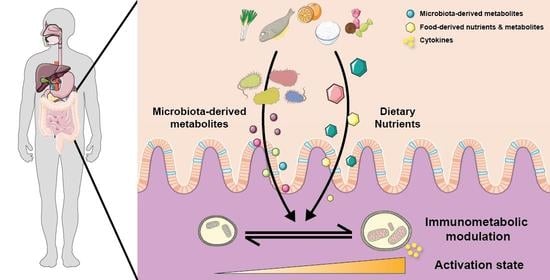
How Changes in the Nutritional Landscape Shape Gut Immunometabolism
Abstract
:1. Introduction
2. Immunometabolism and Impact on Immune Cell Differentiation and Function
2.1. Glycolysis Mediates Proinflammatory Effects
2.2. Pro-Inflammatory Effects of the Pentose Phosphate Pathway
2.3. Gluconeogenesis Drives the Effector Function of Immune Cells by Supporting Glycogenolysis
2.4. Anti-Inflammatory Effects of Fatty Acid Metabolism by Beta Oxidation
2.5. Pro-Inflammatory Effects of Fatty Acid Synthesis and Lipogenesis
2.6. The Tricarboxylic Acid Cycle (TCA) and Its Intermediates Have either Pro- or Anti-Inflammatory Effects
2.6.1. Immunomodulation through Epigenetic Changes
2.6.2. Immunomodulation through Post-Translational Changes
2.6.3. Immunomodulation through G Protein-Coupled Receptor Signalling
2.7. Amino Acid Metabolism and the GABA Pathway Mediates Both Pro- and Anti-Inflammatory Effects
2.8. Mevalonate and Cholesterol Synthesis Pathway Promote Anti-Inflammatory Effects
2.9. The Ketogenic Pathway Mediates Anti-Inflammatory Effects
3. Overview of the Gut Immune System
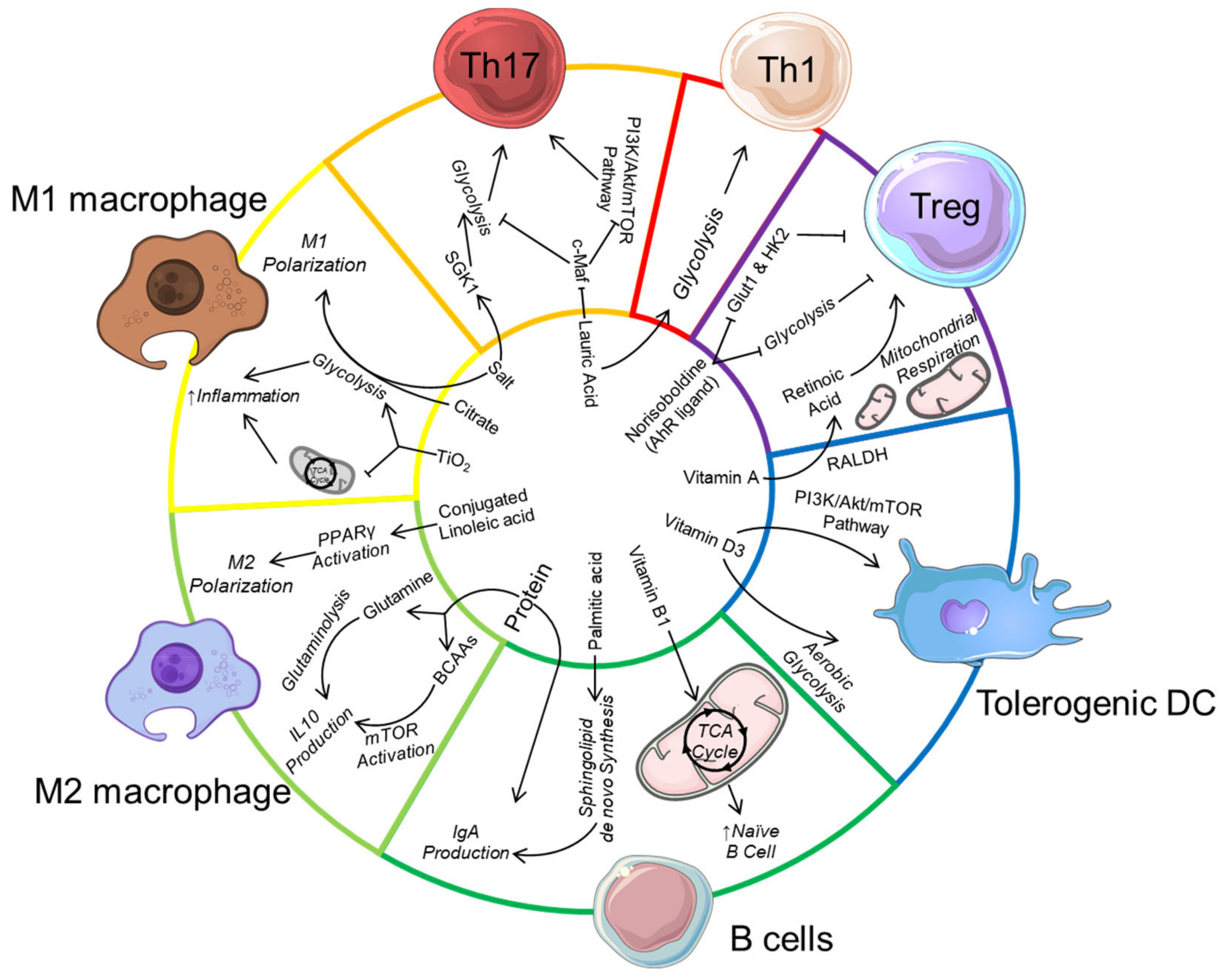
4. Nutrient Sensing and Gut Immunometabolism
4.1. Dietary Protein and Gut Immunity
4.2. Dietary Lipids and Gut Immunity
4.3. Dietary Carbohydrates and Gut Immunity
4.4. Dietary AhR Ligands and AhR Activation
4.5. Vitamins
4.6. Impact of Food Additive on Gut Immunity and Immunometabolism
5. Dietary-Induced Bacterial Metabolites and Gut Immunometabolism
5.1. Microbial Metabolism of Complex Carbohydrate Produces Short-Chain Fatty Acid with Anti-Inflammatory Properties
5.2. Microbial Metabolism of Amino Acid Produces Metabolites with Immunomodulatory Properties
5.3. Impact of Secondary Bile Acids on Gut Immune Profile and Immuno-Metabolism
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venn, B.J. Macronutrients and Human Health for the 21st Century. Nutrients 2020, 12, 2363. [Google Scholar] [CrossRef]
- Huskisson, E.; Maggini, S.; Ruf, M. The Role of Vitamins and Minerals in Energy Metabolism and Well-Being. J. Int. Med. Res. 2007, 35, 277–289. [Google Scholar] [CrossRef]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western Diet” in Inflammatory Autoimmune Diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.K.; McKenzie, C.; Mariño, E.; Macia, L.; Mackay, C.R. Metabolite-Sensing G Protein–Coupled Receptors—Facilitators of Diet-Related Immune Regulation. Annu. Rev. Immunol. 2017, 35, 371–402. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.M.; Boothby, M.R.; Chi, H. Metabolic Coordination of T Cell Quiescence and Activation. Nat. Rev. Immunol. 2020, 20, 55–70. [Google Scholar] [CrossRef]
- Wensveen, F.M.; van Gisbergen, K.P.J.M.; Eldering, E. The Fourth Dimension in Immunological Space: How the Struggle for Nutrients Selects High-Affinity Lymphocytes. Immunol. Rev. 2012, 249, 84–103. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.L.; Finlay, D.K. Immunometabolism and Natural Killer Cell Responses. Nat. Rev. Immunol. 2019, 19, 282–290. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Kishton, R.J.; Rathmell, J. A Guide to Immunometabolism for Immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Lunt, S.Y.; Dayton, T.L.; Fiske, B.P.; Israelsen, W.J.; Mattaini, K.R.; Vokes, N.I.; Stephanopoulos, G.; Cantley, L.C.; Metallo, C.M.; et al. Metabolic Pathway Alterations That Support Cell Proliferation. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Curi, R.; de Siqueira Mendes, R.; de Campos Crispin, L.A.; Norata, G.D.; Sampaio, S.C.; Newsholme, P. A Past and Present Overview of Macrophage Metabolism and Functional Outcomes. Clin. Sci. 2017, 131, 1329–1342. [Google Scholar] [CrossRef] [Green Version]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Le, Z.; Jessie Yanxiang, G.; et al. Glucose Feeds the TCA Cycle via Circulating Lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and Competition in the Evolution of ATP-Producing Pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. HIF-1 Mediates Metabolic Responses to Intratumoral Hypoxia and Oncogenic Mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Hollinshead, K.E.R.; Hao, Y.; Au, C.; Kroehling, L.; Ng, C.; Lin, W.-Y.; Li, D.; Silva, H.M.; Shin, J.; et al. Niche-Selective Inhibition of Pathogenic Th17 Cells by Targeting Metabolic Redundancy. Cell 2020, 182, 641–654.e20. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate Reduces Liver and Pancreatic Injury in Toll-like Receptor- and Inflammasome-Mediated Inflammation via GPR81-Mediated Suppression of Innate Immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef] [Green Version]
- Errea, A.; Cayet, D.; Marchetti, P.; Tang, C.; Kluza, J.; Offermanns, S.; Sirard, J.-C.; Rumbo, M. Lactate Inhibits the Pro-Inflammatory Response and Metabolic Reprogramming in Murine Macrophages in a GPR81-Independent Manner. PLoS ONE 2016, 11, e0163694. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, P.; Shanmugam, A.; Swafford, D.; Suryawanshi, A.; Bhattacharjee, P.; Hussein, M.S.; Koni, P.A.; Prasad, P.D.; Kurago, Z.B.; Thangaraju, M.; et al. GPR81, a Cell-Surface Receptor for Lactate, Regulates Intestinal Homeostasis and Protects Mice from Experimental Colitis. J. Immunol. 2018, 200, 1781–1789. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory Effect of Tumor Cell-Derived Lactic Acid on Human T Cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Tu, D.; Gao, Y.; Yang, R.; Guan, T.; Hong, J.-S.; Gao, H.-M. The Pentose Phosphate Pathway Regulates Chronic Neuroinflammation and Dopaminergic Neurodegeneration. J. Neuroinflamm. 2019, 16, 255. [Google Scholar] [CrossRef] [Green Version]
- Baardman, J.; Verberk, S.G.S.; Prange, K.H.M.; van Weeghel, M.; van der Velden, S.; Ryan, D.G.; Wüst, R.C.I.; Neele, A.E.; Speijer, D.; Denis, S.W.; et al. A Defective Pentose Phosphate Pathway Reduces Inflammatory Macrophage Responses during Hypercholesterolemia. Cell Rep. 2018, 25, 2044–2052.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, E.P.; Rochael, N.C.; Guimarães-Costa, A.B.; de Souza-Vieira, T.S.; Ganilho, J.; Saraiva, E.M.; Palhano, F.L.; Foguel, D. A Metabolic Shift toward Pentose Phosphate Pathway Is Necessary for Amyloid Fibril- and Phorbol 12-Myristate 13-Acetate-Induced Neutrophil Extracellular Trap (NET) Formation. J. Biol. Chem. 2015, 290, 22174–22183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadiku, P.; Willson, J.A.; Ryan, E.M.; Sammut, D.; Coelho, P.; Watts, E.R.; Grecian, R.; Young, J.M.; Bewley, M.; Arienti, S.; et al. Neutrophils Fuel Effective Immune Responses through Gluconeogenesis and Glycogenesis. Cell Metab. 2020. [Google Scholar] [CrossRef]
- Ma, R.; Ji, T.; Zhang, H.; Dong, W.; Chen, X.; Xu, P.; Chen, D.; Liang, X.; Yin, X.; Liu, Y.; et al. A Pck1-Directed Glycogen Metabolic Program Regulates Formation and Maintenance of Memory CD8+ T Cells. Nat. Cell Biol. 2018, 20, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Thwe, P.; Pelgrom, L.; Cooper, R.; Beauchamp, S.; Reisz, J.A.; D’Alessandro, A.; Everts, B.; Amiel, E. Cell-Intrinsic Glycogen Metabolism Supports Early Glycolytic Reprogramming Required for Dendritic Cell Immune Responses. Cell Metab. 2017, 26, 558–567.e5. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wei, K.; Liu, J.; Tang, K.; Zhang, H.; Zhu, L.; Chen, J.; Li, F.; Xu, P.; Chen, J.; et al. Glycogen Metabolism Regulates Macrophage-Mediated Acute Inflammatory Responses. Nat. Commun. 2020, 11, 1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Targeting Immunometabolism as an Anti-Inflammatory Strategy. Cell Res. 2020, 30, 300–314. [Google Scholar] [CrossRef] [Green Version]
- Van den Bossche, J.; van der Windt, G.J.W. Fatty Acid Oxidation in Macrophages and T Cells: Time for Reassessment? Cell Metab. 2018, 28, 538–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, E.L.; Walsh, M.C.; Cejas, P.J.; Harms, G.M.; Shen, H.; Wang, L.-S.; Jones, R.G.; Choi, Y. Enhancing CD8 T Cell Memory by Modulating Fatty Acid Metabolism. Nature 2009, 460, 103–107. [Google Scholar] [CrossRef]
- Raud, B.; Roy, D.G.; Divakaruni, A.S.; Tarasenko, T.N.; Franke, R.; Ma, E.H.; Samborska, B.; Hsieh, W.Y.; Wong, A.H.; Stüve, P.; et al. Etomoxir Actions on Regulatory and Memory T Cells Are Independent of Cpt1a-Mediated Fatty Acid Oxidation. Cell Metab. 2018, 28, 504–515.e7. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, D.A.; Proctor, E.A.; Agrawal, M.; Belkina, A.C.; Van Nostrand, S.C.; Panneerseelan-Bharath, L.; Jones, A.R.; Raval, F.; Ip, B.C.; Zhu, M.; et al. Fatty Acid Metabolites Combine with Reduced β Oxidation to Activate Th17 Inflammation in Human Type 2 Diabetes. Cell Metab. 2019, 30, 447–461.e5. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.M.; Davies, L.C.; Subleski, J.J.; Maio, N.; Gonzalez-Cotto, M.; Andrews, C.; Patel, N.L.; Palmieri, E.M.; Weiss, J.M.; Lee, J.-M.; et al. Tumour-Elicited Neutrophils Engage Mitochondrial Metabolism to Circumvent Nutrient Limitations and Maintain Immune Suppression. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, C.S.; Baixauli, F.; Kyle, R.L.; Puleston, D.J.; Cameron, A.M.; Sanin, D.E.; Hippen, K.L.; Loschi, M.; Thangavelu, G.; Corrado, M.; et al. Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab. 2020, 31, 422–437.e5. [Google Scholar] [CrossRef] [Green Version]
- Weisel, F.J.; Mullett, S.J.; Elsner, R.A.; Menk, A.V.; Trivedi, N.; Luo, W.; Wikenheiser, D.; Hawse, W.F.; Chikina, M.; Smita, S.; et al. Germinal Center B Cells Selectively Oxidize Fatty Acids for Energy While Conducting Minimal Glycolysis. Nat. Immunol. 2020, 21, 331–342. [Google Scholar] [CrossRef]
- Berod, L.; Friedrich, C.; Nandan, A.; Freitag, J.; Hagemann, S.; Harmrolfs, K.; Sandouk, A.; Hesse, C.; Castro, C.N.; Bähre, H.; et al. De Novo Fatty Acid Synthesis Controls the Fate between Regulatory T and T Helper 17 Cells. Nat. Med. 2014, 20, 1327–1333. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, X.; Li, C.; Li, Y.; Ye, Z.; Shapiro, V.S.; Copland, J.A.; Hitosugi, T.; Bernlohr, D.A.; Sun, J.; et al. Stearoyl-CoA Desaturase-Mediated Monounsaturated Fatty Acid Availability Supports Humoral Immunity. Cell Rep. 2021, 34, 108601. [Google Scholar] [CrossRef]
- Dufort, F.J.; Gumina, M.R.; Ta, N.L.; Tao, Y.; Heyse, S.A.; Scott, D.A.; Richardson, A.D.; Seyfried, T.N.; Chiles, T.C. Glucose-Dependent de Novo Lipogenesis in B Lymphocytes: A Requirement for Atp-Citrate Lyase in Lipopolysaccharide-Induced Differentiation. J. Biol. Chem. 2014, 289, 7011–7024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodhi, I.J.; Wei, X.; Yin, L.; Feng, C.; Adak, S.; Abou-Ezzi, G.; Hsu, F.-F.; Link, D.C.; Semenkovich, C.F. Peroxisomal Lipid Synthesis Regulates Inflammation by Sustaining Neutrophil Membrane Phospholipid Composition and Viability. Cell Metab. 2015, 21, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate Is an Anti-Inflammatory Metabolite That Activates Nrf2 via Alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Bambouskova, M.; Gorvel, L.; Lampropoulou, V.; Sergushichev, A.; Loginicheva, E.; Johnson, K.; Korenfeld, D.; Mathyer, M.E.; Kim, H.; Huang, L.-H.; et al. Electrophilic Properties of Itaconate and Derivatives Regulate the IκBζ-ATF3 Inflammatory Axis. Nature 2018, 556, 501–504. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [Green Version]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules That Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Pollard, P.J. Succinate: A New Epigenetic Hacker. Cancer Cell 2013, 23, 709–711. [Google Scholar] [CrossRef] [Green Version]
- Morales-Nebreda, L.; McLafferty, F.S.; Singer, B.D. DNA Methylation as a Transcriptional Regulator of the Immune System. Transl. Res. J. Lab. Clin. Med. 2019, 204, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Joosten, L.A.B.; Netea, M.G. Immunometabolic Circuits in Trained Immunity. Semin. Immunol. 2016, 28, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate Is an Inflammatory Signal That Induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-Citrate Lyase Links Cellular Metabolism to Histone Acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Liu, M.; Feng, X.; Wang, Z.; Das, I.; Xu, Y.; Zhou, X.; Sun, Y.; Guan, K.-L.; Xiong, Y.; et al. Glyceraldehyde-3-Phosphate Dehydrogenase Is Activated by Lysine 254 Acetylation in Response to Glucose Signal. J. Biol. Chem. 2014, 289, 3775–3785. [Google Scholar] [CrossRef] [Green Version]
- Guarente, L. The Logic Linking Protein Acetylation and Metabolism. Cell Metab. 2011, 14, 151–153. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Que, K.-T.; Zhang, Z.; Yi, Z.J.; Zhao, P.X.; You, Y.; Gong, J.-P.; Liu, Z.-J. Iron Overloaded Polarizes Macrophage to Proinflammation Phenotype through ROS/Acetyl-P53 Pathway. Cancer Med. 2018, 7, 4012–4022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Rao, Y.-H.; Inoue, M.; Hao, R.; Lai, C.-H.; Chen, D.; McDonald, S.L.; Choi, M.-C.; Wang, Q.; Shinohara, M.L.; et al. Microtubule Acetylation Amplifies P38 Kinase Signalling and Anti-Inflammatory IL-10 Production. Nat. Commun. 2014, 5, 3479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, J.; Wu, E.; Lowe, C.; Srinivasan, S.; McCord, R.; Wagle, M.-C.; Jayakar, S.; Edick, M.G.; Eastham-Anderson, J.; Liu, B.; et al. CBP/P300 Drives the Differentiation of Regulatory T Cells through Transcriptional and Non-Transcriptional Mechanisms. Cancer Res. 2019, 79, 3916–3927. [Google Scholar] [CrossRef]
- Busslinger, M.; Tarakhovsky, A. Epigenetic Control of Immunity. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Rubic, T.; Lametschwandtner, G.; Jost, S.; Hinteregger, S.; Kund, J.; Carballido-Perrig, N.; Schwärzler, C.; Junt, T.; Voshol, H.; Meingassner, J.G.; et al. Triggering the Succinate Receptor GPR91 on Dendritic Cells Enhances Immunity. Nat. Immunol. 2008, 9, 1261–1269. [Google Scholar] [CrossRef]
- Keiran, N.; Ceperuelo-Mallafré, V.; Calvo, E.; Hernández-Alvarez, M.I.; Ejarque, M.; Núñez-Roa, C.; Horrillo, D.; Maymó-Masip, E.; Rodríguez, M.M.; Fradera, R.; et al. SUCNR1 Controls an Anti-Inflammatory Program in Macrophages to Regulate the Metabolic Response to Obesity. Nat. Immunol. 2019, 20, 581–592. [Google Scholar] [CrossRef]
- Littlewood-Evans, A.; Sarret, S.; Apfel, V.; Loesle, P.; Dawson, J.; Zhang, J.; Muller, A.; Tigani, B.; Kneuer, R.; Patel, S.; et al. GPR91 Senses Extracellular Succinate Released from Inflammatory Macrophages and Exacerbates Rheumatoid Arthritis. J. Exp. Med. 2016, 213, 1655–1662. [Google Scholar] [CrossRef]
- Lei, W.; Ren, W.; Ohmoto, M.; Urban, J.F.; Matsumoto, I.; Margolskee, R.F.; Jiang, P. Activation of Intestinal Tuft Cell-Expressed Sucnr1 Triggers Type 2 Immunity in the Mouse Small Intestine. Proc. Natl. Acad. Sci. USA 2018, 115, 5552–5557. [Google Scholar] [CrossRef] [Green Version]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- Quintana, F.J.; Basso, A.S.; Iglesias, A.H.; Korn, T.; Farez, M.F.; Bettelli, E.; Caccamo, M.; Oukka, M.; Weiner, H.L. Control of T(Reg) and T(H)17 Cell Differentiation by the Aryl Hydrocarbon Receptor. Nature 2008, 453, 65–71. [Google Scholar] [CrossRef]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.D.; Hou, D.-Y.; Liu, Y.; Koni, P.A.; Metz, R.; Chandler, P.; Mellor, A.L.; He, Y.; Munn, D.H. Indoleamine 2,3-Dioxygenase Controls Conversion of Foxp3+ Tregs to TH17-like Cells in Tumor-Draining Lymph Nodes. Blood 2009, 113, 6102–6111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matteoli, G.; Mazzini, E.; Iliev, I.D.; Mileti, E.; Fallarino, F.; Puccetti, P.; Chieppa, M.; Rescigno, M. Gut CD103+ Dendritic Cells Express Indoleamine 2,3-Dioxygenase Which Influences T Regulatory/T Effector Cell Balance and Oral Tolerance Induction. Gut 2010, 59, 595–604. [Google Scholar] [CrossRef]
- Jones, R.G.; Pearce, E.J. MenTORing Immunity: MTOR Signaling in the Development and Function of Tissue-Resident Immune Cells. Immunity 2017, 46, 730–742. [Google Scholar] [CrossRef] [Green Version]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-Tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, W.; Xia, Y.; Chen, S.; Wu, G.; Bazer, F.W.; Zhou, B.; Tan, B.; Zhu, G.; Deng, J.; Yin, Y. Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes. Adv. Nutr. 2019, 10, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine Uptake and Metabolism Are Coordinately Regulated by ERK/MAPK during T Lymphocyte Activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-K.; Yang, K.D.; Chuang, H.; Jan, J.-T.; Shaio, M.-F. Glutamine Protects Activated Human T Cells from Apoptosis by Up-Regulating Glutathione and Bcl-2 Levels. Clin. Immunol. 2002, 104, 151–160. [Google Scholar] [CrossRef]
- Kono, M.; Yoshida, N.; Maeda, K.; Tsokos, G.C. Transcriptional Factor ICER Promotes Glutaminolysis and the Generation of Th17 Cells. Proc. Natl. Acad. Sci. USA 2018, 115, 2478–2483. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.O.; Wolf, M.M.; Madden, M.Z.; Andrejeva, G.; Sugiura, A.; Contreras, D.C.; Maseda, D.; Liberti, M.V.; Paz, K.; Kishton, R.J.; et al. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell 2018, 175, 1780–1795.e19. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Mendu, S.K.; Birnir, B. GABA Is an Effective Immunomodulatory Molecule. Amino Acids 2013, 45, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.K.; Kim, Y.S.; Lee, H.-M.; Jin, H.S.; Neupane, C.; Kim, S.; Lee, S.-H.; Min, J.-J.; Sasai, M.; Jeong, J.-H.; et al. GABAergic Signaling Linked to Autophagy Enhances Host Protection against Intracellular Bacterial Infections. Nat. Commun. 2018, 9, 4184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017, 25, 345–357. [Google Scholar] [CrossRef]
- Kurniawan, H.; Franchina, D.G.; Guerra, L.; Bonetti, L.; Soriano-Baguet, L.; Grusdat, M.; Schlicker, L.; Hunewald, O.; Dostert, C.; Merz, M.P.; et al. Glutathione Restricts Serine Metabolism to Preserve Regulatory T Cell Function. Cell Metab. 2020, 31, 920–936.e7. [Google Scholar] [CrossRef]
- Son, S.M.; Park, S.J.; Lee, H.; Siddiqi, F.; Lee, J.E.; Menzies, F.M.; Rubinsztein, D.C. Leucine Signals to MTORC1 via Its Metabolite Acetyl-Coenzyme A. Cell Metab. 2019, 29, 192–201.e7. [Google Scholar] [CrossRef] [Green Version]
- Akula, M.K.; Shi, M.; Jiang, Z.; Foster, C.E.; Miao, D.; Li, A.S.; Zhang, X.; Gavin, R.M.; Forde, S.D.; Germain, G.; et al. Control of the Innate Immune Response by the Mevalonate Pathway. Nat. Immunol. 2016, 17, 922–929. [Google Scholar] [CrossRef]
- Mausner-Fainberg, K.; Luboshits, G.; Mor, A.; Maysel-Auslender, S.; Rubinstein, A.; Keren, G.; George, J. The Effect of HMG-CoA Reductase Inhibitors on Naturally Occurring CD4+CD25+ T Cells. Atherosclerosis 2008, 197, 829–839. [Google Scholar] [CrossRef]
- Perucha, E.; Melchiotti, R.; Bibby, J.A.; Wu, W.; Frederiksen, K.S.; Roberts, C.A.; Hall, Z.; LeFriec, G.; Robertson, K.A.; Lavender, P.; et al. The Cholesterol Biosynthesis Pathway Regulates IL-10 Expression in Human Th1 Cells. Nat. Commun. 2019, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Kistowska, M.; Rossy, E.; Sansano, S.; Gober, H.-J.; Landmann, R.; Mori, L.; De Libero, G. Dysregulation of the Host Mevalonate Pathway during Early Bacterial Infection Activates Human TCR Gamma Delta Cells. Eur. J. Immunol. 2008, 38, 2200–2209. [Google Scholar] [CrossRef]
- Gober, H.-J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T Cell Receptor Γδ Cells Recognize Endogenous Mevalonate Metabolites in Tumor Cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Bibby, J.A.; Purvis, H.A.; Hayday, T.; Chandra, A.; Okkenhaug, K.; Rosenzweig, S.; Aksentijevich, I.; Wood, M.; Lachmann, H.J.; Kemper, C.; et al. Cholesterol Metabolism Drives Regulatory B Cell IL-10 through Provision of Geranylgeranyl Pyrophosphate. Nat. Commun. 2020, 11, 3412. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Asher, J.L.; Molony, R.D.; Shaw, A.C.; Zeiss, C.J.; Wang, C.; Morozova-Roche, L.A.; Herzog, R.I.; Iwasaki, A.; Dixit, V.D. β-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep. 2017, 18, 2077–2087. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. Ketone Body β-Hydroxybutyrate Blocks the NLRP3 Inflammasome-Mediated Inflammatory Disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Hong, S.-W.; Han, D.; Yi, J.; Jung, J.; Yang, B.-G.; Lee, J.Y.; Lee, M.; Surh, C.D. Dietary Antigens Limit Mucosal Immunity by Inducing Regulatory T Cells in the Small Intestine. Science 2016, 351, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S.; Gordon, J.I.; Glimcher, L.H. Homeostasis and Inflammation in the Intestine. Cell 2010, 140, 859–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and Disease Markers Correlate with Gut Microbiome Composition across Thousands of People. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The Gut Microbiome: Relationships with Disease and Opportunities for Therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Kunisawa, J.; Sugiura, Y.; Wake, T.; Nagatake, T.; Suzuki, H.; Nagasawa, R.; Shikata, S.; Honda, K.; Hashimoto, E.; Suzuki, Y.; et al. Mode of Bioenergetic Metabolism during B Cell Differentiation in the Intestine Determines the Distinct Requirement for Vitamin B1. Cell Rep. 2015, 13, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Harris, N.L.; Spoerri, I.; Schopfer, J.F.; Nembrini, C.; Merky, P.; Massacand, J.; Urban, J.F.; Lamarre, A.; Burki, K.; Odermatt, B.; et al. Mechanisms of Neonatal Mucosal Antibody Protection. J. Immunol. 2006, 177, 6256–6262. [Google Scholar] [CrossRef] [Green Version]
- Guerra, L.; Bonetti, L.; Brenner, D. Metabolic Modulation of Immunity: A New Concept in Cancer Immunotherapy. Cell Rep. 2020, 32, 107848. [Google Scholar] [CrossRef]
- Stark, J.M.; Tibbitt, C.A.; Coquet, J.M. The Metabolic Requirements of Th2 Cell Differentiation. Front. Immunol. 2019, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Garrett, W.S. The Gut Microbiota and Mucosal T Cells. Front. Microbiol. 2011, 2, 111. [Google Scholar] [CrossRef] [Green Version]
- Probert, C.S.J.; Williams, A.M.; Stepankova, R.; Tlaskalova-Hogenova, H.; Phillips, A.; Bland, P.W. The Effect of Weaning on the Clonality of Alpha Beta T-Cell Receptor T Cells in the Intestine of GF and SPF Mice. Dev. Comp. Immunol. 2007, 31, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Konjar, Š.; Veldhoen, M. Dynamic Metabolic State of Tissue Resident CD8 T Cells. Front. Immunol. 2019, 10, 1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agace, W.W.; McCoy, K.D. Regionalized Development and Maintenance of the Intestinal Adaptive Immune Landscape. Immunity 2017, 46, 532–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Cano, F.J.; Castellote, C.; González-Castro, A.M.; Pelegrí, C.; Castell, M.; Franch, A. Developmental Changes in Intraepithelial T Lymphocytes and NK Cells in the Small Intestine of Neonatal Rats. Pediatr. Res. 2005, 58, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Galgani, M.; De Rosa, V.; La Cava, A.; Matarese, G. Role of Metabolism in the Immunobiology of Regulatory T Cells. J. Immunol. 2016, 197, 2567–2575. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Déjardin, F.; Sparwasser, T.; Bérard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288.e5. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Xue, F.; Qin, H.; Chen, X.; Liu, N.; Fleming, C.; Hu, X.; Zhang, H.; Chen, F.; Zheng, J.; et al. Differential Roles of the MTOR-STAT3 Signaling in Dermal Γδ T Cell Effector Function in Skin Inflammation. Cell Rep. 2019, 27, 3034–3048.e5. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. Γδ T Cells in Homeostasis and Host Defence of Epithelial Barrier Tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chou, K.; Fuchs, E.; Havran, W.L.; Boismenu, R. Protection of the Intestinal Mucosa by Intraepithelial Gamma Delta T Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14338–14343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.S.; Severson, K.M.; Vaishnava, S.; Behrendt, C.L.; Yu, X.; Benjamin, J.L.; Ruhn, K.A.; Hou, B.; DeFranco, A.L.; Yarovinsky, F.; et al. Gammadelta Intraepithelial Lymphocytes Are Essential Mediators of Host-Microbial Homeostasis at the Intestinal Mucosal Surface. Proc. Natl. Acad. Sci. USA 2011, 108, 8743–8748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzaki, A.R.B.M.; Soncin, I.; Setiagani, Y.A.; Sheng, J.; Tetlak, P.; Karjalainen, K.; Ruedl, C. Long-Lived Innate IL-17–Producing γ/δ T Cells Modulate Antimicrobial Epithelial Host Defense in the Colon. J. Immunol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, A.; Mota-Santos, T.; Itohara, S.; Degermann, S.; Heusser, C.; Tonegawa, S.; Coutinho, A. Localization of Gamma/Delta T Cells to the Intestinal Epithelium Is Independent of Normal Microbial Colonization. J. Exp. Med. 1990, 172, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Webb, T.J.; Carey, G.B.; East, J.E.; Sun, W.; Bollino, D.R.; Kimball, A.S.; Brutkiewicz, R.R. Alterations in Cellular Metabolism Modulate CD1d-Mediated NKT-Cell Responses. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [Green Version]
- Middendorp, S.; Nieuwenhuis, E.E.S. NKT Cells in Mucosal Immunity. Mucosal Immunol. 2009, 2, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingender, G.; Kronenberg, M. Role of NKT Cells in the Digestive System. IV. The Role of Canonical Natural Killer T Cells in Mucosal Immunity and Inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1–G8. [Google Scholar] [CrossRef] [Green Version]
- Zeissig, S.; Kaser, A.; Dougan, S.K.; Nieuwenhuis, E.E.S.; Blumberg, R.S. Role of NKT Cells in the Digestive System. III. Role of NKT Cells in Intestinal Immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1101–G1105. [Google Scholar] [CrossRef]
- O’Sullivan, T.E.; Sun, J.C. Innate Lymphoid Cell Immunometabolism. J. Mol. Biol. 2017, 429, 3577–3586. [Google Scholar] [CrossRef]
- Sanos, S.L.; Bui, V.L.; Mortha, A.; Oberle, K.; Heners, C.; Johner, C.; Diefenbach, A. RORgammat and Commensal Microflora Are Required for the Differentiation of Mucosal Interleukin 22-Producing NKp46+ Cells. Nat. Immunol. 2009, 10, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.B.; Vanherwegen, A.-S.; Eelen, G.; Gutiérrez, A.C.F.; Van Lommel, L.; Marchal, K.; Verlinden, L.; Verstuyf, A.; Nogueira, T.; Georgiadou, M.; et al. Vitamin D3 Induces Tolerance in Human Dendritic Cells by Activation of Intracellular Metabolic Pathways. Cell Rep. 2015, 10, 711–725. [Google Scholar] [CrossRef] [Green Version]
- Giovanelli, P.; Sandoval, T.A.; Cubillos-Ruiz, J.R. Dendritic Cell Metabolism and Function in Tumors. Trends Immunol. 2019, 40, 699–718. [Google Scholar] [CrossRef]
- Stagg, A.J. Intestinal Dendritic Cells in Health and Gut Inflammation. Front. Immunol. 2018, 9, 2883. [Google Scholar] [CrossRef] [Green Version]
- Niess, J.H.; Adler, G. Enteric Flora Expands Gut Lamina Propria CX3CR1+ Dendritic Cells Supporting Inflammatory Immune Responses under Normal and Inflammatory Conditions. J. Immunol. 2010, 184, 2026–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, E.L.; O’Neill, L.A. Reprogramming Mitochondrial Metabolism in Macrophages as an Anti-Inflammatory Signal. Eur. J. Immunol. 2016, 46, 13–21. [Google Scholar] [CrossRef]
- Kang, B.; Alvarado, L.J.; Kim, T.; Lehmann, M.L.; Cho, H.; He, J.; Li, P.; Kim, B.-H.; Larochelle, A.; Kelsall, B.L. Commensal Microbiota Drive the Functional Diversification of Colon Macrophages. Mucosal Immunol. 2020, 13, 216–229. [Google Scholar] [CrossRef] [Green Version]
- Bain, C.C.; Bravo-Blas, A.; Scott, C.L.; Perdiguero, E.G.; Geissmann, F.; Henri, S.; Malissen, B.; Osborne, L.C.; Artis, D.; Mowat, A.M. Constant Replenishment from Circulating Monocytes Maintains the Macrophage Pool in the Intestine of Adult Mice. Nat. Immunol. 2014, 15, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Dikshit, M. Metabolic Insight of Neutrophils in Health and Disease. Front. Immunol. 2019, 10, 2099. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.M.; Monticelli, L.A.; Sonnenberg, G.F. Metabolic Regulation of Innate and Adaptive Lymphocyte Effector Responses. Immunol. Rev. 2018, 286, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Cong, J. Metabolism of Natural Killer Cells and Other Innate Lymphoid Cells. Front. Immunol. 2020, 11, 1989. [Google Scholar] [CrossRef] [PubMed]
- Cording, S.; Medvedovic, J.; Cherrier, M.; Eberl, G. Development and Regulation of RORγt(+) Innate Lymphoid Cells. FEBS Lett. 2014, 588, 4176–4181. [Google Scholar] [CrossRef] [Green Version]
- Killig, M.; Glatzer, T.; Romagnani, C. Recognition Strategies of Group 3 Innate Lymphoid Cells. Front. Immunol. 2014, 5, 142. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.A.; Barlow, J.L.; McKenzie, A.N.J. Innate Lymphoid Cells—How Did We Miss Them? Nat. Rev. Immunol. 2013, 13, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Dunsford, B.R.; Haensly, W.E.; Knabe, D.A. Effects of Diet on Acidic and Neutral Goblet Cell Populations in the Small Intestine of Early Weaned Pigs. Am. J. Vet. Res. 1991, 52, 1743–1746. [Google Scholar] [PubMed]
- Lan, A.; Andriamihaja, M.; Blouin, J.-M.; Liu, X.; Descatoire, V.; Desclée de Maredsous, C.; Davila, A.-M.; Walker, F.; Tomé, D.; Blachier, F. High-Protein Diet Differently Modifies Intestinal Goblet Cell Characteristics and Mucosal Cytokine Expression in Ileum and Colon. J. Nutr. Biochem. 2015, 26, 91–98. [Google Scholar] [CrossRef]
- Wlodarska, M.; Willing, B.; Keeney, K.M.; Menendez, A.; Bergstrom, K.S.; Gill, N.; Russell, S.L.; Vallance, B.A.; Finlay, B.B. Antibiotic Treatment Alters the Colonic Mucus Layer and Predisposes the Host to Exacerbated Citrobacter Rodentium-Induced Colitis. Infect. Immun. 2011, 79, 1536–1545. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Kim, T.-Y.; Kim, Y.; Lee, S.-H.; Kim, S.; Kang, S.W.; Yang, J.-Y.; Baek, I.-J.; Sung, Y.H.; Park, Y.-Y.; et al. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe 2018, 24, 833–846.e6. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.; Oelschlaeger, T.A.; Wullaert, A.; Vlantis, K.; Pasparakis, M.; Wehkamp, J.; Stange, E.F.; Gersemann, M. Bacteria Regulate Intestinal Epithelial Cell Differentiation Factors Both in Vitro and in Vivo. PLoS ONE 2013, 8, e55620. [Google Scholar] [CrossRef]
- Simmonds, N.; Furman, M.; Karanika, E.; Phillips, A.; Bates, A.W.H. Paneth Cell Metaplasia in Newly Diagnosed Inflammatory Bowel Disease in Children. BMC Gastroenterol. 2014, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- McKinley, E.T.; Sui, Y.; Al-Kofahi, Y.; Millis, B.A.; Tyska, M.J.; Roland, J.T.; Santamaria-Pang, A.; Ohland, C.L.; Jobin, C.; Franklin, J.L.; et al. Optimized Multiplex Immunofluorescence Single-Cell Analysis Reveals Tuft Cell Heterogeneity. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Schneider, C.; O’Leary, C.E.; von Moltke, J.; Liang, H.-E.; Ang, Q.Y.; Turnbaugh, P.J.; Radhakrishnan, S.; Pellizzon, M.; Ma, A.; Locksley, R.M. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 2018, 174, 271–284.e14. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.; Yamakami-Kimura, M.; Obata, Y.; Hase, K.; Kitamura, H.; Ohno, H.; Iwanaga, T. Visualization of the Entire Differentiation Process of Murine M Cells: Suppression of Their Maturation in Cecal Patches. Mucosal Immunol. 2015, 8, 650–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva Menezes, J.; de Sousa Mucida, D.; Cara, D.C.; Alvarez-Leite, J.I.; Russo, M.; Vaz, N.M.; de Faria, A.M.C. Stimulation by Food Proteins Plays a Critical Role in the Maturation of the Immune System. Int. Immunol. 2003, 15, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Nagai, M.; Noguchi, R.; Takahashi, D.; Morikawa, T.; Koshida, K.; Komiyama, S.; Ishihara, N.; Yamada, T.; Kawamura, Y.I.; Muroi, K.; et al. Fasting-Refeeding Impacts Immune Cell Dynamics and Mucosal Immune Responses. Cell 2019, 178, 1072–1087.e14. [Google Scholar] [CrossRef]
- Daïen, C.I.; Pinget, G.V.; Tan, J.K.; Macia, L. Detrimental Impact of Microbiota-Accessible Carbohydrate-Deprived Diet on Gut and Immune Homeostasis: An Overview. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Ochi, T.; Feng, Y.; Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Teitelbaum, D.H.; Kamada, N. Diet-Dependent, Microbiota-Independent Regulation of IL-10-Producing Lamina Propria Macrophages in the Small Intestine. Sci. Rep. 2016, 6, 27634. [Google Scholar] [CrossRef] [Green Version]
- Nose, K.; Yang, H.; Sun, X.; Nose, S.; Koga, H.; Feng, Y.; Miyasaka, E.; Teitelbaum, D.H. Glutamine Prevents Total Parenteral Nutrition-Associated Changes to Intraepithelial Lymphocyte Phenotype and Function: A Potential Mechanism for the Preservation of Epithelial Barrier Function. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2010, 30, 67–80. [Google Scholar] [CrossRef]
- Ohtani, M.; Hoshii, T.; Fujii, H.; Koyasu, S.; Hirao, A.; Matsuda, S. Cutting Edge: MTORC1 in Intestinal CD11c+ CD11b+ Dendritic Cells Regulates Intestinal Homeostasis by Promoting IL-10 Production. J. Immunol. 2012, 188, 4736–4740. [Google Scholar] [CrossRef] [Green Version]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [Green Version]
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Jiraskova Zakostelska, Z.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-Inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Lletjós, S.; Andriamihaja, M.; Blais, A.; Grauso, M.; Lepage, P.; Davila, A.-M.; Viel, R.; Gaudichon, C.; Leclerc, M.; Blachier, F.; et al. Dietary Protein Intake Level Modulates Mucosal Healing and Mucosa-Adherent Microbiota in Mouse Model of Colitis. Nutrients 2019, 11, 514. [Google Scholar] [CrossRef] [Green Version]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.-H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef] [Green Version]
- Neumann, C.; Blume, J.; Roy, U.; Teh, P.P.; Vasanthakumar, A.; Beller, A.; Liao, Y.; Heinrich, F.; Arenzana, T.L.; Hackney, J.A.; et al. C-Maf-Dependent Treg Cell Control of Intestinal TH17 Cells and IgA Establishes Host-Microbiota Homeostasis. Nat. Immunol. 2019, 20, 471–481. [Google Scholar] [CrossRef]
- Kunisawa, J.; Hashimoto, E.; Inoue, A.; Nagasawa, R.; Suzuki, Y.; Ishikawa, I.; Shikata, S.; Arita, M.; Aoki, J.; Kiyono, H. Regulation of Intestinal IgA Responses by Dietary Palmitic Acid and Its Metabolism. J. Immunol. 2014, 193, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
- Bassaganya-Riera, J.; Reynolds, K.; Martino-Catt, S.; Cui, Y.; Hennighausen, L.; Gonzalez, F.; Rohrer, J.; Benninghoff, A.U.; Hontecillas, R. Activation of PPAR Gamma and Delta by Conjugated Linoleic Acid Mediates Protection from Experimental Inflammatory Bowel Disease. Gastroenterology 2004, 127, 777–791. [Google Scholar] [CrossRef]
- Hontecillas, R.; Horne, W.T.; Climent, M.; Guri, A.J.; Evans, C.; Zhang, Y.; Sobral, B.W.; Bassaganya-Riera, J. Immunoregulatory Mechanisms of Macrophage PPAR-γ in Mice with Experimental Inflammatory Bowel Disease. Mucosal Immunol. 2011, 4, 304–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, V.L.; Nguyen, H.C.B.; Garcìa-Cañaveras, J.C.; Briggs, E.R.; Ho, W.Y.; DiSpirito, J.R.; Marinis, J.M.; Hill, D.A.; Lazar, M.A. PPARγ Is a Nexus Controlling Alternative Activation of Macrophages via Glutamine Metabolism. Genes Dev. 2018, 32, 1035–1044. [Google Scholar] [CrossRef]
- Ramírez-Orozco, R.E.; Franco Robles, E.; Pérez Vázquez, V.; Ramírez Emiliano, J.; Hernández Luna, M.A.; López Briones, S. Diet-Induced Obese Mice Exhibit Altered Immune Responses to Early Salmonella Typhimurium Oral Infection. J. Microbiol. 2018, 56, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Heras, V.L.; Clooney, A.G.; Ryan, F.J.; Cabrera-Rubio, R.; Casey, P.G.; Hueston, C.M.; Pinheiro, J.; Rudkin, J.K.; Melgar, S.; Cotter, P.D.; et al. Short-Term Consumption of a High-Fat Diet Increases Host Susceptibility to Listeria Monocytogenes Infection. Microbiome 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jin, W.; Wu, R.; Li, J.; Park, S.-A.; Tu, E.; Zanvit, P.; Xu, J.; Liu, O.; Cain, A.; et al. High Glucose Intake Exacerbates Autoimmunity through Reactive-Oxygen-Species-Mediated TGF-β Cytokine Activation. Immunity 2019, 51, 671–681.e5. [Google Scholar] [CrossRef] [PubMed]
- Fajstova, A.; Galanova, N.; Coufal, S.; Malkova, J.; Kostovcik, M.; Cermakova, M.; Pelantova, H.; Kuzma, M.; Sediva, B.; Hudcovic, T.; et al. Diet Rich in Simple Sugars Promotes Pro-Inflammatory Response via Gut Microbiota Alteration and TLR4 Signaling. Cells 2020, 9, 2701. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl Hydrocarbon Receptor and Intestinal Immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Wang, K.; Qiao, S.; Yang, L.; Xin, Y.; Dai, Y.; Wei, Z. Norisoboldine, a Natural AhR Agonist, Promotes Treg Differentiation and Attenuates Colitis via Targeting Glycolysis and Subsequent NAD+/SIRT1/SUV39H1/H3K9me3 Signaling Pathway. Cell Death Dis. 2018, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Shi, C.; Qiao, S.; Cao, N.; Guan, C.; Dai, Y.; Wei, Z. Alpinetin Exerts Anti-Colitis Efficacy by Activating AhR, Regulating MiR-302/DNMT-1/CREB Signals, and Therefore Promoting Treg Differentiation. Cell Death Dis. 2018, 9, 1–25. [Google Scholar] [CrossRef]
- Huerta-Yepez, S.; Tirado-Rodriguez, A.; Montecillo-Aguado, M.R.; Yang, J.; Hammock, B.D.; Hankinson, O. Aryl Hydrocarbon Receptor-Dependent Inductions of Omega-3 and Omega-6 Polyunsaturated Fatty Acid Metabolism Act Inversely on Tumor Progression. Sci. Rep. 2020, 10, 7843. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kayama, H.; Kusu, T.; Yamaguchi, T.; Kunisawa, J.; Kiyono, H.; Sakaguchi, S.; Takeda, K. Dietary Folic Acid Promotes Survival of Foxp3+ Regulatory T Cells in the Colon. J. Immunol. 2012, 189, 2869–2878. [Google Scholar] [CrossRef] [Green Version]
- Bono, M.R.; Tejon, G.; Flores-Santibañez, F.; Fernandez, D.; Rosemblatt, M.; Sauma, D. Retinoic Acid as a Modulator of T Cell Immunity. Nutrients 2016, 8, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howie, D.; Cobbold, S.P.; Adams, E.; Ten Bokum, A.; Necula, A.S.; Zhang, W.; Huang, H.; Roberts, D.J.; Thomas, B.; Hester, S.S.; et al. Foxp3 Drives Oxidative Phosphorylation and Protection from Lipotoxicity. JCI Insight 2017, 2, e89160. [Google Scholar] [CrossRef] [Green Version]
- Cantorna, M.T.; Lin, Y.-D.; Arora, J.; Bora, S.; Tian, Y.; Nichols, R.G.; Patterson, A.D. Vitamin D Regulates the Microbiota to Control the Numbers of RORγt/FoxP3+ Regulatory T Cells in the Colon. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed Food and Chronic Noncommunicable Diseases: A Systematic Review and Meta-Analysis of 43 Observational Studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [Green Version]
- de Koning, L.; Malik, V.S.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sugar-Sweetened and Artificially Sweetened Beverage Consumption and Risk of Type 2 Diabetes in Men. Am. J. Clin. Nutr. 2011, 93, 1321–1327. [Google Scholar] [CrossRef] [Green Version]
- Leandro, J.G.B.; Espindola-Netto, J.M.; Vianna, M.C.F.; Gomez, L.S.; DeMaria, T.M.; Marinho-Carvalho, M.M.; Zancan, P.; Paula Neto, H.A.; Sola-Penna, M. Exogenous Citrate Impairs Glucose Tolerance and Promotes Visceral Adipose Tissue Inflammation in Mice. Br. J. Nutr. 2016, 115, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Kelly, B.; O’Neill, L.A.J. Metabolic Reprogramming in Macrophages and Dendritic Cells in Innate Immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashbrook, M.J.; McDonough, K.L.; Pituch, J.J.; Christopherson, P.L.; Cornell, T.T.; Selewski, D.T.; Shanley, T.P.; Blatt, N.B. Citrate Modulates Lipopolysaccharide-Induced Monocyte Inflammatory Responses. Clin. Exp. Immunol. 2015, 180, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, S.L.F.; Miranda, M.C.G.; Guimarães, M.A.F.; Santiago, H.C.; Queiroz, C.P.; da Silva Cunha, P.; Cara, D.C.; Foureaux, G.; Ferreira, A.J.; Cardoso, V.N.; et al. High-Salt Diet Induces IL-17-Dependent Gut Inflammation and Exacerbates Colitis in Mice. Front. Immunol. 2017, 8, 1969. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.F.; Aden, L.A.; Barbaro, N.R.; Van Beusecum, J.P.; Xiao, L.; Simmons, A.J.; Warden, C.; Pasic, L.; Himmel, L.E.; Washington, M.K.; et al. High Dietary Salt-Induced Dendritic Cell Activation Underlies Microbial Dysbiosis-Associated Hypertension. JCI Insight 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Binger, K.J.; Gebhardt, M.; Heinig, M.; Rintisch, C.; Schroeder, A.; Neuhofer, W.; Hilgers, K.; Manzel, A.; Schwartz, C.; Kleinewietfeld, M.; et al. High Salt Reduces the Activation of IL-4– and IL-13–Stimulated Macrophages. J. Clin. Investig. 2015, 125, 4223–4238. [Google Scholar] [CrossRef]
- Hucke, S.; Eschborn, M.; Liebmann, M.; Herold, M.; Freise, N.; Engbers, A.; Ehling, P.; Meuth, S.G.; Roth, J.; Kuhlmann, T.; et al. Sodium Chloride Promotes Pro-Inflammatory Macrophage Polarization Thereby Aggravating CNS Autoimmunity. J. Autoimmun. 2016, 67, 90–101. [Google Scholar] [CrossRef]
- Tubbs, A.L.; Liu, B.; Rogers, T.D.; Sartor, R.B.; Miao, E.A. Dietary Salt Exacerbates Experimental Colitis. J. Immunol. 2017, 199, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ip, W.K.E.; Medzhitov, R. Macrophages Monitor Tissue Osmolarity and Induce Inflammatory Response through NLRP3 and NLRC4 Inflammasome Activation. Nat. Commun. 2015, 6, 6931. [Google Scholar] [CrossRef]
- Willebrand, R.; Kleinewietfeld, M. The Role of Salt for Immune Cell Function and Disease. Immunology 2018, 154, 346–353. [Google Scholar] [CrossRef]
- Lang, F.; Stournaras, C.; Zacharopoulou, N.; Voelkl, J.; Alesutan, I. Serum- and Glucocorticoid-Inducible Kinase 1 and the Response to Cell Stress. Cell Stress 2019, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-Responsive Gut Commensal Modulates TH17 Axis and Disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-Grade TiO2 Impairs Intestinal and Systemic Immune Homeostasis, Initiates Preneoplastic Lesions and Promotes Aberrant Crypt Development in the Rat Colon. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, N.; Zhu, M.; Lu, J.; Zhong, H.; Xue, X.; Guo, S.; Li, M.; Wei, X.; Tao, Y.; et al. TiO2 Nanoparticles Cause Mitochondrial Dysfunction, Activate Inflammatory Responses, and Attenuate Phagocytosis in Macrophages: A Proteomic and Metabolomic Insight. Redox Biol. 2018, 15, 266–276. [Google Scholar] [CrossRef]
- Viennois, E.; Merlin, D.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifier-Induced Low-Grade Inflammation Promotes Colon Carcinogenesis. Cancer Res. 2017, 77, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Swidsinski, A.; Ung, V.; Sydora, B.C.; Loening-Baucke, V.; Doerffel, Y.; Verstraelen, H.; Fedorak, R.N. Bacterial Overgrowth and Inflammation of Small Intestine after Carboxymethylcellulose Ingestion in Genetically Susceptible Mice. Inflamm. Bowel Dis. 2009, 15, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress-Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium Chloride Drives Autoimmune Disease by the Induction of Pathogenic TH17 Cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L.; Ji, W.-J.; Yuan, F.; Guo, Z.-Z.; Pang, B.; Luo, T.; Liu, X.; Zhang, W.-C.; Jiang, T.-M.; et al. Variation in Dietary Salt Intake Induces Coordinated Dynamics of Monocyte Subsets and Monocyte-Platelet Aggregates in Humans: Implications in End Organ Inflammation. PLoS ONE 2013, 8, e60332. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, A.L.; Kitz, A.; Wu, C.; Lowther, D.E.; Rodriguez, D.M.; Vudattu, N.; Deng, S.; Herold, K.C.; Kuchroo, V.K.; Kleinewietfeld, M.; et al. Sodium Chloride Inhibits the Suppressive Function of FOXP3+ Regulatory T Cells. J. Clin. Investig. 2015, 125, 4212–4222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.H.; Istomine, R.; Alvarez, F.; Al-Aubodah, T.-A.; Shi, X.Q.; Takano, T.; Thornton, A.M.; Shevach, E.M.; Zhang, J.; Piccirillo, C.A. Salt Sensing by Serum/Glucocorticoid-Regulated Kinase 1 Promotes Th17-like Inflammatory Adaptation of Foxp3+ Regulatory T Cells. Cell Rep. 2020, 30, 1515–1529.e4. [Google Scholar] [CrossRef] [PubMed]
- Jobin, K.; Stumpf, N.E.; Schwab, S.; Eichler, M.; Neubert, P.; Rauh, M.; Adamowski, M.; Babyak, O.; Hinze, D.; Sivalingam, S.; et al. A High-Salt Diet Compromises Antibacterial Neutrophil Responses through Hormonal Perturbation. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. Gut Microbiome Response to Sucralose and Its Potential Role in Inducing Liver Inflammation in Mice. Front. Physiol. 2017, 8, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, M.; Boyle, J.J.; Powell, J.J.; Playford, R.J.; Ghosh, S. Dietary Microparticles Implicated in Crohn’s Disease Can Impair Macrophage Phagocytic Activity and Act as Adjuvants in the Presence of Bacterial Stimuli. Inflamm. Res. 2007, 56, 353–361. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium Dioxide Nanoparticles Exacerbate DSS-Induced Colitis: Role of the NLRP3 Inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef] [Green Version]
- Balmer, M.L.; Ma, E.H.; Bantug, G.R.; Grählert, J.; Pfister, S.; Glatter, T.; Jauch, A.; Dimeloe, S.; Slack, E.; Dehio, P.; et al. Memory CD8(+) T Cells Require Increased Concentrations of Acetate Induced by Stress for Optimal Function. Immunity 2016, 44, 1312–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-Activated PPAR-γ Signaling Inhibits Dysbiotic Enterobacteriaceae Expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.K.; Colonna, M. Innate Lymphoid Cells in Mucosal Immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl Hydrocarbon Receptor-Induced Signals up-Regulate IL-22 Production and Inhibit Inflammation in the Gastrointestinal Tract. Gastroenterology 2011, 141, 237–248.e1. [Google Scholar] [CrossRef]
- Chun, E.; Lavoie, S.; Fonseca-Pereira, D.; Bae, S.; Michaud, M.; Hoveyda, H.R.; Fraser, G.L.; Gallini Comeau, C.A.; Glickman, J.N.; Fuller, M.H.; et al. Metabolite-Sensing Receptor Ffar2 Regulates Colonic Group 3 Innate Lymphoid Cells and Gut Immunity. Immunity 2019, 51, 871–884.e6. [Google Scholar] [CrossRef]
- Bhatt, B.; Zeng, P.; Zhu, H.; Sivaprakasam, S.; Li, S.; Xiao, H.; Dong, L.; Shiao, P.; Kolhe, R.; Patel, N.; et al. Gpr109a Limits Microbiota-Induced IL-23 Production To Constrain ILC3-Mediated Colonic Inflammation. J. Immunol. 2018, 200, 2905–2914. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Cho, B.-H.; Kiyono, H.; Jang, Y.-S. Microbiota-Derived Butyrate Suppresses Group 3 Innate Lymphoid Cells in Terminal Ileal Peyer’s Patches. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal Microbiota-Derived Short-Chain Fatty Acids Regulation of Immune Cell IL-22 Production and Gut Immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [Green Version]
- Scott, N.A.; Andrusaite, A.; Andersen, P.; Lawson, M.; Alcon-Giner, C.; Leclaire, C.; Caim, S.; Le Gall, G.; Shaw, T.; Connolly, J.P.R.; et al. Antibiotics Induce Sustained Dysregulation of Intestinal T Cell Immunity by Perturbing Macrophage Homeostasis. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, C.; Bommakanti, G.; Gardinassi, L.; Loebbermann, J.; Johnson, M.J.; Hakimpour, P.; Hagan, T.; Benitez, L.; Todor, A.; Machiah, D.; et al. MTOR Regulates Metabolic Adaptation of APCs in the Lung and Controls the Outcome of Allergic Inflammation. Science 2017, 357, 1014–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B Cell-Intrinsic Epigenetic Modulation of Antibody Responses by Dietary Fiber-Derived Short-Chain Fatty Acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [Green Version]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The Short-Chain Fatty Acid Pentanoate Suppresses Autoimmunity by Modulating the Metabolic-Epigenetic Crosstalk in Lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-Chain Fatty Acids Induce Both Effector and Regulatory T Cells by Suppression of Histone Deacetylases and Regulation of the MTOR-S6K Pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8+ T Cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, C.L.; Williams, P.A.; Dalby, M.J.; Garden, K.; Thomson, L.M.; Richardson, A.J.; Gratz, S.W.; Ross, A.W. Different Types of Soluble Fermentable Dietary Fibre Decrease Food Intake, Body Weight Gain and Adiposity in Young Adult Male Rats. Nutr. Metab. 2014, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Connors, J.; Dawe, N.; Van Limbergen, J. The Role of Succinate in the Regulation of Intestinal Inflammation. Nutrients 2018, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hou, Y.; Wang, G.; Zheng, X.; Hao, H. Gut Microbial Metabolites of Aromatic Amino Acids as Signals in Host-Microbe Interplay. Trends Endocrinol. Metab. TEM 2020, 31, 818–834. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Babu, S.T.; Niu, X.; Raetz, M.; Savani, R.C.; Hooper, L.V.; Mirpuri, J. Maternal High-Fat Diet Results in Microbiota-Dependent Expansion of ILC3s in Mice Offspring. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus Reuteri Induces Gut Intraepithelial CD4+CD8αα+ T Cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, B.; Doñas, C.; Wuggenig, P.; Diaz, O.E.; Morales, R.A.; Melhem, H.; Swiss IBD Cohort Investigators; Hernández, P.P.; Kaymak, T.; Das, S.; et al. Lysophosphatidic Acid-Mediated GPR35 Signaling in CX3CR1+ Macrophages Regulates Intestinal Homeostasis. Cell Rep. 2020, 32, 107979. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Larraufie, P.; Jarry, A.; Béguet-Crespel, F.; Marinelli, L.; Ledue, F.; Reimann, F.; Blottière, H.M.; Lapaque, N. Butyrate Produced by Commensal Bacteria Down-Regulates Indolamine 2,3-Dioxygenase 1 (IDO-1) Expression via a Dual Mechanism in Human Intestinal Epithelial Cells. Front. Immunol. 2018, 9, 2838. [Google Scholar] [CrossRef]
- Rosser, E.C.; Piper, C.J.M.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020, 31, 837–851.e10. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Hernandez-Galan, L.; Galipeau, H.J.; Constante, M.; Clarizio, A.; Jury, J.; Breyner, N.M.; Caminero, A.; Rueda, G.; Hayes, C.L.; et al. Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is Decreased in Celiac Disease Leading to Intestinal Inflammation. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile Salt Biotransformations by Human Intestinal Bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneno, K.; Hisamatsu, T.; Shimamura, K.; Kamada, N.; Ichikawa, R.; Kitazume, M.T.; Mori, M.; Uo, M.; Namikawa, Y.; Matsuoka, K.; et al. TGR5 Signalling Inhibits the Production of Pro-Inflammatory Cytokines by in Vitro Differentiated Inflammatory and Intestinal Macrophages in Crohn’s Disease. Immunology 2013, 139, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Bou Sleiman, M.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968.e8. [Google Scholar] [CrossRef]
- Perino, A.; Pols, T.W.H.; Nomura, M.; Stein, S.; Pellicciari, R.; Schoonjans, K. TGR5 Reduces Macrophage Migration through MTOR-Induced C/EBPβ Differential Translation. J. Clin. Investig. 2014, 124, 5424–5436. [Google Scholar] [CrossRef] [Green Version]
- Gadaleta, R.M.; van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.L.; Renooij, W.; Murzilli, S.; Klomp, L.W.J.; Siersema, P.D.; Schipper, M.E.I.; Danese, S.; et al. Farnesoid X Receptor Activation Inhibits Inflammation and Preserves the Intestinal Barrier in Inflammatory Bowel Disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial Bile Acid Metabolites Modulate Gut RORγ+ Regulatory T Cell Homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile Acid Metabolites Control TH17 and Treg Cell Differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef]
- Argüello, R.J.; Combes, A.J.; Char, R.; Gigan, J.-P.; Baaziz, A.I.; Bousiquot, E.; Camosseto, V.; Samad, B.; Tsui, J.; Yan, P.; et al. SCENITH: A Flow Cytometry-Based Method to Functionally Profile Energy Metabolism with Single-Cell Resolution. Cell Metab. 2020, 32, 1063–1075.e7. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Le Couteur, D.G.; James, D.E.; George, J.; Gunton, J.E.; Solon-Biet, S.M.; Raubenheimer, D. The Geometric Framework for Nutrition as a Tool in Precision Medicine. Nutr. Healthy Aging 2017, 4, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cell Type/ Markers | Anatomical Location in the Gut | Functions | Metabolic Profile | Dietary Impact | Microbial Impact | Reference | |
|---|---|---|---|---|---|---|---|
| Antibiotic Impact | Germ-Free Conditions | ||||||
| B cells | Lamina propria, Peyer’s patches, mesenteric lymph node | Immunoglobulin production (IgA, IgG, IgM) Host-microbiota mutualism | Intestinal naïve B cell utilizes TCA-OXPHOS IgA+ plasma cell utilizes glycolysis-TCA-OXPHOS | High fibre diet increases plasma cells ↑ IgA after weaning ↑ Germinal centre B cell development after weaning ↑ IgA+ plasma cell after weaning | ↓ in SI, colon and Peyer’s patches; similar in mesenteric lymph node | ↓ IgA and IgG production in SI | [89,90] |
| Regulatory B cell | Lamina propria, mesenteric lymph node | Intestinal inflammation suppression through IL-10 production and repression of IL-1 and STAT3-related inflammatory cascades | Little is known about the development and metabolism of Breg cells but they can increase glycolysis during activation | [91] | |||
| CD4+ T cell | Lamina propria, Peyer’s patches, mesenteric lymph node | Th1: anti-viral and -bacterial responses Th2: anti-parasite responses Th17: anti-fungi and anti-bacterial responses | Glycolysis is dominant during activation of Th cells TCA cycle/OXPHOS is dominant for long-lived and naïve CD4+ T cells | Th2-bias responses and lack of Th1 responses before weaning TCR repertoire is polyclonal before weaning but become restricted/oligoclonal after weaning | ↓ in Peyer’s patch, and SI; similar or ↓ in mesenteric lymph node; ↓ memory CD4+ T cell in SI, colon and mesenteric lymph node | ↓ in SI, and mesenteric lymph node; ↓ or similar in colon | [92,93,94] |
| CD8+ T cell | Lamina propria, Peyer’s patches, mesenteric lymph node | Cytotoxicity Mucosal defence | Naive: FAO and OXPHOS Activated: Glycolysis and OXPHOS | ↑ in intestine recruitment after weaning (mostly CD8αβ+ TCR+) | ↓ in SI, and colon, ↑ or similar in mesenteric lymph node and Peyer’s patch Similar IFNγ production in SI | ↓ in mesenteric lymph node and similar in SI ↓ IFNγ production in SI, colon and mesenteric lymph node | [95,96] |
| CD4+ CD8αα+ T cell | SI epithelium | Tolerance to dietary antigens | ? | Promoted by dietary tryptophan & indole derivatives ↑ in SI after weaning | ↓ in SI | ↓ in SI | [97] |
| Regulatory T cell | Lamina propria, Peyer’s patches, mesenteric lymph node | Intestinal inflammation suppression through IL-10 and TGFβ production Tolerance to dietary antigens | FAO, TCA, OXPHOS | High fibre promotes Treg ↑ in colon and SI after weaning | ↓ in colon; ↓ or similar in SI, Peyer’s patch; inconsistent in mesenteric lymph node ↓ in RORγt Treg (peripheral induced Treg) in colon while Helios+ thymic ones mostly similar | ↓ in colon and Peyer’s patch; similar in mesenteric lymph node; ↓ in RORγt Treg in colon, SI and mesenteric lymph node; ↓ or similar Helios+ and GATA3+ Treg in colon | [98,99,100] |
| γδ T cell | Lamina propria, Peyer’s patches, mesenteric lymph node, Intestinal epithelium | Intestinal inflammation suppression through IL10 and TGFβ production Epithelial repair and protection through KGF-1 and IL-17 production Host-microbiota mutualism maintenance through antimicrobials production scavenger receptor 2 (SCART-2) positive γδT cells produce IL-17 in the colon of mice to control antimicrobial epithelial responses | IFNγ-producing γδ T cells: glycolysis IL-17 producing γδ T cells: TCA OXPHOS | Similar IL-17 production in SI, ↓ antimicrobials production in SI | Similar or ↑ in SI; ↓ IL-17 production in SI; ↓ antimicrobials production in SI Minor impact on gut intraepithelial lymphocyte population | [101,102,103,104,105,106] | |
| NK T cell | Lamina propria, Intestinal epithelium | Defence against microbial pathogen Host-microbiota mutualism Th1 and Th2 cytokines production IL-17 and IL-22 production | Generally, blocking glycolysis results in NKT activation | Similar in mesenteric lymph node and Peyer’s patch; ↑ in colon | ↑ in colon | [107,108,109,110] | |
| NK cell | GALT, mesenteric lymph node, intestinal epithelium, lamina propria | Cytotoxicity, IL-22 production to modulate epithelial survival and remodelling, IFN-γ production | Resting NK cells: OXPHOS Activated NK cells: glycolysis | ↓ in SI after weaning ↓ NK cell activity after weaning | Similar in Peyer’s patch, and mesenteric lymph node | RORγtneg–intNK1.1high similar, but RORγthighNK1.1int ↓ in lamina propria ↓ IL-22 production | [97,111,112] |
| Dendritic cell | GALT, mesenteric lymph node, intestinal epithelium, lamina propria | Antigen sampling and presenting Gut tropism imprinting Regulatory and effector T cell induction Food/oral/microbiota tolerance | Tolerogenic DC: OXPHOS Immunogenic DC: glycolysis | ↓ in SI, colon and mesenteric lymph node | ↓ or similar in mesenteric lymph node ↓ type I IFN production ↓ or similar in colon ↓ in SI CX3CR1+ DC ↓ in SI CD103+ DC ↓ in mesenteric lymph node | [113,114,115,116] | |
| Macrophage | GALT, mesenteric lymph node, intestinal epithelium, lamina propria | Intestinal inflammation suppression through IL10 production Host-microbiota mutualism Apoptotic or damaged cell cleavage Treg vs Th17 balance | M1 pro-inflammatory: highly glycolytic, fatty acid synthesis, reduced TCA cycle M2 anti-inflammatory: FAO, OXPHOS, decreased glycolysis | Yolk sac/fetal liver-derived Macrophage would be diluted after weaning by accumulation of circulating Ly6Chigh monocytes | ↓ in SI, and colon, similar in Peyer’s patches, and mesenteric lymph node | ↓ in colon | [117,118,119] |
| Neutrophil | Antimicrobials production, host-microbiota mutualism, immune cell activation and recruitment, mucosal/epithelial repairing | Immature, c-Kit+ neutrophils: OXPHOS Mature neutrophils: glycolysis | [31,120] | ||||
| Innate lymphoid cell | Intestinal epithelium, mucosal surface | Type 1 innate lymphoid cells (ILC1): IFNγ and TNFα production, anti-virus, -cancer, -intracellular pathogen responses Type 2 innate lymphoid cells (ILC2): IL5 and IL13 production, anti-helminth responses, tissue repairing Type 3 innate lymphoid cells (ILC3): IL17 and IL22 production, intestinal lymphoid organ development, host-microbiota mutualism and host defence | Resting ILC1: ? Active ILC1: ? resting ILC2s: OXPHOS or FAO Active ILCs: glycolysis and high rates of OXPHOS Resting ILC3: ? suggested glycolysis- still largely unclear Active ILC3: FAO and synthesis but unclear | ILC3: LTi cells developed in fetus would be diluted by post-birth develop LTi-like ILC3 after weaning in intestinal lamina propria | ↓ ILC3 and ILC1 in Peyer’s patch; ↑ ILC3 in terminal ileum Peyer’s patch; ↓ GM-CSF+ ILC3 in colon ILC1 and ILC2 expression become ILC3-like | Similar ILC1 in SI; ↑ ILC2 in SI; similar or ↑ ILC3 in SI | [111,121,122,123,124,125] |
| Goblet cell | SI, colon epithelium | Mucus secretion | OXPHOS is necessary for goblet cell differentiation | Weaning effect in pigs High protein diet increases goblet cell number and promotes mucus secretion in ileum and alter goblet cell distribution in colon | Similar in colon ↓ in SI ↓ mucus secretion in colon upon Metronidazole treatment | Similar in colon | [126,127,128,129,130] |
| Paneth cell | SI (crypt)+++, colon+ | Antimicrobial peptides and cytokines production Stem cell niche support | Highly glycolytic | ↓ in SI Distorted crypts and fewer granules in cells | ↓ in SI ↓ Reg3γ expression in SI | [131] | |
| Tuft cell | Intestinal epithelium | Chemosensation, IL-25 production to promote ILC2 expansion, anti-parasitic responses | ? | ↑ in SI and colon upon fasting and refeeding ↑ after weaning ↑ in SI after succinate feeding | Similar in colon | [58,132,133] | |
| Microfold cell | GALT, Peyer’s patch, mesenteric lymph node, | (Antigen) Transportation through trans-cellular endocytosis, cytokines/costimulatory signal (IL1) secretion Antigen uptake | ? | Similar in Peyer’s patch | [134] | ||
| Additive | Immune Effects | Reference |
|---|---|---|
| Citrate | Promoted adipose tissue inflammation (IL-6, TNF, IL-1β) and insulin resistance when orally administered in combination with a high sucrose diet in mice. | [166] |
| High levels potentiated inflammatory response in LPS- stimulated THP-1 cells in vitro. | [168] | |
| Emulsifiers: Polysorbate-80 and Carboxymethylcellulose (CMC) | Both P80 and CMC promoted low grade intestinal inflammation (increased MPO, CXCL1, CXCL2 and TNF expression), exacerbating colitis and carcinogenesis in an AOM/DSS mouse model. | [181] |
| Heightened low grade inflammation, induced via microbial changes, resulting in metabolic syndrome and IBD. | [164] | |
| CMC promoted bacterial overgrowth within the small intestine of in IL-10 deficient mice (a model for spontaneous colitis). Did not show significant difference in intestinal inflammation. | [182] | |
| Emulsifiers: Maltodextrin (MDX) | Induced upregulation of genes involved in lipid and carbohydrate metabolism in intestinal epithelial cells in vivo. Induced ER stress in intestinal epithelial cells, greater TNF and IL-1β expression and worse DSS colitis. Inhibition of endoplasmic reticulum (ER) stress in maltodextrin fed mice protected from worsened colitis. This effect was ameliorated with ER stress inhibition. | [183] |
| Sodium chloride | Activated p38/MAPK pathway during cytokine induced Th17 cell polarisation, leading to Th17 bias. Th17 cells polarised in high NaCl conditions produced more GM-CSF, TNF and IL-2. This led to more severe experimental autoimmune encephalomyelitis (EAE) in high salt intake mice. | [184] |
| Worsened pathology in the IL-10-/- mouse model of colitis. Elevated colonic expression of TNF, IL-12β, IL-23α, IL-1β. Mice infected with Salmonella typhimurium had greater IL-17A, IL-12α, IL-12β, TNF, IL-23α, IL-6 and IFNγ mRNA expression on high sodium diet. | [173] | |
| A small human study found activation of CD14++ monocytes and expansion of “intermediate” CD14++CD16+ monocyte populations in the peripheral blood. In vitro follow up studies demonstrated ROS activation of CD14++ monocytes in high sodium media. | [185] | |
| In vitro high NaCl media supressed the induction of AKT and mTOR signalling and increased oxidative phosphorylation and glycolysis in macrophages. High NaCl also dampened the ability of M2 macrophages to supress CD4+ and CD8+ effector T cell proliferation in vitro but enhanced Nos2 expression in M1 macrophages when stimulated with LPS. Impaired wound healing in mice. | [171] | |
| A high NaCl environment induced IL-1β secretion in bone marrow derived macrophages in vitro via through NLRP3 inflammasome and subsequent caspase-1 activation. Mice on a high NaCl diet had an enhanced IL-17 production when immunised with OVA and LPS. This was caspase-1 specific. | [174] | |
| High NaCl blocked the suppressive capacity of human Tregs in vitro. Tregs in high salt fed mice switched to an IFNγ producing phenotype and similarly lost suppressive function. Treg suppressor function was regained by blocking IFNγ or by silencing serum and glucocorticoid-regulated kinase 1 (SGK1) in Tregs. | [186] | |
| High salt in vivo induced IL-17 like Tregs in the thymus and promoted the generation of peripheral RORγt+ iTregs in Th17 polarising conditions in an SGK1 dependant manner. | [187] | |
| Mice fed a high salt diet for 1 week had impaired neutrophil function and more severe pyelonephritis | [188] | |
| Worsened EAE symptoms in mice with high NaCl intake. Enhanced expression of TNF, IL-12, IL-1β, iNOS and IL-6 in the CNS of EAE mice. Promoted pro-inflammatory M1 macrophage activation and activation of NF-κB signalling. | [172] | |
| Sucralose | Glucose intolerance via actions on microbiome. | [189] |
| Promoted a pro-inflammatory environment in the liver as measured by TNF and iNOS transcription. Also altered microbiome composition and microbial metabolic profile to one which is associated with inflammation. | [190] | |
| Titanium dioxide (E171) | Promoted macrophages to produce TNF and IL-10 ex-vivo in the presence of LPS. | [191] |
| Increased macrophage and CD8+ T cells in the colon of orally exposed mice. | [178] | |
| Long term exposure led to spontaneous preneoplastic development in the small intestine of rats. Also resulted in increased IL-17 and IFNγ production when cells from Peyer’s patches were restimulated in vitro and increased IL-10, TNF-a, IL8, IL-6, IL-1β and IFNγ in colonic mucosa or orally exposed mice. | [179] | |
| Chronic exposure worsened DSS- induced psoriasis via activation of NLRP3 inflammasome | [192] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Ni, D.; Ribeiro, R.V.; Pinget, G.V.; Macia, L. How Changes in the Nutritional Landscape Shape Gut Immunometabolism. Nutrients 2021, 13, 823. https://doi.org/10.3390/nu13030823
Tan J, Ni D, Ribeiro RV, Pinget GV, Macia L. How Changes in the Nutritional Landscape Shape Gut Immunometabolism. Nutrients. 2021; 13(3):823. https://doi.org/10.3390/nu13030823
Chicago/Turabian StyleTan, Jian, Duan Ni, Rosilene V. Ribeiro, Gabriela V. Pinget, and Laurence Macia. 2021. "How Changes in the Nutritional Landscape Shape Gut Immunometabolism" Nutrients 13, no. 3: 823. https://doi.org/10.3390/nu13030823