Nutrition in the First Week after Stroke Is Associated with Discharge to Home
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Setting
2.2. Data Collection
2.3. Nutrition Intake
2.4. Outcome Measurement
2.5. Sample Size Calculation and Statistical Analysis
2.6. Ethics
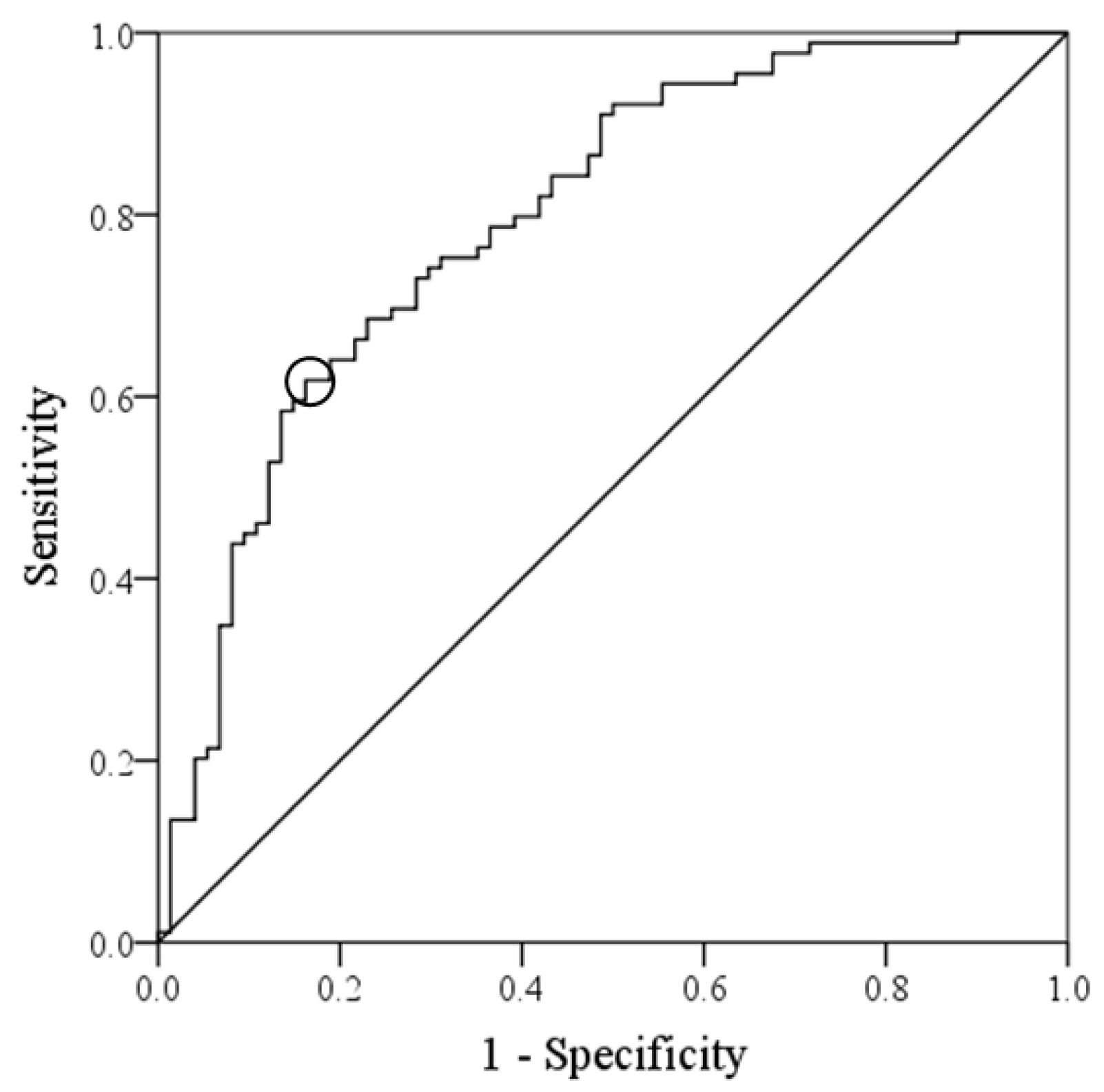
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dávalos, A.; Ricart, W.; Gonzalez-Huix, F.; Soler, S.; Marrugat, J.; Molins, A.; Suñer, R.; Genís, D. Effect of malnutrition after acute stroke on clinical outcome. Stroke 1996, 27, 1028–1032. [Google Scholar] [CrossRef]
- Collaboration, F.T. Poor nutritional status on admission predicts poor outcomes after stroke: Observational data from the FOOD trial. Stroke 2003, 34, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Nii, M.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Tanaka, A. Nutritional improvement and energy intake are associated with functional recovery in patients after cerebrovascular disorders. J. Stroke Cerebrovasc. Dis. 2016, 25, 57–62. [Google Scholar] [CrossRef]
- Chen, N.; Li, Y.; Fang, J.; Lu, Q.; He, L. Risk factors for malnutrition in stroke patients: A meta-analysis. Clin. Nutr. 2019, 38, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Pichard, C.; Jeejeebhoy, K.N. Muscle dysfunction in malnourished patients. Q. J. Med. 1988, 69, 1021–1045. [Google Scholar]
- Shiraishi, A.; Yoshimura, Y.; Wakabayashi, H.; Tsuji, Y. Prevalence of stroke-related sarcopenia and its association with poor oral status in post-acute stroke patients: Implications for oral sarcopenia. Clin. Nutr. 2018, 37, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Tanoue, M. Prevalence of sarcopenia and its association with activities of daily living and dysphagia in convalescent rehabilitation ward inpatients. Clin. Nutr. 2018, 37, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.Q.; PrvuBettger, J.; Guerrier, T.; Hirsch, M.A.; Thomas, J.G.; Pugh, T.M.; Rhoads, C.F., 3rd. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch. Phys. Med. Rehabil. 2015, 96, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Kenmuir, C.L.; Hammer, M.; Jovin, T.; Reddy, V.; Wechsler, L.; Jadhav, A. Predictors of outcome in patients presenting with acute ischemic stroke and mild stroke scale scores. J. Stroke Cerebrovasc. Dis. 2015, 24, 1685–1689. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tsubakino, S.; Fujii, H. Eating and grooming abilities predict outcomes in patients with early middle cerebral infarction: A retrospective cohort study. Occup. Ther. Int. 2020, 2020, 1374527. [Google Scholar] [CrossRef]
- Sato, M.; Ido, Y.; Yoshimura, Y.; Mutai, H. Relationship of malnutrition during hospitalization with functional recovery and postdischarge destination in elderly stroke patients. J. Stroke Cerebrovasc. Dis. 2019, 28, 1866–1872. [Google Scholar] [CrossRef]
- Nozoe, M.; Kanai, M.; Kubo, H.; Yamamoto, M.; Shimada, S.; Mase, K. Prestroke sarcopenia and functional outcomes in elderly patients who have had an acute stroke: A prospective cohort study. Nutrition 2019, 66, 44–47. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Bise, T.; Shimazu, S.; Shiraishi, A. Chair-stand exercise improves post-stroke dysphagia. Geriatr. Gerontol. Int. 2020, 20, 885–891. [Google Scholar] [CrossRef]
- Shimazu, S.; Yoshimura, Y.; Kudo, M.; Nagano, F.; Bise, T.; Shiraishi, A.; Sunahara, T. Frequent and personalized nutritional support leads to improved nutritional status, activities of daily living, and dysphagia after stroke. Nutrition 2020, 83, 111091. [Google Scholar] [CrossRef] [PubMed]
- Kokura, Y.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Higashi, S. High nutritional-related risk on admission predicts less improvement of functional independence measure in geriatric stroke patients: A retrospective cohort study. J. Stroke Cerebrovasc. Dis. 2016, 25, 1335–1341. [Google Scholar] [CrossRef]
- Nishioka, S.; Okamoto, T.; Takayama, M.; Urushihara, M.; Watanabe, M.; Kiriya, Y.; Shintani, K.; Nakagomi, H.; Kageyama, N. Malnutrition risk predicts recovery of full oral intake among older adult stroke patients undergoing enteral nutrition: Secondary analysis of a multicentre survey (the APPLE study). Clin. Nutr. 2017, 36, 1089–1096. [Google Scholar] [CrossRef]
- Abe, T.; Iwata, K.; Yoshimura, Y.; Shinoda, T.; Inagaki, Y.; Ohya, S.; Yamada, K.; Oyanagi, K.; Maekawa, Y.; Honda, A.; et al. Low muscle mass is associated with walking function in patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105259. [Google Scholar] [CrossRef] [PubMed]
- Kokura, Y.; Wakabayashi, H.; Nishioka, S.; Maeda, K. Nutritional intake is associated with activities of daily living and complications in older inpatients with stroke. Geriatr. Gerontol. Int. 2018, 18, 1334–1339. [Google Scholar] [CrossRef]
- Kokura, Y.; Kato, M.; Taniguchi, Y.; Kimoto, K.; Okada, Y. Energy intake during the acute phase and changes in femoral muscle thickness in older hemiplegic inpatients with stroke. Nutrition 2020, 70, 110582. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Bise, T.; Shimazu, S.; Kudo, M.; Shiraishi, A. Sarcopenic obesity is associated with activities of daily living and home discharge in post-acute rehabilitation. J. Am. Med. Dir. Assoc. 2020, 21, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.M.; Niewczyk, P.; DiVita, M.; Markello, S.; Granger, C. Predictors of discharge to acute care after inpatient rehabilitation in severely affected stroke patients. Arch. Phys. Med. Rehabil. 2012, 91, 387–392. [Google Scholar]
- Yoshimura, Y.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Systemic Inflammation in the Recovery Stage of Stroke: Its Association with Sarcopenia and Poor Functional Rehabilitation Outcomes. Prog. Rehabil. Med. 2018, 3, 20180011. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition 2019, 61, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Nip, W.F.; Perry, L.; McLaren, S.; Mackenzie, A. Dietary intake, nutritional status and rehabilitation outcomes of stroke patients in hospital. J. Hum. Nutr. Diet 2011, 24, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Vluggen, T.; van Haastregt, J.C.M.; Tan, F.E.S.; Kempen, G.; Schols, J.; Verbunt, J.A. Factors associated with successful home discharge after inpatient rehabilitation in frail older stroke patients. BMC Geriatr. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for Treating Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Am. Med. Dir. Assoc. 2017, 18, 553.e1–533.e16. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Uchida, K.; Jeong, S.; Yamaga, M. Effects of nutritional supplements on muscle mass and activities of daily living in elderly rehabilitation patients with decreased muscle mass: A randomized controlled trial. J. Nutr. Health Aging 2016, 20, 185–191. [Google Scholar] [CrossRef]
- Takeuchi, I.; Yoshimura, Y.; Shimazu, S.; Jeong, S.; Yamaga, M.; Koga, H. Effects of branched-chain amino acids and vitamin D supplementation on physical function, muscle mass and strength, and nutritional status in sarcopenic older adults undergoing hospital-based rehabilitation: A multicenter randomized controlled trial. Geriatr. Gerontol. Int. 2019, 19, 12–17. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef]

| Total (n = 163) | Home (n = 89) | Other (n = 74) | p–Value | |
|---|---|---|---|---|
| Age (year) | 75.2 (12.6) | 71.4 (12.6) | 78.4 (11.8) | <0.001 |
| Sex (male/female) | 103/60 | 64/25 | 39/35 | 0.011 |
| Body mass index (kg/m2) | 22.8 (3.9) | 23.1 (3.9) | 22.3 (3.9) | 0.205 |
| NIHSS score | 5 (2–9) | 2 (1–5) | 9 (6–14) | <0.001 |
| Stroke type (infarct/hemorrhage) | 129/34 | 75/14 | 54/20 | 0.077 |
| Comorbidity (%) | ||||
| Hypertension | 109 (66.9) | 61 (68.5) | 48 (64.9) | 0.547 |
| Diabetes | 54 (33.1) | 26 (29.2) | 28 (37.8) | 0.244 |
| Previous stroke | 19 (11.7) | 7 (7.9) | 12 (16.2) | 0.098 |
| Atrial fibrillation | 50 (30.7) | 23 (25.8) | 27 (36.5) | 0.142 |
| Side of lesion (right/left/both) | 65/89/9 | 38/45/6 | 27/44/3 | 0.401 |
| BRS | ||||
| Upper limb | 5 (3–6) | 6 (5–6) | 3 (2–5) | <0.001 |
| Hand-finger | 5 (3–6) | 5 (5–6) | 3 (2–5) | <0.001 |
| Lower limb | 5 (4–6) | 6 (5–6) | 4 (2–5) | <0.001 |
| Days from onset (day) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.989 |
| Length of hospital stay (day) | 19 (11–28) | 14 (10–19) | 27 (20–34) | <0.001 |
| Laboratory data | ||||
| Albumin (g/dL) | 4.0 (0.5) | 4.1 (0.5) | 30.9 (00.5) | 0.079 |
| Hemoglobin (g/dL) | 13.3 (2.1) | 13.5 (2.1) | 130.1 (20.2) | 0.268 |
| GNRI | 103.3 (95.2–108.9) | 104.7 (96.7–110.5) | 101.0 (93.6–107.8) | 0.275 |
| SMI (kg/m2) | 7.0 (6.0–8.1) | 7.2 (6.3–8.6) | 6.4 (5.4–7.6) | <0.001 |
| Handgrip strength (kg) | 22.9 (14.0–30.2) | 25.0 (18.5–33.0) | 15.2 (8.6–20.0) | <0.001 |
| FOIS | 5 (2–6) | 6 (5–7) | 2 (1–5) | <0.001 |
| Rehabilitation time (min/day) | 60.4 (45.8–76.0) | 54.8 (37.9–74.5) | 64.0 (52.5–77.4) | 0.022 |
| FIM at admission | 58 (32–83) | 80 (66–93) | 32 (20–51) | <0.001 |
| FIM eating at discharge | 7 (5–7) | 7 (7–7) | 4 (2–6) | <0.001 |
| FIM gain | 24 (11–40) | 22 (11–40) | 28 (11–39) | 0.001 |
| Energy intake (kcal/kg/day) | 18.3 (12.1–24.7) | 23.5 (16.7–26.6) | 12.4 (9.3–18.4) | <0.001 |
| Protein intake (g/kg/day) | 0.9 (0.6–1.0) | 0.9 (0.8–1.1) | 0.7 (0.5–0.9) | <0.001 |
| Home–Discharge #1 | FIM at Discharge #2 | |||
|---|---|---|---|---|
| OR (95% CI) | p–Value | β | p-Value | |
| Age | 0.966 (0.903–1.034) | 0.323 | −0.065 | 0.131 |
| Gender (male) | 1.160 (0.214–6.293) | 0.864 | −0.048 | 0.302 |
| Stroke type(infarction) | 0.466 (0.079–2.729) | 0.397 | −0.084 | 0.109 |
| NIHSS | 0.836 (0.637–1.098) | 0.198 | −0.164 | 0.020 |
| Length of stay | 0.974 (0.937–1.012) | 0.180 | 0.108 | 0.041 |
| GNRI | 1.012 (0.977–1.048) | 0.514 | 0.060 | 0.205 |
| SMI | 1.137 (0.551–2.345) | 0.729 | 0.164 | 0.019 |
| Handgrip strength | 1.057 (0.922–1.212) | 0.425 | 0.166 | 0.028 |
| FOIS | 1.450 (1.036–2.544) | 0.036 | 0.070 | 0.278 |
| FIM at admission | 1.039 (1.003–1.075) | 0.033 | 0.349 | <0.001 |
| FIM eating at discharge | 1.651 (1.952–2.063) | 0.045 | ||
| FIM gain | 1.042 (0.979–1.109) | 0.193 | ||
| BRS-lower limb | 0.819 (0.432–1.549) | 0.539 | 0.123 | 0.049 |
| Rehabilitation therapy | 0.968 (0.941–1.083) | 0.074 | 0.109 | 0.121 |
| Energy intake | 1.146 (1.029–1.276) | 0.013 | 0.131 | 0.025 |
| Protein intake | 0.104 (0.006–1.739) | 0.115 | 0.063 | 0.264 |
| High Group (n = 67) | Low Group (n = 96) | p-Value | |
|---|---|---|---|
| Length of hospital stay (day) | 14 (10–24) | 22 (16–31) | 0.012 |
| GNRI | 103.3 (94.7–108.3) | 102.9 (95.1–109.4) | 0.270 |
| FOIS | 6 (5–7) | 4 (1–5) | <0.001 |
| FIM at discharge | 122 (97–126) | 78 (40–111) | <0.001 |
| Discharge to home (%) | 55 (82.1) | 34 (35.4) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Yoshimura, Y.; Abe, T. Nutrition in the First Week after Stroke Is Associated with Discharge to Home. Nutrients 2021, 13, 943. https://doi.org/10.3390/nu13030943
Sato Y, Yoshimura Y, Abe T. Nutrition in the First Week after Stroke Is Associated with Discharge to Home. Nutrients. 2021; 13(3):943. https://doi.org/10.3390/nu13030943
Chicago/Turabian StyleSato, Yoichi, Yoshihiro Yoshimura, and Takafumi Abe. 2021. "Nutrition in the First Week after Stroke Is Associated with Discharge to Home" Nutrients 13, no. 3: 943. https://doi.org/10.3390/nu13030943
APA StyleSato, Y., Yoshimura, Y., & Abe, T. (2021). Nutrition in the First Week after Stroke Is Associated with Discharge to Home. Nutrients, 13(3), 943. https://doi.org/10.3390/nu13030943







