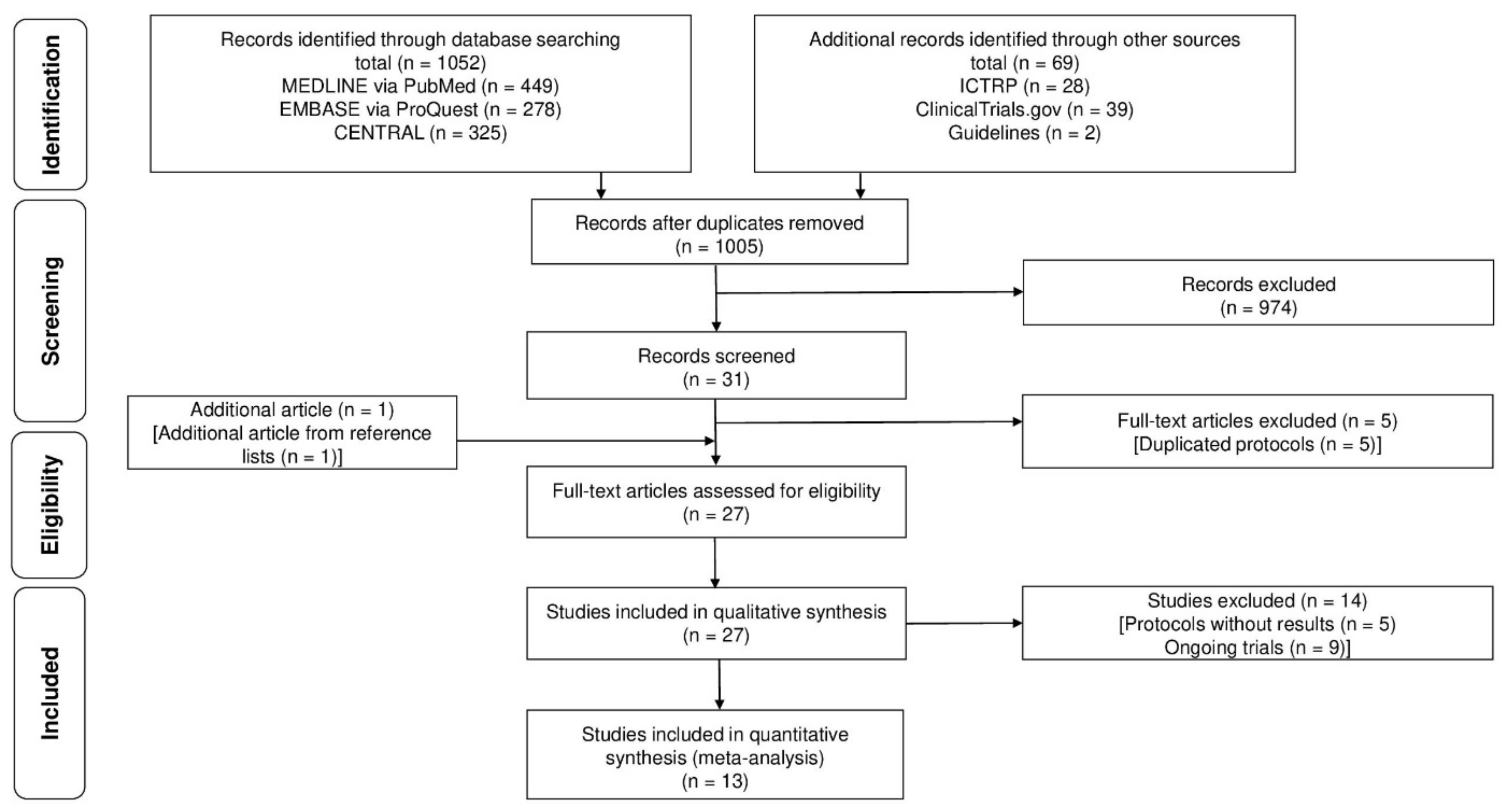
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 3 |
| 16b | Cite studies that might appear to meet the inclusion criteria but were excluded and explain why they were excluded. | 3 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 4 |
| Risk of bias in studies | 18 | Present assessments of the risk of bias for each included study. | 4, Table 2, Appendix D and Appendix E |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 4, Table 1 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and the risk of bias among contributing studies. | 5, 6, 7, 8 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | 5, 6, 7, 8 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | 5, 6, 7, 8 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | 8 | |
| Reporting biases | 21 | Present assessments of the risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | 8 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Table 3 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 8 |
| 23b | Discuss any limitations of the evidence included in the review. | 9 | |
| 23c | Discuss any limitations of the review processes used. | 9 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 9 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including the register name and the registration number, or state that the review was not registered. | 2 |
| 24b | Indicate where the review protocol can be accessed or state that a protocol was not prepared. | 2 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | 2 | |
| Support | 25 | Describe sources of financial or non-financial support for the review and the role of the funders or sponsors in the review. | 9 |
| Competing interests | 26 | Declare any competing interests of review authors. | 10 |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms, data extracted from included studies, data used for all analyses, analytic code, and any other materials used in the review. | 24, 25, 26 |
Appendix B. Search Strategy for Electronic Databases
Appendix C. Search Strategy for Clinical Trial Registries
Appendix D
| Authors [ref no.] | Risk of Bias 2 Tool Assessment | |||||
|---|---|---|---|---|---|---|
| Bias Arising from the Randomization Process | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Outcome Data | Bias in the Measurement of the Outcome | Bias in the Selection of the Reported Results | Overall Risk of Bias | |
| Müller [20] | Low | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Dulskas [21] | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Piric [22] | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Güngördük [24] | Some concerns | Low | Low | Some concerns | High | High |
| Mohamed [25] | Some concerns | High | High | Some concerns | Some concerns | High |
| Rabiepoor [26] | Some concerns | Low | Low | Some concerns | Some concerns | Some concerns |
| Hasler-Gehrer [27] | Low | Some concerns | Some concerns | Some concerns | High | High |
| Hayashi [28] | Low | Low | Low | Some concerns | Low | High |
| Güngördük [30] | Low | Some concerns | Some concerns | Some concerns | High | High |
| Parnasa [32] | Low | Some concerns | Some concerns | Some concerns | Low | Some concerns |
Appendix E
| Authors [ref no.] | Risk of Bias 2 Tool Assessment | |||||
|---|---|---|---|---|---|---|
| Bias Arising from the Randomization Process | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Outcome Data | Bias in the Measurement of the Outcome | Bias in the Selection of the Reported Results | Overall Risk of Bias | |
| Müller [20] | Low | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Dulskas [21] | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Piric [22] | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Güngördük [24] | Some concerns | Low | Low | Some concerns | High | High |
| Mohamed [25] | Some concerns | High | High | Some concerns | Some concerns | High |
| Hasler-Gehrer [27] | Low | Some concerns | Some concerns | Some concerns | High | High |
| Bozkurt Koseoglu [29] | Low | Some concerns | Some concerns | Some concerns | High | High |
| Güngördük [30] | Low | Some concerns | Some concerns | Some concerns | High | High |
| Parnasa [32] | Low | Some concerns | Some concerns | Some concerns | High | High |


References
- Wolff, B.G.; Viscusi, E.R.; Delaney, C.P.; Du, W.; Techner, L. Patterns of gastrointestinal recovery after bowel resection and total abdominal hysterectomy: Pooled results from the placebo arms of alvimopan phase III North American clinical trials. J. Am. Coll. Surg. 2007, 205, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, J.E.; Schumacher, J.; Kent, K.C.; Heise, C.P.; Greenberg, C.C. Associations of specific postoperative complications with outcomes after elective colon resection: A procedure-targeted approach toward surgical quality improvement. JAMA Surg. 2017, 152, e164681. [Google Scholar] [CrossRef]
- Gero, D.; Gié, O.; Hübner, M.; Demartines, N.; Hahnloser, D. Postoperative ileus: In search of an international consensus on definition, diagnosis, and treatment. Langenbecks Arch. Surg. 2017, 402, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Saunders, W.B.; Stemkowski, S. Economic burden of postoperative ileus associated with colectomy in the United States. J. Manag. Care Pharm. 2009, 15, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgeirsson, T.; El-Badawi, K.I.; Mahmood, A.; Barletta, J.; Luchtefeld, M.; Senagore, A.J. Postoperative ileus: It costs more than you expect. J. Am. Coll. Surg. 2010, 210, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.G.; Pattamatta, M.; Smeets, B.J.J.; Brinkman, D.J.; Evers, S.M.A.A.; de Jonge, W.J.; Hiligsmann, M.; Luyer, M.D.P. The clinical and economical impact of postoperative ileus in patients undergoing colorectal surgery. Neurogastroenterol. Motil. 2020, 32, e13862. [Google Scholar] [CrossRef]
- Abalo, R. Coffee and caffeine consumption for human health. Nutrients 2021, 13, 2918. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V. Effects of caffeine and coffee consumption on cardiovascular disease and risk factors. Future Cardiol. 2007, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Joós, G.; Jákim, J.; Kiss, B.; Szamosi, R.; Papp, T.; Felszeghy, S.; Sághy, T.; Nagy, G.; Szondy, Z. Involvement of adenosine A3 receptors in the chemotactic navigation of macrophages towards apoptotic cells. Immunol. Lett. 2017, 183, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [Green Version]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations—2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Eamudomkarn, N.; Kietpeerakool, C.; Kaewrudee, S.; Jampathong, N.; Ngamjarus, C.; Lumbiganon, P. Effect of postoperative coffee consumption on gastrointestinal function after abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2018, 8, 17349. [Google Scholar] [CrossRef] [PubMed]
- Gkegkes, I.D.; Minis, E.E.; Iavazzo, C. Effect of caffeine intake on postoperative ileus: A systematic review and meta-analysis. Dig. Surg. 2020, 37, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, H.L.; Edwards, B.A.; Curran, J.F.; Boyce, S. Coffee to go? The effect of coffee on resolution of ileus following abdominal surgery: A systematic review and meta-analysis of randomised controlled trials. Clin. Nutr. 2020, 39, 1385–1394. [Google Scholar] [CrossRef]
- Kane, T.D.; Tubog, T.D.; Schmidt, J.R. The use of coffee to decrease the incidence of postoperative ileus: A systematic review and meta-analysis. J. Perianesth. Nurs. 2020, 35, 171–177.e1. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. 2021. Available online: https://training.cochrane.org/handbook/current (accessed on 29 September 2021).
- Furukawa, T.A.; Barbui, C.; Cipriani, A.; Brambilla, P.; Watanabe, N. Imputing missing standard deviations in meta-analyses can provide accurate results. J. Clin. Epidemiol. 2006, 59, 7–10. [Google Scholar] [CrossRef]
- Müller, S.A.; Rahbari, N.N.; Schneider, F.; Warschkow, R.; Simon, T.; von Frankenberg, M.; Bork, U.; Weitz, J.; Schmied, B.M.; Büchler, M.W. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br. J. Surg. 2012, 99, 1530–1538. [Google Scholar] [CrossRef]
- Dulskas, A.; Klimovskij, M.; Vitkauskiene, M.; Samalavicius, N.E. Effect of coffee on the length of postoperative ileus after elective laparoscopic left-sided colectomy: A randomized, prospective single-center study. Dis. Colon Rectum. 2015, 58, 1064–1069. [Google Scholar] [CrossRef]
- Piric, M.; Pasic, F.; Rifatbegovic, Z.; Konjic, F. The Effects of Drinking Coffee While Recovering from Colon and Rectal Resection Surgery. Med. Arch. 2015, 69, 357–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goymen, A.; Simsek, Y.; Ozkaplan, S.E.; Ozdurak, H.I.; Akpak, Y.K.; Semiz, A.; Oral, S. Effect of gum chewing and coffee consumption on intestinal motility in caesarean sections. J. Clin. Anal. Med. 2017, 8, 411e5. [Google Scholar] [CrossRef] [Green Version]
- Güngördük, K.; Özdemir, İ.A.; Güngördük, Ö.; Gülseren, V.; Gokçü, M.; Sancı, M. Effects of coffee consumption on gut recovery after surgery of gynecological cancer patients: A randomized controlled trial. Am. J. Obstet Gynecol. 2017, 216, 145.e1–145.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, S. Immediate Postoperative Coffee Consumption Stimulates Early Gut Recovery after Cesarean Section. 2018. Available online: https://www.semanticscholar.org/paper/Immediate-Postoperative-Coffee-Consumption-Early-Sherbeny/8b790eb67f979bea8affae03fe5010167ae166c0?p2df (accessed on 1 October 2021).
- Rabiepoor, S.; Yas, A.; Navaei, J.; Khalkhali, H.R. Does coffee affect the bowel function after caesarean section? Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 220, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Hasler-Gehrer, S.; Linecker, M.; Keerl, A.; Slieker, J.; Descloux, A.; Rosenberg, R.; Seifert, B.; Nocito, A. Does coffee intake reduce postoperative ileus after laparoscopic elective colorectal surgery? A prospective, randomized controlled study: The coffee study. Dis. Colon Rectum 2019, 62, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Tsunoda, A.; Shiraishi, A.; Kusanagi, H. Quantification of the effects of coffee on postoperative ileus after laparoscopic ventral rectopexy: A randomized controlled trial. Eur. Surg. 2019, 51, 325–332. [Google Scholar] [CrossRef]
- Bozkurt Koseoglu, S.; Korkmaz Toker, M.; Gokbel, I.; Celikkol, O.; Gungorduk, K. Can coffee consumption be used to accelerate the recovery of bowel function after cesarean section? Randomized prospective trial. Ginekol. Pol. 2020, 91, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Gungorduk, K.; Paskal, E.K.; Demirayak, G.; Köseoğlu, S.B.; Akbaba, E.; Ozdemir, I.A. Coffee consumption for recovery of intestinal function after laparoscopic gynecological surgery: A randomized controlled trial. Int. J. Surg. 2020, 82, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Kanza Gül, D.; Şolt Kırca, A. Effects of acupressure, gum chewing and coffee consumption on the gastrointestinal system after caesarean section under spinal anaesthesia. J. Obstet Gynaecol. 2021, 41, 573–580. [Google Scholar] [CrossRef]
- Parnasa, S.Y.; Marom, G.; Bdolah-Abram, T.; Gefen, R.; Luques, L.; Michael, S.; Mizrahi, I.; Abu-Gazala, M.; Rivkind, A.I.; Mintz, Y.; et al. Does caffeine enhance bowel recovery after elective colorectal resection? A prospective double-blinded randomized clinical trial. Tech. Coloproctol. 2021, 25, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V.; Jitmungngan, R.; Chadbunchachai, W.; Ungprasert, P. Enhanced recovery after surgery in emergency resection for obstructive colorectal cancer: A systematic review and meta-analysis. Int. J. Colorectal. Dis. 2020, 35, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, T.; Anpalagan, T.; Ichhpuniani, S.; Lee, Y.; Ramji, K.; Eskicioglu, C. Selective opioid antagonists following bowel resection for prevention of postoperative ileus: A systematic review and meta-analysis. J. Gastrointest. Surg. 2021, 25, 1601–1624. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Migaldi, M.; Farinetti, A. Coffee in hypertensive women with asymptomatic peripheral arterial disease: A potential nutraceutical effect. J. Cardiovasc. Med. 2018, 19, 183–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine’s Vascular Mechanisms of Action. Int. J. Vasc. Med. 2010, 2010, 834060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattioli, A.V.; Nasi, M.; Farinetti, A.; Gelmini, R. Effects of caffeine on colon: A potential clinical use of coffee in surgical patients. Dig. Surg. 2020, 37, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.Q.; Wang, S.T.; Pan, D.; Chang, B.; Sang, L.X. Caffeine and its main targets of colorectal cancer. World J. Gastrointest. Oncol. 2020, 12, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jing, X.; Cao, S.; Liu, X.; Tan, X.; Jiang, H.; Niu, Z.; Su, M.; Zhang, J.; Zhang, X.; et al. Impact of drinking Chinese green tea on postoperative short outcomes for gastric cancer: A randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; de Souza, G.E. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [Green Version]
- Krakauer, T. The polyphenol chlorogenic acid inhibits staphylococcal exotoxin-induced inflammatory cytokines and chemokines. Immunopharmacol. Immunotoxicol. 2002, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Sun, X.; Yin, Z.; Li, H.; Cheng, C.; Liu, L.; Zhang, R.; Liu, F.; Zhou, Q.; et al. Caffeinated and decaffeinated coffee consumption and risk of all-cause mortality: A dose-response meta-analysis of cohort studies. J. Hum. Nutr. Diet. 2019, 32, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.J.; Roderick, P.; Buchanan, R.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: A systematic review and dose-response meta-analysis. BMJ Open 2017, 7, e013739. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, E.; Bongiovì, S.; Paleari, C.D.; Petucco, S.; Boni, M.; Colangeli, G.; Penzo, M.; Pessina, A.C. Haemodynamic effects of coffee and caffeine in normal volunteers: A placebo-controlled clinical study. J. Intern. Med. 1991, 229, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.M.; Alves, M.; Costa, J.; Ferreira, J.J.; Pinto, F.J.; Caldeira, D. Safety of coffee consumption after myocardial infarction: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2146–2158. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.J.; Alfirevic, Z.; Ghosh, A. Outpatient versus inpatient induction of labour for improving birth outcomes. Cochrane Database Syst. Rev. 2013, 11, CD007372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira Gomes Morais, E.; Riera, R.; Porfírio, G.J.; Macedo, C.R.; Sarmento Vasconcelos, V.; de Souza Pedrosa, A.; Torloni, M.R. Chewing gum for enhancing early recovery of bowel function after caesarean section. Cochrane Database Syst. Rev. 2016, 10, CD011562. [Google Scholar] [CrossRef]
- Hamel, J.F.; Sabbagh, C.; Alves, A.; Regimbeau, J.M.; Vignaud, T.; Venara, A. Comparison of treatment to improve gastrointestinal functions after colorectal surgery within enhanced recovery programmes: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 7423. [Google Scholar] [CrossRef]
| Authors [ref. no.] | Year | Country | No. | Age, Year | Male, % | Surgical | Coffee | Volume, mL | Frequency | Control |
|---|---|---|---|---|---|---|---|---|---|---|
| Müller [21] | 2012 | Germany | 79 | 61 | 56 | CRS | Caffeine | 100 | TDS | Water |
| Dulskas [22] | 2015 | Lithuania | 90 | 65 | 53 | CRS | Caffeine, Decaf | 100 | TDS | Water |
| Piric [23] | 2015 | Bosnia and Herzegovina | 58 | 63 | 59 | CRS | Caffeine | 100 | TDS | Tea |
| Göymen [24] | 2016 | Turkey | 75 | 50 | 0 | CS | Decaf | 100 | TDS | Water, no intervention |
| Güngördük [25] | 2017 | Turkey | 114 | 55 | 0 | GS | Caffeine | 100 | TDS | No intervention |
| Mohamed [26] | 2018 | Egypt | 210 | NR | 0 | CS | NR | NR | NR | No intervention |
| Rabiepoor [27] | 2018 | Iran | 100 | 28 | 0 | CS | Caffeine | 100 | TDS | Water |
| Hasler-Gehrer [28] | 2019 | Switzerland | 115 | 66 | 51 | CRS | Caffeine | 150 | TDS | Tea |
| Hayashi [29] | 2019 | Japan | 46 | 77 | 26 | CRS | Caffeine | 100 | TDS | Water |
| Bozkurt Koseoglu [30] | 2020 | Turkey | 113 | 29 | 0 | CS | Caffeine | 100 | TDS | No intervention |
| Güngördük [31] | 2020 | Turkey | 96 | 60 | 0 | GS | Caffeine | 150 | TDS | Water |
| Kanza Gül [32] | 2021 | Turkey | 80 | 28 | 0 | CS | Decaf | NR | TDS | No intervention |
| Parnasa [33] | 2021 | Israel | 70 | 56 | 50 | CRS | Caffeine | 50 * | TDS | Placebo |
| Authors [ref. no.] | Risk of Bias 2 Tool Assessment | |||||
|---|---|---|---|---|---|---|
| Bias Arising from the Randomization Process | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Outcome Data | Bias in the Measurement of the Outcome | Bias in the Selection of the Reported Results | Overall Risk of Bias | |
| Müller [21] | Low | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Dulskas [22] | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Piric [23] | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Göymen [24] | Some concerns | Low | Low | Some concerns | Some concerns | Some concerns |
| Güngördük [25] | Some concerns | Low | Low | Some concerns | High | High |
| Mohamed [26] | Some concerns | High | High | Some concerns | Some concerns | High |
| Rabiepoor [27] | Some concerns | Low | Low | Some concerns | Some concerns | Some concerns |
| Hasler-Gehrer [28] | Low | Some concerns | Some concerns | Some concerns | Low | Some concerns |
| Hayashi [29] | Low | Low | Low | Some concerns | Low | Some concerns |
| Bozkurt Koseoglu [30] | Low | Some concerns | Some concerns | Some concerns | Low | Some concerns |
| Güngördük [31] | Low | Some concerns | Some concerns | Some concerns | Low | Some concerns |
| Kanza Gül [32] | Low | Low | Low | Some concerns | Some concerns | Some concerns |
| Parnasa [33] | Low | Some concerns | Some concerns | Some concerns | Low | Some concerns |
| Effect of Postoperative Coffee Consumption after Abdominal Surgery | ||||||
|---|---|---|---|---|---|---|
| Patient: Adults after Abdominal Surgery; Setting: In-Patients; Intervention: Coffee; Comparison: Control | ||||||
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | Patient Number (Studies) | Certainty of the Evidence (GRADE) | Comments | |
| Risk with control | Risk with coffee | |||||
| Time to first defecation | The median time was 42 h. | MD −10 h (−14 to −5.6) | - | 1209 (13 RCTs) | Moderate a | Coffee reduced the time to first defecation. |
| Length of hospital stay | The median stay was 6 days. | MD −1.5 days (−2.7 to −0.3) | - | 905 (9 RCTs) | Low a,b | Coffee reduced the length of hospital stay. |
| Postoperative ileus | 165 per 1000. | 69 per 1000 (43 to 114) | RR 0.42 (0.26 to 0.69) | 913 (8 RCTs) | Low a,b | Coffee reduced postoperative ileus. |
| Time to first flatus | The median time was 30 h. | MD −4.3 h (−8.5 to −0.07) | - | 1113 (12 RCT) | Low a,b | Coffee reduced the time to first flatus. |
| Time to first bowel sound | The median time was 10 h. | MD −4.3 h (−7.1 to −1.5) | - | 683 (6 RCTs) | Very low a,b,c | Coffee reduced the time to first bowel sound. |
| Time to tolerance of solid food | The median time was 48 h. | MD −9.9 h (−14 to −5.9) | - | 833 (8 RCTs) | Low a,b | Coffee reduced the time to first tolerance of solid food. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, J.; Miki, A.; Koizumi, M.; Kotani, K.; Sata, N. Effect of Postoperative Coffee Consumption on Postoperative Ileus after Abdominal Surgery: An Updated Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4394. https://doi.org/10.3390/nu13124394
Watanabe J, Miki A, Koizumi M, Kotani K, Sata N. Effect of Postoperative Coffee Consumption on Postoperative Ileus after Abdominal Surgery: An Updated Systematic Review and Meta-Analysis. Nutrients. 2021; 13(12):4394. https://doi.org/10.3390/nu13124394
Chicago/Turabian StyleWatanabe, Jun, Atsushi Miki, Masaru Koizumi, Kazuhiko Kotani, and Naohiro Sata. 2021. "Effect of Postoperative Coffee Consumption on Postoperative Ileus after Abdominal Surgery: An Updated Systematic Review and Meta-Analysis" Nutrients 13, no. 12: 4394. https://doi.org/10.3390/nu13124394
APA StyleWatanabe, J., Miki, A., Koizumi, M., Kotani, K., & Sata, N. (2021). Effect of Postoperative Coffee Consumption on Postoperative Ileus after Abdominal Surgery: An Updated Systematic Review and Meta-Analysis. Nutrients, 13(12), 4394. https://doi.org/10.3390/nu13124394







