Change in Nutrient and Dietary Intake in European Children with Cystic Fibrosis after a 6-Month Intervention with a Self-Management mHealth Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Food Recording with the App
2.3. Statistical Analysis
3. Results
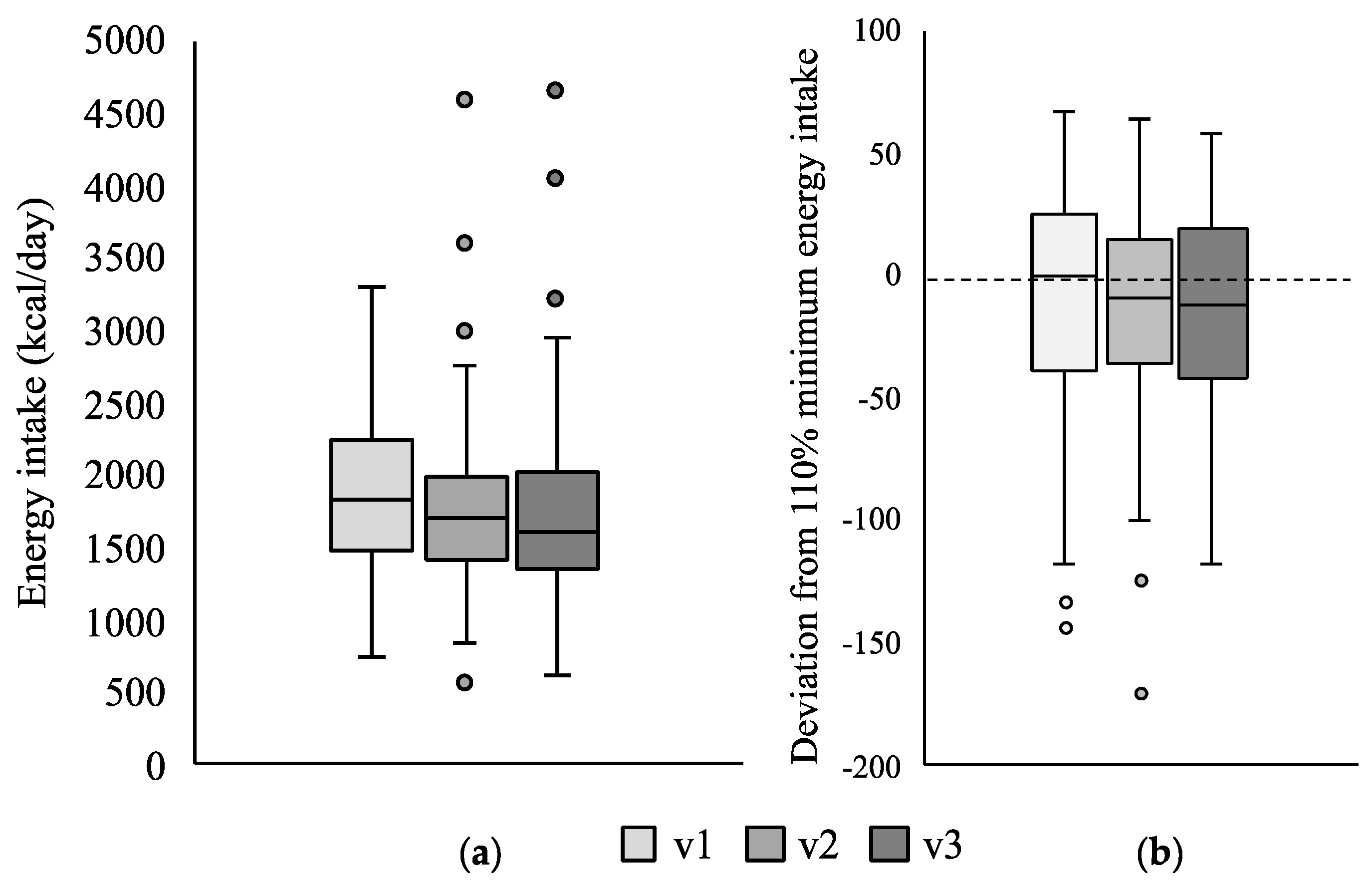
3.1. Change in Energy Intake
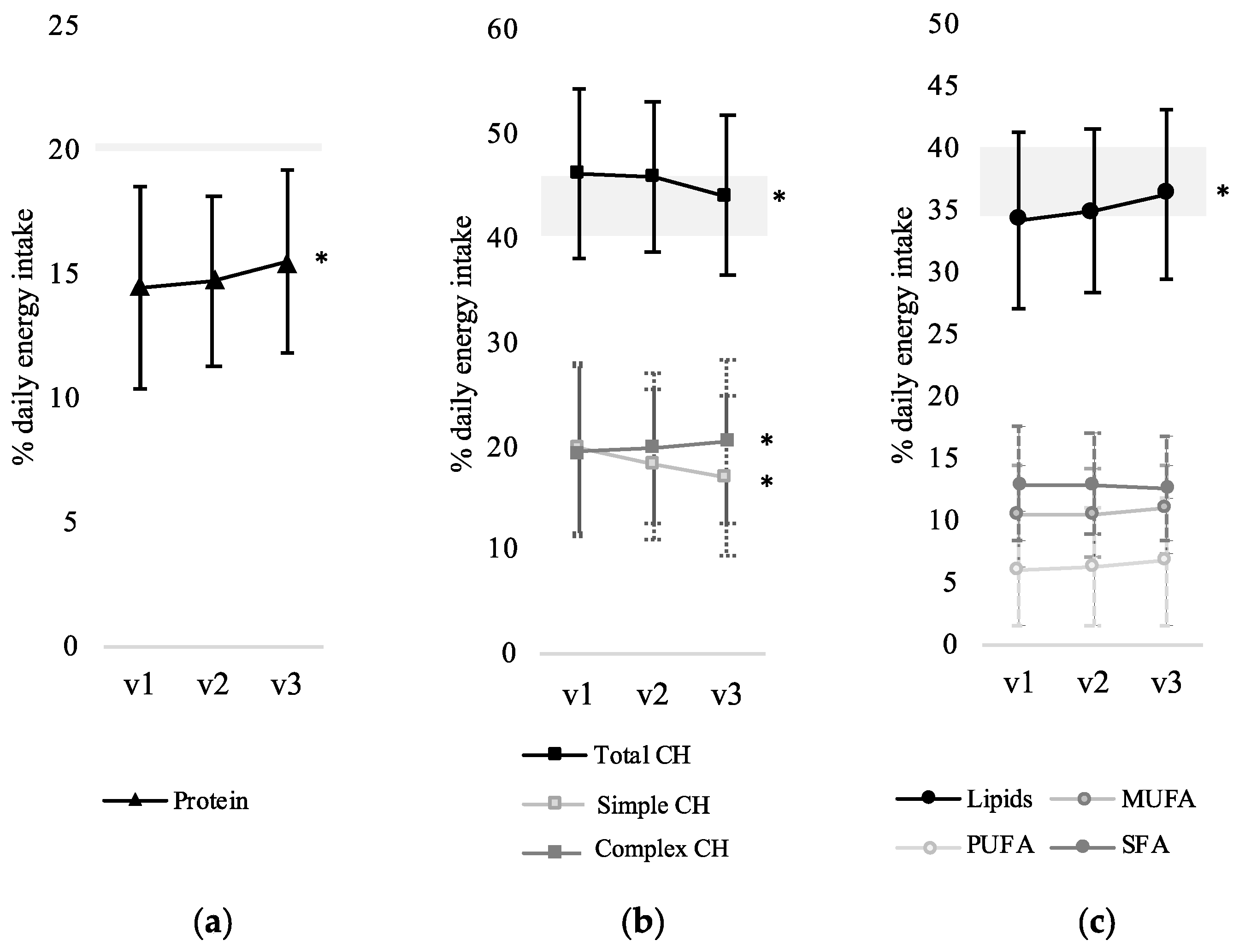
3.2. Change in Macronutrient Distribution
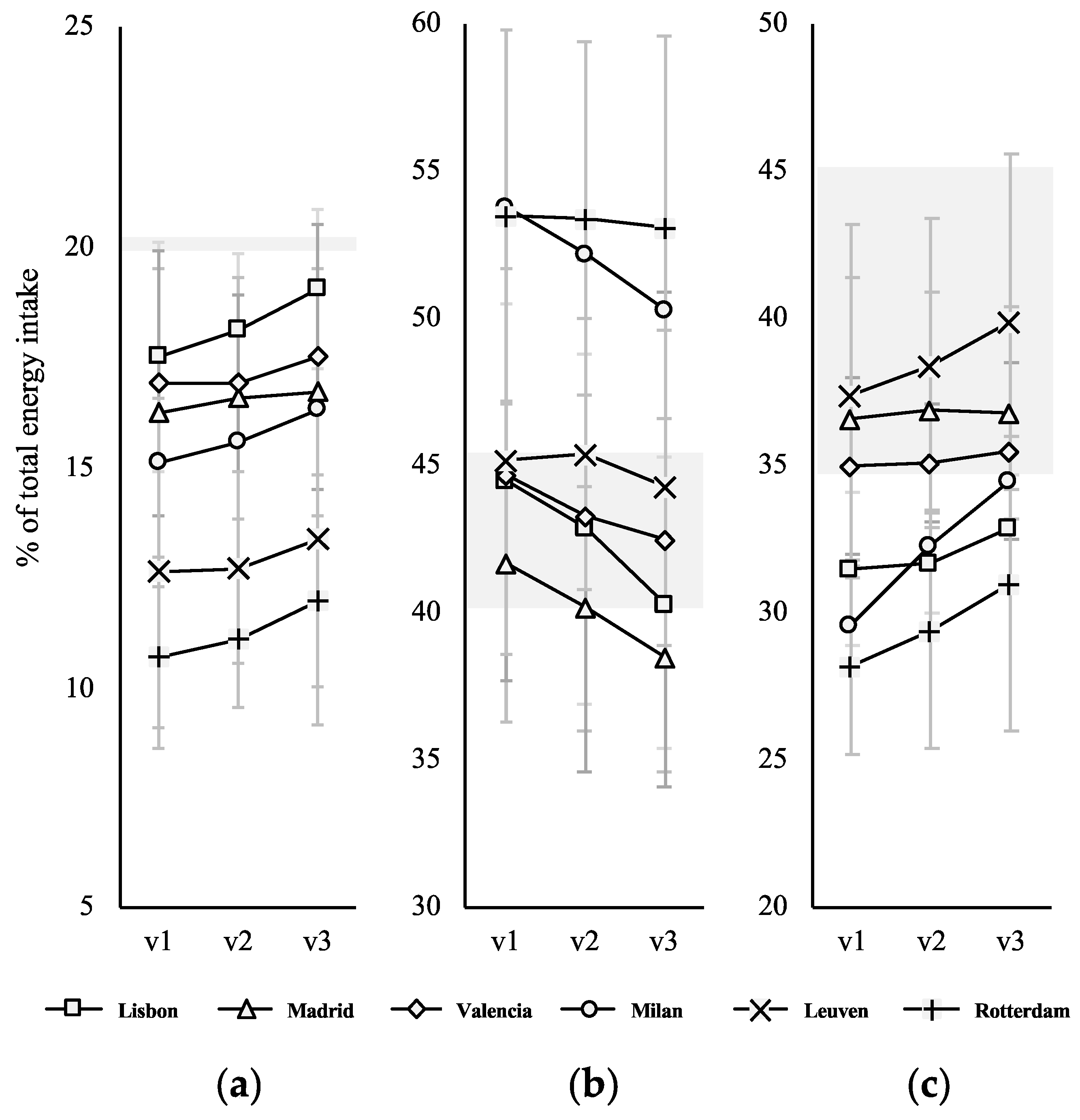
3.3. Change in Dietary Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Somerset, S. Digestive system dysfunction in cystic fibrosis: Challenges for nutrition therapy. Dig. Liver Dis. 2014, 46, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Corey, M.; Mclaughlin, F.J.; Williams, M.; Levkon, H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J. Clin. Epidemiol. 1988, 41, 583–591. [Google Scholar] [CrossRef]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Yen, E.H.; Quinton, H.; Borowitz, D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J. Pediatr. 2013, 162, 530–535. [Google Scholar] [CrossRef]
- Amin, R.; Ratjen, F. Emerging drugs for cystic fibrosis. Expert Opin. Emerg. Drugs 2014, 19, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Sinaasappel, M.; Stern, M.; Littlewood, J.; Wolfe, S.; Steinkamp, G.; Heijerman, H.G.; Robberecht, E.; Döring, G. Nutrition in patients with cystic fibrosis: A European Consensus. J. Cyst. Fibros. 2002, 1, 51–75. [Google Scholar] [CrossRef] [Green Version]
- Woestenenk, J.W.; Castelijns, S.J.A.M.; Van der Ent, C.K.; Houwen, R.H.J. Dietary intake in children and adolescents with cystic fibrosis. Clin. Nutr. 2014, 33, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.; Katz, T.; Liu, V.; Quintano, J.; Brunner, R.; Tong, C.W.; Collins, C.E.; Ooi, C.Y. Dietary intake of energy-dense, nutrient-poor and nutrient-dense food sources in children with cystic fibrosis. J. Cyst. Fibros. 2018, 17, 804–810. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Hulst, J.; Boon, M.; Martins, T.; Ruperto, M.; Colombo, C.; Fornés-Ferrer, V.; Woodcock, S.; Claes, I.; Asseiceira, I.; et al. The relative contribution of food groups to macronutrient intake in children with cystic fibrosis: A European multicenter assessment. J. Acad. Nutr. Diet. 2019, 119, 1305–1319. [Google Scholar] [CrossRef]
- Joint WHO/FAO Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO technical report series; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Woestenenk, J.W.; Van Der Ent, C.K.; Houwen, R.H.J. Pancreatic enzyme replacement therapy and coefficient cystic fibrosis. Pancreatology 2015, 61, 355–360. [Google Scholar]
- Calvo-Lerma, J.; Martinez-Jimenez, C.P.; Lázaro-Ramos, J.P.; Andrés, A.; Crespo-Escobar, P.; Stav, E.; Schauber, C.; Pannese, L.; Hulst, J.M.; Suarez, L.; et al. Innovative approach for self-management and social welfare of children with cystic fibrosis in Europe: Development, validation and implementation of an mHealth tool (MyCyFAPP). BMJ Open 2017, 7, e014931. [Google Scholar] [CrossRef]
- Vilarinho, T.; Floch, J.; Stav, E. Co-designing a mHealth Application for Self-management of Cystic Fibrosis. In Proceedings of the IFIP Conference on Human-Computer Interaction, Mumbai, India, 25–29 September 2017; Springer: Cham, Switzerland, 2017; pp. 3–22. [Google Scholar]
- Calvo-Lerma, J.; Fornés-Ferrer, V.; Peinado, I.; Heredia, A.; Ribes-Koninckx, C.; Andrés, A. A first approach for an evidence-based in vitro digestion method to adjust pancreatic enzyme replacement therapy in cystic fibrosis. PLoS ONE 2019, 14, e0212459. [Google Scholar] [CrossRef]
- Boon, M.; Calvo-Lerma, J.; Claes, I.; Havermans, T.; Asseiceira, I.; Bulfamante, A.; Garriga, M.; Masip, E.; Van Schijndel, B.; Fornes, V.; et al. Use of a mobile application for self-management of pancreatic enzyme replacement therapy is associated with improved gastro-intestinal related quality of life in children with Cystic Fibrosis. J. Cyst. Fibros. 2020, 19, 562–568. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Boon, M.; Colombo, C.; de Koning, B.; Asseiceira, I.; Garriga, M.; Roca, M.; Claes, I.; Bulfamante, A.; Walet, S.; et al. Clinical evaluation of an evidence-based method based on food characteristics to adjust pancreatic enzyme supplements dose in cystic fibrosis. J. Cyst. Fibros. 2020, in press. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; da Costa Louzada, M.L.; Machado, P.P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019. [Google Scholar]
- Mendonça, R.D.D.; Lopes, A.C.S.; Pimenta, A.M.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Ultra-processed food consumption and the incidence of hypertension in a Mediterranean cohort: The Seguimiento Universidad de Navarra Project. Am. J. Hypertens. 2017, 30, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.O.; Wyatt, H.R.; Reed, G.W.; Peters, J.C. Obesity and the environment: Where do we go from here? Science 2003, 299, 853–855. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Galvano, F. Mediterranean diet adherence in children and adolescents in southern European countries. NFS J. 2016, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Graffe, M.I.M.; Pala, V.; De Henauw, S.; Eiben, G.; Hadjigeorgiou, C.; Iacoviello, L.; Intemann, T.; Jilani, H.; Molnar, D.; Russo, P.; et al. Dietary sources of free sugars in the diet of European children: The IDEFICS Study. Eur. J. Nutr. 2020, 59, 979–989. [Google Scholar] [CrossRef]
- Rosi, A.; Paolella, G.; Biasini, B.; Scazzina, F.; Alicante, P.; De Blasio, F.; Russo, M.D.; Rendina, D.; Tabacchi, G.; Cairella, G.; et al. Dietary habits of adolescents living in North America, Europe or Oceania: A review on fruit, vegetable and legume consumption, sodium intake, and adherence to the Mediterranean Diet. Nutr. Metab. Cardiovasc. 2019, 29, 544–560. [Google Scholar] [CrossRef]
- Floch, J.; Vilarinho, T.; Zettl, A.; Ibanez-Sanchez, G.; Calvo-Lerma, J.; Stav, E.; Haro, P.H.; Aalberg, A.L.; Fides-Valero, A.; Montón, J.L.B. Users’ Experiences of a Mobile Health Self-Management Approach for the Treatment of Cystic Fibrosis: Mixed Methods Study. JMIR eHalth uHealth 2020, 8, e15896. [Google Scholar] [CrossRef]
- Watson, H.; Bilton, D.; Truby, H. A randomized controlled trial of a new behavioral home-based nutrition education program, “Eat Well with CF,” in adults with cystic fibrosis. J. Acad. Nutr. Diet. 2008, 108, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Stark, L.J.; Quittner, A.L.; Powers, S.W.; Opipari-Arrigan, L.; Bean, J.A.; Duggan, C.; Stallings, V.A. Randomized clinical trial of behavioral intervention and nutrition education to improve caloric intake and weight in children with cystic fibrosis. Arch. Pediatr. Adolesc. Med. 2009, 163, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Woestenenk, J.W.; Castelijns, S.J.A.M.; Van der Ent, C.K.; Houwen, R.H.J. Nutritional intervention in patients with cystic fibrosis: A systematic review. J. Cyst. Fibros. 2013, 12, 102–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| % of Total Energy Intake | Difference from v1 to v3 | Odds Ratio, p-Value | 95% Confidence Interval |
|---|---|---|---|
| % Protein intake | +1.0% | 1.09, p = 0.001 | (1.03, 1.15) |
| % Lipid intake | +2.0% | 1.09, p = 0.02 | (1.01, 1.18) |
| % SFA intake | −0.4% | 0.97, p = 0.42 | (0.90, 1.04) |
| % MUFA intake | +0.6% | 1.05, p = 0.28 | (0.96, 1.14) |
| % PUFA intake | +0.6% | 1.09, p = 0.22 | (0.95, 1.25) |
| % CH intake | −2.5% | 0.88, p = 0.001 | (0.81, 0.95) |
| % Simple CH intake | −2.4% | 0.81, p < 0.001 | (0.73, 0.89) |
| % Complex CH intake | +1.0% | 1.03, p = 0.003 | (1.01, 1.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Lerma, J.; Boon, M.; Hulst, J.; Colombo, C.; Asseiceira, I.; Garriga, M.; Masip, E.; Claes, I.; Bulfamante, A.; Janssens, H.M.; et al. Change in Nutrient and Dietary Intake in European Children with Cystic Fibrosis after a 6-Month Intervention with a Self-Management mHealth Tool. Nutrients 2021, 13, 1801. https://doi.org/10.3390/nu13061801
Calvo-Lerma J, Boon M, Hulst J, Colombo C, Asseiceira I, Garriga M, Masip E, Claes I, Bulfamante A, Janssens HM, et al. Change in Nutrient and Dietary Intake in European Children with Cystic Fibrosis after a 6-Month Intervention with a Self-Management mHealth Tool. Nutrients. 2021; 13(6):1801. https://doi.org/10.3390/nu13061801
Chicago/Turabian StyleCalvo-Lerma, Joaquim, Mieke Boon, Jessie Hulst, Carla Colombo, Inês Asseiceira, María Garriga, Etna Masip, Ine Claes, Anna Bulfamante, Hettie M. Janssens, and et al. 2021. "Change in Nutrient and Dietary Intake in European Children with Cystic Fibrosis after a 6-Month Intervention with a Self-Management mHealth Tool" Nutrients 13, no. 6: 1801. https://doi.org/10.3390/nu13061801






