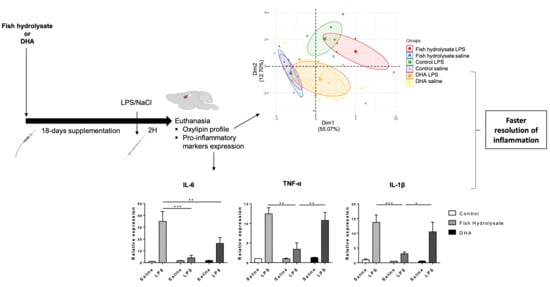
Fish Hydrolysate Supplementation Containing n-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation
Abstract
Share and Cite
Chataigner, M.; Martin, M.; Lucas, C.; Pallet, V.; Layé, S.; Mehaignerie, A.; Bouvret, E.; Dinel, A.-L.; Joffre, C. Fish Hydrolysate Supplementation Containing n-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation. Nutrients 2021, 13, 824. https://doi.org/10.3390/nu13030824
Chataigner M, Martin M, Lucas C, Pallet V, Layé S, Mehaignerie A, Bouvret E, Dinel A-L, Joffre C. Fish Hydrolysate Supplementation Containing n-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation. Nutrients. 2021; 13(3):824. https://doi.org/10.3390/nu13030824
Chicago/Turabian StyleChataigner, Mathilde, Marie Martin, Céline Lucas, Veronique Pallet, Sophie Layé, Alexis Mehaignerie, Elodie Bouvret, Anne-Laure Dinel, and Corinne Joffre. 2021. "Fish Hydrolysate Supplementation Containing n-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation" Nutrients 13, no. 3: 824. https://doi.org/10.3390/nu13030824
APA StyleChataigner, M., Martin, M., Lucas, C., Pallet, V., Layé, S., Mehaignerie, A., Bouvret, E., Dinel, A.-L., & Joffre, C. (2021). Fish Hydrolysate Supplementation Containing n-3 Long Chain Polyunsaturated Fatty Acids and Peptides Prevents LPS-Induced Neuroinflammation. Nutrients, 13(3), 824. https://doi.org/10.3390/nu13030824