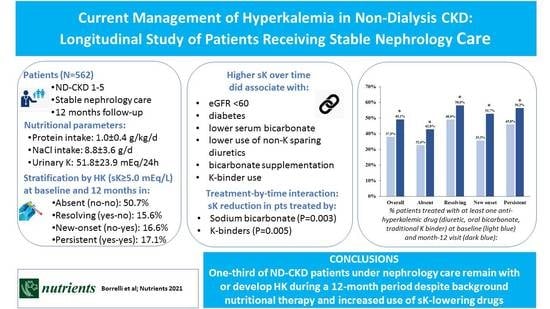
Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Methods
3. Results
3.1. Clinical Features
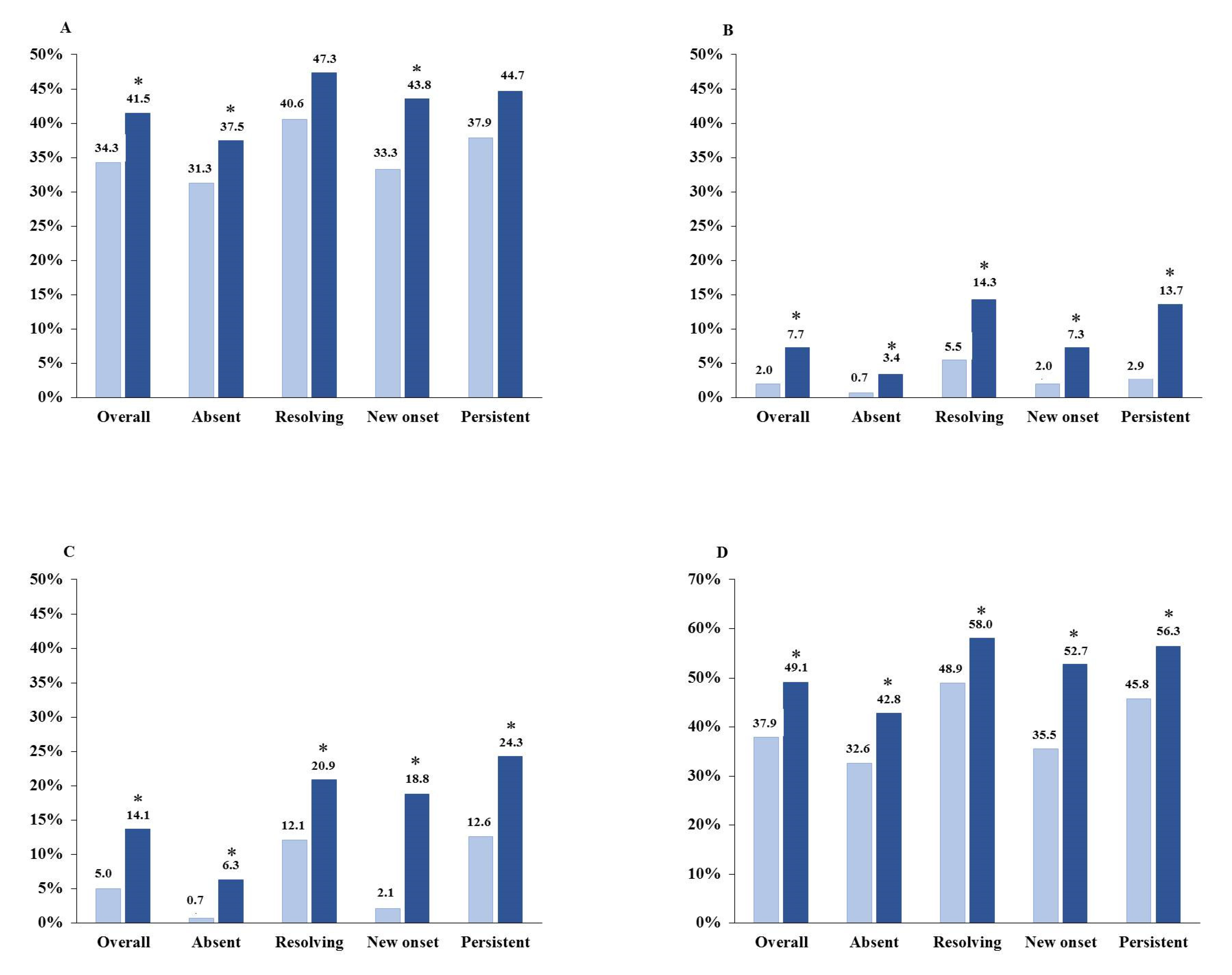
3.2. Therapeutic Features
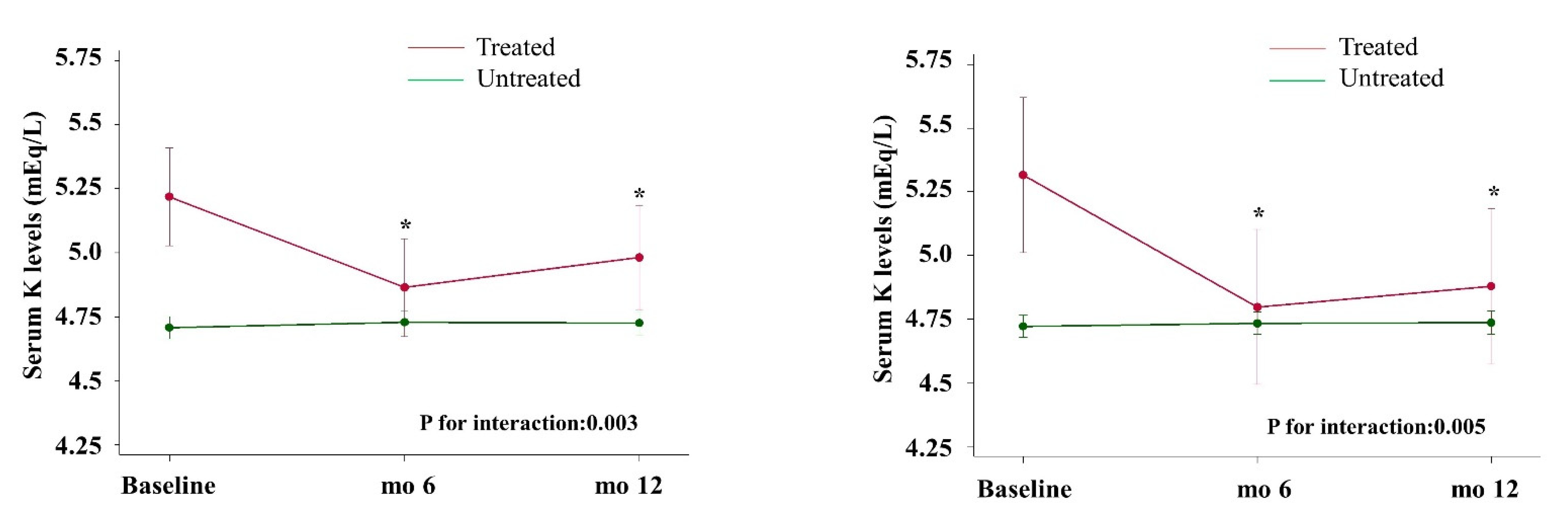
3.3. Time-Dependent Serum K Levels and Interaction in the Whole Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Nicola, L.; Di Lullo, L.; Paoletti, E.; Cupisti, A.; Bianchi, S. Chronic hyperkalemia in non-dialysis CKD: Controversial issues in nephrology practice. J. Nephrol. 2018, 31, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al. Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur. Heart J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Bayés-Genís, A.; Zannad, F.; Rossignol, P.; Núñez, E.; Bodí, V.; Miñana, G.; Santas, E.; Chorro, F.J.; Mollar, A.; et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality. Circulation 2018, 137, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; De Francesco, M.; Iannazzo, S.; Garofalo, C.; Andreucci, M.; Genualdo, R.; Borrelli, S.; Minutolo, R.; Conte, G.; De Nicola, L. Cost-analysis of persistent hyperkalaemia in non-dialysis chronic kidney disease patients under nephrology care in Italy. Int. J. Clin. Pract. 2020, 74, e13475. [Google Scholar] [CrossRef]
- Betts, K.A.; Woolley, J.M.; Mu, F.; Wang, Y.; Wang, Y.; Dua, A.; Wu, E.Q. Postdischarge Health Care Costs and Readmission in Patients With Hyperkalemia-Related Hospitalizations. Kidney Int. Rep. 2020, 5, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Aucella, F.; De Nicola, L.; Genovesi, S.; Paoletti, E.; Regolisti, G. Management of hyperkalemia in patients with kidney disease: A position paper endorsed by the Italian Society of Nephrology. J. Nephrol. 2019, 32, 499–516. [Google Scholar] [CrossRef]
- Rossi, G.M.; Regolisti, G.; Peyronel, F.; Fiaccadori, E. Recent insights into sodium and potassium handling by the aldosterone-sensitive distal nephron: A review of the relevant physiology. J. Nephrol. 2020, 33, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Bovee, D.M.; Roksnoer, L.C.W.; van Kooten, C.; Rotmans, J.I.; Vogt, L.; de Borst, M.H.; Zietse, R.; Danser, A.H.J.; Hoorn, E.J. Effect of sodium bicarbonate supplementation on the renin-angiotensin system in patients with chronic kidney disease and acidosis: A randomized clinical trial. J. Nephrol. 2020. [Google Scholar] [CrossRef]
- Capelli, I.; Gasperoni, L.; Ruggeri, M.; Donati, G.; Baraldi, O.; Sorrenti, G.; Caletti, M.T.; Aiello, V.; Cianciolo, G.; La Manna, G. New mineralocorticoid receptor antagonists: Update on their use in chronic kidney disease and heart failure. J. Nephrol. 2020, 33, 37–48. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Y.; Perkovic, V.; Li, X.; Ninomiya, T.; Hou, W.; Zhao, N.; Liu, L.; Lv, J.; Zhang, H.; et al. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients With CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. Am. J. Kidney Dis. 2016, 67, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Lakkis, J.I.; Jaar, B.; Rocco, M.V.; Choi, M.J.; Kramer, H.J.; Ku, E. Use of Renin-Angiotensin System Blockade in Advanced CKD: An NKF-KDOQI Controversies Report. Am. J. Kidney Dis. 2018, 72, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.L.; Clase, C.M.; Evans, M.; Lindholm, B.; Rotmans, J.I.; Dekker, F.W.; van Diepen, M.; Carrero, J.J. Comparative Effectiveness of Renin-Angiotensin System Inhibitors and Calcium Channel Blockers in Individuals With Advanced CKD: A Nationwide Observational Cohort Study. Am. J. Kidney Dis. 2020. [Google Scholar] [CrossRef]
- Qiao, Y.; Shin, J.I.; Chen, T.K.; Inker, L.A.; Coresh, J.; Alexander, G.C.; Jackson, J.W.; Chang, A.R.; Grams, M.E. Association Between Renin-Angiotensin System Blockade Discontinuation and All-Cause Mortality Among Persons With Low Estimated Glomerular Filtration Rate. JAMA Intern. Med. 2020, 180, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Blacklock, R.; Wood, E.; Rumjon, A.; Simmonds, S.; Fletcher-Rogers, J.; Ariyanayagam, R.; Al-Yassin, A.; Sharpe, C.; Vinen, K. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin. J. Am. Soc. Nephrol. 2012, 7, 1234–1241. [Google Scholar] [CrossRef]
- Hayes, J.; Kalantar-Zadeh, K.; Lu, J.L.; Turban, S.; Anderson, J.E.; Kovesdy, C.P. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: The role of race. Nephron Clin. Pract. 2012, 120, c8–c16. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Cumetti, A.; Pardini, E.; Mannucci, C.; Serio, P.; Morganti, R.; Cupisti, A. Prevalence and correlates of hyperkalemia in a renal nutrition clinic. Intern. Emerg. Med. 2021, 16, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Sang, Y.; Leddy, J.; Yahya, T.; Kirchner, H.L.; Inker, L.A.; Matsushita, K.; Ballew, S.H.; Coresh, J.; Grams, M.E. Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large Health System. Hypertension 2016, 67, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Minutolo, R.; Chiodini, P.; Bellizzi, V.; Nappi, F.; Russo, D.; Borrelli, S.; Garofalo, C.; Iodice, C.; De Stefano, T.; et al. Competing-Risk Analysis of Death and End Stage Kidney Disease by Hyperkalaemia Status in Non-Dialysis Chronic Kidney Disease Patients Receiving Stable Nephrology Care. J. Clin. Med. 2018, 7, 499. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Meyer, H.E.; Johansson, L.; Eggen, A.E.; Johansen, H.; Holvik, K. Sodium and Potassium Intake Assessed by Spot and 24-h Urine in the Population-Based Tromso Study 2015–2016. Nutrients 2019, 11, 1619. [Google Scholar] [CrossRef]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef]
- Dwyer, J.; Kenler, S.R. Assessment of nutritional status in renal disease. In Nutrition and the Kidney, 2nd ed.; Mitch, W.E., Klahr, S., Eds.; Little, Brown: Boston, MA, USA, 1993; pp. 61–95. [Google Scholar]
- Cnaan, A.; Laird, N.M.; Slasor, P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 1997, 16, 2349–2380. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer Publishing Company: Cham, Switzerland, 2014; pp. 99–103. [Google Scholar]
- Donfrancesco, C.; Ippolito, R.; Lo Noce, C.; Palmieri, L.; Iacone, R.; Russo, O.; Vanuzzo, D.; Galletti, F.; Galeone, D.; Giampaoli, S.; et al. Excess dietary sodium and inadequate potassium intake in Italy: Results of the MINISAL study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Guastadisegni, C.; Donfrancesco, C.; Palmieri, L.; Grioni, S.; Krogh, V.; Vanuzzo, D.; Strazzullo, P.; Vannucchi, S.; Onder, G.; Giampaoli, S. Nutrients Intake in Individuals with Hypertension, Dyslipidemia, and Diabetes: An Italian Survey. Nutrients 2020, 12, 923. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Murphy, D.P.; Drawz, P.E.; Foley, R.N. Trends in Angiotensin-Converting Enzyme Inhibitor and Angiotensin II Receptor Blocker Use among Those with Impaired Kidney Function in the United States. J. Am. Soc. Nephrol. 2019, 30, 1314–1321. [Google Scholar] [CrossRef]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: Evidence-based advances in monitoring and treatment. Kidney Int. 2020, 98, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Hundemer, G.L.; Talarico, R.; Tangri, N.; Leon, S.J.; Bota, S.E.; Rhodes, E.; Knoll, G.A.; Sood, M.M. Ambulatory Treatments for RAAS Inhibitor-Related Hyperkalemia and the 1-Year Risk of Recurrence. Clin. J. Am. Soc. Nephrol. 2021. [Google Scholar] [CrossRef]
- Rossignol, P.; Lainscak, M.; Crespo-Leiro, M.G.; Laroche, C.; Piepoli, M.F.; Filippatos, G.; Rosano, G.; Savarese, G.; Anker, S.D.; Seferovic, P.M.; et al. Unravelling the interplay between hyperkalaemia, renin-angiotensin-aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC-HFA-EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2020, 22, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.G.; Cabral, J.V.; El-Feghaly, W.B.; de Sousa, L.S.; Nunes, A.B. Hyporeninemic hypoaldosteronism and diabetes mellitus: Pathophysiology assumptions, clinical aspects and implications for management. World J. Diabetes 2016, 7, 101–111. [Google Scholar] [CrossRef]
- Adrogue, H.J.; Madias, N.E. Veverimer: An Emerging Potential Treatment Option for Managing the Metabolic Acidosis of CKD. Am. J. Kidney Dis. 2020, 76, 861–867. [Google Scholar] [CrossRef]
- Noel, J.A.; Bota, S.E.; Petrcich, W.; Garg, A.X.; Carrero, J.J.; Harel, Z.; Tangri, N.; Clark, E.G.; Komenda, P.; Sood, M.M. Risk of Hospitalization for Serious Adverse Gastrointestinal Events Associated With Sodium Polystyrene Sulfonate Use in Patients of Advanced Age. JAMA Intern. Med. 2019, 179, 1025–1033. [Google Scholar] [CrossRef]
- Laureati, P.; Xu, Y.; Trevisan, M.; Schalin, L.; Mariani, I.; Bellocco, R.; Sood, M.M.; Barany, P.; Sjolander, A.; Evans, M.; et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: A nationwide study. Nephrol. Dial. Transplant. 2020, 35, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Dennis, B.; Stamler, J.; Buzzard, M.; Conway, R.; Elliott, P.; Moag-Stahlberg, A.; Okayama, A.; Okuda, N.; Robertson, C.; Robinson, F.; et al. INTERMAP: The dietary data—Process and quality control. J. Hum. Hypertens. 2003, 17, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Mount, D.B.; Curhan, G.C. DASH-style diet and 24-hour urine composition. Clin. J. Am. Soc. Nephrol. 2010, 5, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Potassium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef]
- Ramos, C.I.; Gonzalez-Ortiz, A.; Espinosa-Cuevas, A.; Avesani, C.M.; Carrero, J.J.; Cuppari, L. Does dietary potassium intake associate with hyperkalemia in patients with chronic kidney disease? Nephrol. Dial. Transplant. 2020. [Google Scholar] [CrossRef] [PubMed]
- Arcand, J.; Floras, J.S.; Azevedo, E.; Mak, S.; Newton, G.E.; Allard, J.P. Evaluation of 2 methods for sodium intake assessment in cardiac patients with and without heart failure: The confounding effect of loop diuretics. Am. J. Clin. Nutr. 2011, 93, 535–541. [Google Scholar] [CrossRef]
- Davidson, J.P.; King, A.J.; Kumaraswamy, P.; Caldwell, J.S.; Korner, P.; Blanks, R.C.; Jacobs, J.W. Evaluation of the Pharmacodynamic Effects of the Potassium Binder RDX7675 in Mice. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 244–253. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef]
- Gritter, M.; Vogt, L.; Yeung, S.M.H.; Wouda, R.D.; Ramakers, C.R.B.; de Borst, M.H.; Rotmans, J.I.; Hoorn, E.J. Rationale and Design of a Randomized Placebo-Controlled Clinical Trial Assessing the Renoprotective Effects of Potassium Supplementation in Chronic Kidney Disease. Nephron 2018, 140, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sussman, E.J.; Singh, B.; Clegg, D.; Palmer, B.F.; Kalantar-Zadeh, K. Let Them Eat Healthy: Can Emerging Potassium Binders Help Overcome Dietary Potassium Restrictions in Chronic Kidney Disease? J. Ren. Nutr. 2020, 30, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Bushinsky, D.A.; Benton, W.W.; Woods, S.D.; Mayo, M.R.; Arthur, S.P.; Pitt, B.; Bakris, G.L. Effect of Patiromer on Hyperkalemia Recurrence in Older Chronic Kidney Disease Patients Taking RAAS Inhibitors. Am. J. Med. 2018, 131, 555–564.e3. [Google Scholar] [CrossRef] [PubMed]
- Spinowitz, B.S.; Fishbane, S.; Pergola, P.E.; Roger, S.D.; Lerma, E.V.; Butler, J.; von Haehling, S.; Adler, S.H.; Zhao, J.; Singh, B.; et al. Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-Month Phase 3 Study. Clin. J. Am. Soc. Nephrol. 2019, 14, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Natale, P.; Palmer, S.C.; Ruospo, M.; Saglimbene, V.M.; Strippoli, G.F. Potassium binders for chronic hyperkalaemia in people with chronic kidney disease. Cochrane Database Syst. Rev. 2020, 6, CD013165. [Google Scholar] [CrossRef]

| Overall (n = 562) | Absent (n = 285) | Resolving (n = 88) | New Onset (n = 93) | Persistent (n = 96) | p-Value | |
|---|---|---|---|---|---|---|
| Age (years) | 66.2 ± 14.5 | 63.6 ± 15.8 | 68.7 ± 13.8 | 69.0 ± 12.7 | 68.9 ± 11.4 | 0.0004 |
| Male (%) | 61.0 | 63.5 | 62.5 | 46.2 | 66.7 | 0.014 |
| BMI (Kg/m2) | 28.7 ± 5.0 | 28.8 ± 4.8 | 28.0 ± 4.5 | 29.0 ± 5.5 | 28.6 ± 5.5 | 0.118 |
| Diabetes (%) | 33.8 | 30.5 | 34.1 | 34.4 | 42.7 | 0.188 |
| CVD (%) | 32.9 | 31.2 | 34.1 | 35.5 | 34.3 | 0.851 |
| Kidney disease (%) | 0.666 | |||||
| HTN | 35.2 | 35.4 | 37.5 | 36.6 | 31.3 | |
| DN | 22.2 | 19.3 | 22.7 | 24.7 | 28.1 | |
| GN | 10.5 | 11.9 | 10.2 | 8.6 | 8.3 | |
| ADPKD | 6.1 | 6.3 | 2.3 | 6.5 | 15.0 | |
| Others | 18.3 | 20.7 | 19.3 | 15.1 | 13.5 | |
| Unknown | 7.7 | 6.3 | 8.0 | 8.6 | 10.4 | |
| CKD stage (%) | <0.001 | |||||
| 1–2 | 13.5 | 23.5 | 3.4 | 5.4 | 1.0 | |
| 3A | 19.8 | 24.6 | 17.1 | 18.3 | 9.4 | |
| 3B | 29.7 | 27.0 | 33.0 | 35.5 | 29.2 | |
| 4 | 28.1 | 20.4 | 37.5 | 30.1 | 40.6 | |
| 5 | 8.9 | 4.6 | 9.1 | 10.8 | 19.8 | |
| SBP (mmHg) | 140 ± 19 | 139 ± 19 | 140 ± 22 | 143 ± 20 | 141 ± 19 | 0.247 |
| sAlbumin (g/dL) | 4.0 ± 0.5 | 4.0 ± 0.6 | 4.0 ± 0.5 | 4.1 ± 0.5 | 3.9 ± 0.5 | 0.192 |
| Hemoglobin (g/dL) | 12.8 ± 1.9 | 13.2 ± 1.8 | 12.4 ± 2.0 | 12.6 ± 1.6 | 11.9 ± 1.7 | <0.001 |
| Proteinuria (g/day) | 0.47 (0.15–0.86) | 0.52 (0.16–0.93) | 0.22 (0.10–0.67) | 0.26 (0.14–0.75) | 0.48 (0.16–0.96) | 0.112 |
| Overall (n = 562) | Absent (n = 285) | Resolving (n = 88) | New Onset (n = 93) | Persistent (n = 96) | p-Value | |
|---|---|---|---|---|---|---|
| Serum K (mEq/L) baseline | 4.74 ± 0.56 | 4.38 ± 0.35 | 5.36 ± 0.37 | 4.58 ± 0.32 | 5.37 ± 0.34 | -- |
| month 12 | 4.74 ± 0.54 | 4.42 ± 0.34 | 4.56 ± 0.34 | 5.24 ± 0.22 | 5.40 ± 0.42 | -- |
| p-value (baseline vs. final) | 0.756 | 0.131 | <0.0001 | <0.0001 | 0.576 | |
| eGFR (ml/min/1.73 m2) baseline | 39.8 ± 21.8 | 47.6 ± 24.2 | 33.5 ± 15.2 | 33.9 ± 16.2 | 27.9 ± 13.8 | <0.001 |
| month 12 | 37.9 ± 21.8 | 45.1 ± 24.3 | 32.9 ± 16.1 | 31.6 ± 16.6 | 27.4 ± 14.1 | <0.001 |
| p-value (baseline vs. final) | <0.0001 | <0.0001 | 0.612 | 0.078 | 0.626 | |
| Serum Bicarbonate (mEq/L) baseline | 23.5 ± 5.7 | 24.7 ± 7.3 | 23.3 ± 4.2 | 22.4 ± 2.7 | 22.1 ± 3.8 | 0.047 |
| month 12 | 24.6 ± 3.0 | 24.8 ± 3.2 | 24.8 ± 2.3 | 24.3 ± 3.0 | 24.4 ± 3.4 | 0.180 |
| p-value (baseline vs. final) | 0.014 | 0.887 | 0.127 | 0.002 | <0.0001 | |
| Serum Bicarbonate < 22 mEq/L (%) baseline | 43.3 | 30.9 | 46.2 | 52.8 | 60.0 | 0.006 |
| month 12 | 22.9 | 20.2 | 11.5 | 30.5 | 28.9 | 0.222 |
| p-value (baseline vs. final) | <0.0001 | 0.077 | 0.007 | 0.059 | 0.003 | |
| Salt intake (g/24 h) baseline | 8.8 ± 3.6 | 8.8 ± 3.7 | 9.1 ± 4.0 | 8.5 ± 3.1 | 8.9 ± 3.8 | 0.636 |
| month 12 | 8.2 ± 3.4 | 8.4 ± 3.1 | 8.3 ± 3.9 | 7.6 ± 3.9 | 8.1 ± 3.5 | 0.358 |
| p-value (baseline vs. final) | 0.009 | 0.247 | 0.295 | 0.064 | 0.119 | |
| Urinary K (mEq/24 h) baseline | 51.8 ± 23.9 | 51.9 ± 24.6 | 47.4 ± 24.1 | 54.5 ± 23.4 | 53.3 ± 22.6 | 0.402 |
| month 12 | 53.4 ± 27.9 | 55.8 ± 27.2 | 47.4 ± 26.3 | 56.4 ± 33.8 | 51.0 ± 23.9 | 0.192 |
| p-value (baseline vs. final) | 0.370 | 0.185 | 0.993 | 0.630 | 0.464 | |
| Protein intake (g/24 h/kg) baseline | 1.0 ± 0.4 | 1.0 ± 0.4 | 0.9 ± 0.3 | 1.0 ± 0.5 | 0.9 ± 0.3 | 0.421 |
| month 12 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.5 | 1.0 ± 0.4 | 0.776 |
| p-value (baseline vs. final) | 0.591 | 0.109 | 0.990 | 0.853 | 0.268 |
| Variables | Βeta | p-Value |
|---|---|---|
| CKD stages (%) | ||
| 1–2 | Ref. | - |
| 3A | 0.15 (0.03/0.27) | 0.015 |
| 3B | 0.28 (0.17/0.39) | <0.001 |
| 4 | 0.41 (0.29/0.53) | <0.001 |
| 5 | 0.49 (0.34/0.65) | <0.001 |
| Age (10 years) | 0.02 (−0.01/0.04) | 0.209 |
| Male | 0.05 (−0.02/0.12) | 0.165 |
| BMI (kg/m2) | 0.00 (−0.00/0.01) | 0.308 |
| Diabetes (yes/no) | 0.08 (0.01/0.16) | 0.026 |
| CV disease (yes/no) | 0.01 (−0.06/0.08) | 0.757 |
| Serum bicarbonate (mEq/L) | −0.01 (−0.02/−0.00) | 0.039 |
| Urinary K (mEq/24 h) | 0.00 (−0.00/0.00) | 0.234 |
| Protein intake (g/day per kg body wt) | 0.01 (−0.08/0.10) | 0.788 |
| Salt intake (g/day) | −0.00 (−0.01/0.01) | 0.576 |
| RAASI (yes/no) | 0.05 (−0.03/0.13) | 0.212 |
| Non-K sparing diuretics (yes/no) | −0.09 (−0.17/−0.02) | 0.014 |
| Bicarbonate supplementation (yes/no) | 0.31 (0.15/0.46) | <0.001 |
| K-binders (yes/no) | 0.27 (0.03/0.50) | 0.025 |
| Time | ||
| Baseline | Ref. | |
| 6 months | 0.00 (−0.05/0.05) | 0.963 |
| 12 months | 0.01 (−0.05/0.6) | 0.855 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrelli, S.; De Nicola, L.; Minutolo, R.; Conte, G.; Chiodini, P.; Cupisti, A.; Santoro, D.; Calabrese, V.; Giannese, D.; Garofalo, C.; et al. Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care. Nutrients 2021, 13, 942. https://doi.org/10.3390/nu13030942
Borrelli S, De Nicola L, Minutolo R, Conte G, Chiodini P, Cupisti A, Santoro D, Calabrese V, Giannese D, Garofalo C, et al. Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care. Nutrients. 2021; 13(3):942. https://doi.org/10.3390/nu13030942
Chicago/Turabian StyleBorrelli, Silvio, Luca De Nicola, Roberto Minutolo, Giuseppe Conte, Paolo Chiodini, Adamasco Cupisti, Domenico Santoro, Vincenzo Calabrese, Domenico Giannese, Carlo Garofalo, and et al. 2021. "Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care" Nutrients 13, no. 3: 942. https://doi.org/10.3390/nu13030942
APA StyleBorrelli, S., De Nicola, L., Minutolo, R., Conte, G., Chiodini, P., Cupisti, A., Santoro, D., Calabrese, V., Giannese, D., Garofalo, C., Provenzano, M., Bellizzi, V., Apicella, L., Piccoli, G. B., Torreggiani, M., & Di Iorio, B. R. (2021). Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care. Nutrients, 13(3), 942. https://doi.org/10.3390/nu13030942