A Non-Probiotic Fermented Soy Product Reduces Total and LDL Cholesterol: A Randomized Controlled Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
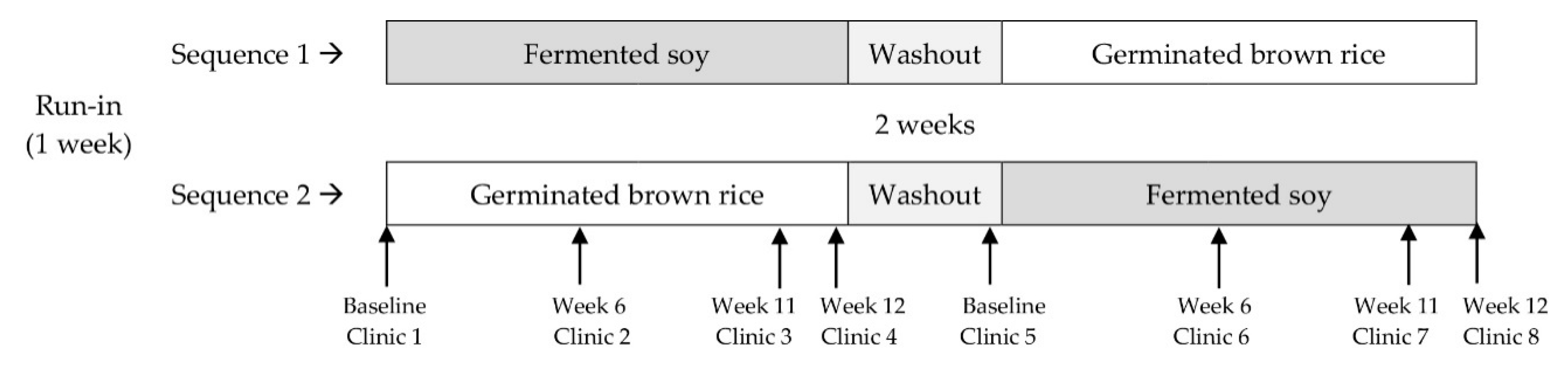
2.1. Study Design
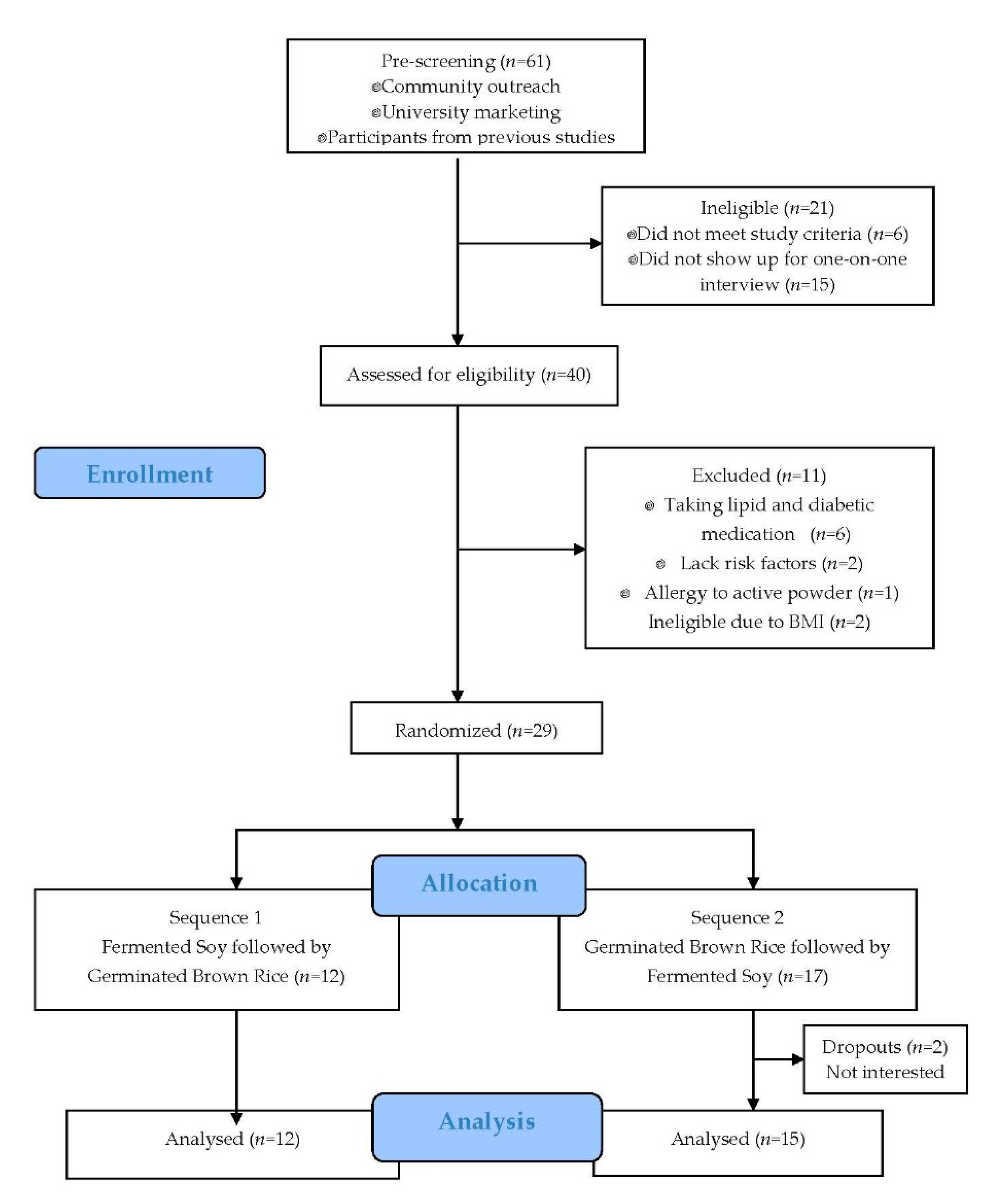
2.2. Participants
2.3. Intervention Products and Compliance
2.4. Anthropometric and Laboratory Measurements
2.5. Statistical Analysis
3. Results
3.1. Participants, Dietary Intake, and Compliance
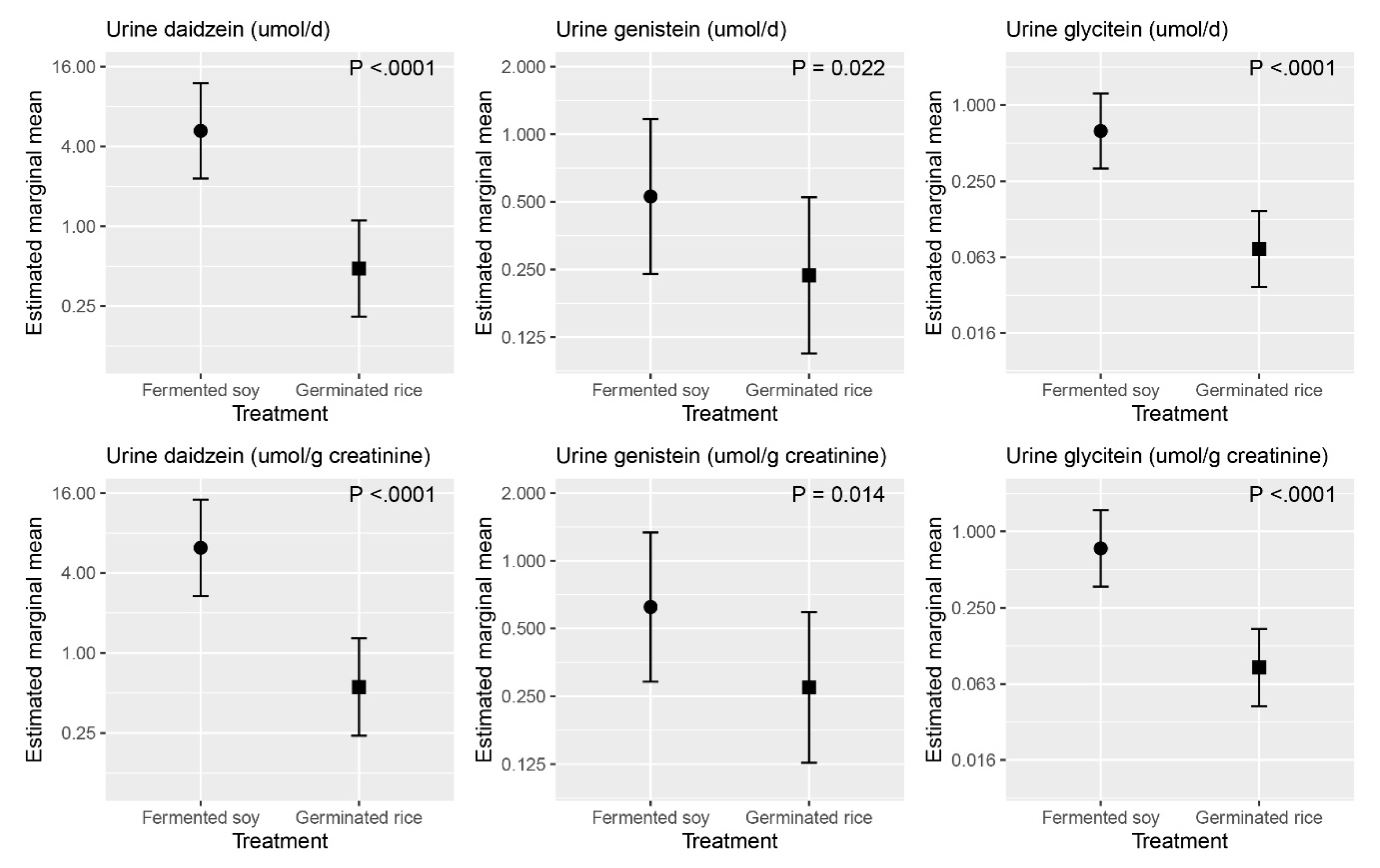
3.2. Laboratory Measures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heron, M. Deaths: Leading Causes for 2017. Natl. Vital Stat. Rep. 2019, 68, 1–77. [Google Scholar]
- Zhang, X.; Shu, X.O.; Gao, Y.T.; Yang, G.; Li, Q.; Li, H.; Jin, F.; Zheng, W. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J. Nutr. 2003, 133, 2874–2878. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, Y.; Iso, H.; Ishihara, J.; Okada, K.; Inoue, M.; Tsugane, S. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: The Japan Public Health Center-based (JPHC) study cohort I. Circulation 2007, 116, 2553–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Zhang, X.; Xiang, Y.B.; Yang, G.; Li, H.; Fazio, S.; Linton, M.; Cai, Q.; Zheng, W.; Gao, Y.T.; et al. Association of soy food intake with risk and biomarkers of coronary heart disease in Chinese men. Int. J. Cardiol. 2014, 172, e285–e287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talaei, M.; Koh, W.P.; van Dam, R.M.; Yuan, J.M.; Pan, A. Dietary soy intake is not associated with risk of cardiovascular disease mortality in Singapore Chinese adults. J. Nutr. 2014, 144, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Mirrahimi, A.; Srichaikul, K.; Berryman, C.E.; Wang, L.; Carleton, A.; Abdulnour, S.; Sievenpiper, J.L.; Kendall, C.W.; Kris-Etherton, P.M. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J. Nutr. 2010, 140, 2302s–2311s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, J.A.; Teng, H.; Erdman, J.W., Jr.; Weigel, R.M.; Klein, B.P.; Persky, V.W.; Freels, S.; Surya, P.; Bakhit, R.M.; Ramos, E.; et al. Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women. Am. J. Clin. Nutr. 1998, 68, 545–551. [Google Scholar] [CrossRef]
- de Kleijn, M.J.; van der Schouw, Y.T.; Wilson, P.W.; Grobbee, D.E.; Jacques, P.F. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S.women: The Framingham study. J. Nutr. 2002, 132, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J. Nutr. 2001, 131, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.R., 3rd; Morgan, T.; Terry, J.G.; Ellis, J.; Vitolins, M.; Burke, G.L. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch. Intern. Med. 1999, 159, 2070–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayagopal, V.; Albertazzi, P.; Kilpatrick, E.S.; Howarth, E.M.; Jennings, P.E.; Hepburn, D.A.; Atkin, S.L. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care 2002, 25, 1709–1714. [Google Scholar] [CrossRef] [Green Version]
- Potter, S.M. Soy protein and cardiovascular disease: The impact of bioactive components in soy. Nutr. Rev. 1998, 56, 231–235. [Google Scholar] [CrossRef]
- Wangen, K.E.; Duncan, A.M.; Xu, X.; Kurzer, M.S. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 225–231. [Google Scholar] [CrossRef]
- Gardner, C.D.; Newell, K.A.; Cherin, R.; Haskell, W.L. The effect of soy protein with or without isoflavones relative to milk protein on plasma lipids in hypercholesterolemic postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Nestel, P.J.; Yamashita, T.; Sasahara, T.; Pomeroy, S.; Dart, A.; Komesaroff, P.; Owen, A.; Abbey, M. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Jalbert, S.M.; Adlercreutz, H.; Goldin, B.R.; Rasmussen, H.; Schaefer, E.J.; Ausman, L.M. Lipoprotein response to diets high in soy or animal protein with and without isoflavones in moderately hypercholesterolemic subjects. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1852–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinwald, S.; Akabas, S.R.; Weaver, C.M. Whole versus the piecemeal approach to evaluating soy. J. Nutr. 2010, 140, 2335s–2343s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Du, B.; Xu, B. A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China. Food Chem. 2015, 174, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Jung, E.S.; Choi, E.K.; Jeong, D.Y.; Jo, S.W.; Jin, J.H.; Lee, J.M.; Park, B.H.; Chae, S.W. Supplementation with Aspergillus oryzae-fermented kochujang lowers serum cholesterol in subjects with hyperlipidemia. Clin. Nutr. (Edinb. Scotl.) 2015, 34, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Dioletis, E.; Paiva, R.; Fields, M.R.; Weiss, T.R.; Secor, E.R.; Ali, A. Fermented Soy Beverage Q-CAN Plus Consumption Improves Serum Cholesterol and Cytokines. J. Med. Food 2020, 23, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Tokede, O.A.; Onabanjo, T.A.; Yansane, A.; Gaziano, J.M.; Djoussé, L. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kayaba, K.; Ishikawa, S. Soy and Soy Products Intake, All-Cause Mortality, and Cause-Specific Mortality in Japan: The Jichi Medical School Cohort Study. Asia Pac. J. Public Health 2015, 27, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Takatsuka, N.; Shimizu, H. Soy and fish oil intake and mortality in a Japanese community. Am. J. Epidemiol. 2002, 156, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Schooling, M.; Hui, L.L.; McGhee, S.M.; Mak, K.H.; Lam, T.H. Soy consumption and mortality in Hong Kong: Proxy-reported case-control study of all older adult deaths in 1998. Prev. Med. 2006, 43, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Goto, Y.; Koda, S.; Kawachi, T.; Tsuji, M.; Nakamura, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2017, 105, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Liu, G.; Ding, M.; Zong, G.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Sun, Q. Isoflavone Intake and the Risk of Coronary Heart Disease in US Men and Women: Results From 3 Prospective Cohort Studies. Circulation 2020, 141, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Agradi, E.; Conti, F.; Mantero, O.; Gatti, E. Soybean-protein diet in the treatment of type-II hyperlipoproteinaemia. Lancet (Lond. Engl.) 1977, 1, 275–277. [Google Scholar] [CrossRef]
- Carroll, K.K.; Giovannetti, P.M.; Huff, M.W.; Moase, O.; Roberts, D.C.; Wolfe, B.M. Hypocholesterolemic effect of substituting soybean protein for animal protein in the diet of healthy young women. Am. J. Clin. Nutr. 1978, 31, 1312–1321. [Google Scholar] [CrossRef] [Green Version]
- Descovich, G.C.; Ceredi, C.; Gaddi, A.; Benassi, M.S.; Mannino, G.; Colombo, L.; Cattin, L.; Fontana, G.; Senin, U.; Mannarino, E.; et al. Multicentre study of soybean protein diet for outpatient hyper-cholesterolaemic patients. Lancet (Lond. Engl.) 1980, 2, 709–712. [Google Scholar] [CrossRef]
- Food labeling: Health claims; soy protein and coronary heart disease. Food and Drug Administration, HHS. Final rule. Fed. Regist. 1999, 64, 57700–57733. [Google Scholar]
- Jenkins, D.J.A.; Blanco Mejia, S.; Chiavaroli, L.; Viguiliouk, E.; Li, S.S.; Kendall, C.W.C.; Vuksan, V.; Sievenpiper, J.L. Cumulative Meta-Analysis of the Soy Effect Over Time. J. Am. Heart Assoc. 2019, 8, e012458. [Google Scholar] [CrossRef] [Green Version]
- Blanco Mejia, S.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P.; et al. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J. Nutr. 2019, 149, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, T.; Fukui, Y.; Yamori, Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal japanese women: A four-week study. J. Am. Coll. Nutr. 2002, 21, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Umegaki, K.; Sato, Y.; Taki, Y.; Endoh, K.; Watanabe, S. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007, 85, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Umegaki, K.; Ishimi, Y.; Watanabe, S. Effects of extracted soy isoflavones alone on blood total and LDL cholesterol: Meta-analysis of randomized controlled trials. Ther. Clin. Risk Manag. 2008, 4, 1097–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padhi, E.M.; Blewett, H.J.; Duncan, A.M.; Guzman, R.P.; Hawke, A.; Seetharaman, K.; Tsao, R.; Wolever, T.M.; Ramdath, D.D. Whole Soy Flour Incorporated into a Muffin and Consumed at 2 Doses of Soy Protein Does Not Lower LDL Cholesterol in a Randomized, Double-Blind Controlled Trial of Hypercholesterolemic Adults. J. Nutr. 2015, 145, 2665–2674. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Sawada, N.; Goto, A.; Yamaji, T.; Iwasaki, M.; Noda, M.; Iso, H.; Tsugane, S. Association of soy and fermented soy product intake with total and cause specific mortality: Prospective cohort study. BMJ (Clin. Res. Ed.) 2020, 368, m34. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.J.; Heo, K.; Park, J.Y.; Lee, K.W.; Park, J.Y.; Joo, S.H.; Kim, J.H. Characterization of AprE176, a fibrinolytic enzyme from Bacillus subtilis HK176. J. Microbiol. Biotechnol. 2015, 25, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Byun, M.-S.; Yu, O.-K.; Park, T.-S. Korean traditional Chungkookjang improves body composition, lipid profiles and atherogenic indices in overweight/obese subjects: A double-blend, randomized, crossover, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016, 70, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yamaguchi, M.; Sobue, T.; Takahashi, T.; Miura, T.; Arai, Y.; Mazur, W.; Wähälä, K.; Adlercreutz, H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). J. Nutr. 1998, 128, 1710–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Sapbamrer, R.; Visavarungroj, N.; Suttajit, M. Effects of dietary traditional fermented soybean on reproductive hormones, lipids, and glucose among postmenopausal women in northern Thailand. Asia Pac. J. Clin. Nutr. 2013, 22, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, D.C.; Manzoni, M.S.; Bedani, R.; Roselino, M.N.; Celiberto, L.S.; Vendramini, R.C.; de Valdez, G.; Abdalla, D.S.; Pinto, R.A.; Rosetto, D.; et al. Probiotic Soy Product Supplemented with Isoflavones Improves the Lipid Profile of Moderately Hypercholesterolemic Men: A Randomized Controlled Trial. Nutrients 2016, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Lovati, M.R.; Manzoni, C.; Gianazza, E.; Arnoldi, A.; Kurowska, E.; Carroll, K.K.; Sirtori, C.R. Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. J. Nutr. 2000, 130, 2543–2549. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Taku, K.; Zhang, Y.; Jia, M.; Wang, Y.; Wang, P. Serum lipid-improving effect of soyabean β-conglycinin in hyperlipidaemic menopausal women. Br. J. Nutr. 2013, 110, 1680–1684. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Two Peptides from Soy β-Conglycinin Induce a Hypocholesterolemic Effect in HepG2 Cells by a Statin-Like Mechanism: Comparative in Vitro and in Silico Modeling Studies. J. Agric. Food Chem. 2015, 63, 7945–7951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siow, R.C.; Li, F.Y.; Rowlands, D.J.; de Winter, P.; Mann, G.E. Cardiovascular targets for estrogens and phytoestrogens: Transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radic. Biol. Med. 2007, 42, 909–925. [Google Scholar] [CrossRef]
- Menazza, S.; Murphy, E. The Expanding Complexity of Estrogen Receptor Signaling in the Cardiovascular System. Circ. Res. 2016, 118, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Tsurugasaki, W.; Nakamura, S.; Osada, K. Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J. Nutr. Biochem. 2005, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Chen, W.H.; Guo, J.J.; Fu, Z.H.; Yi, C.; Zhang, M.; Na, X.L. Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women--a meta-analysis. Nutrition (Burbank Los Angeles County Calif.) 2013, 29, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Chen, Y.M.; Ho, S.C.; Ho, Y.P.; Woo, J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: A 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am. J. Clin. Nutr. 2010, 91, 1394–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.B.; Chen, A.L.; Lu, W.; Zhuo, S.Y.; Liu, J.; Guan, J.H.; Deng, W.P.; Fang, S.; Li, Y.B.; Chen, Y.M. Daidzein and genistein fail to improve glycemic control and insulin sensitivity in Chinese women with impaired glucose regulation: A double-blind, randomized, placebo-controlled trial. Mol. Nutr. Food Res. 2015, 59, 240–249. [Google Scholar] [CrossRef]
- Sarkar, M.; Hossain, S.; Hussain, J.; Hasan, M.; Bhowmick, S.; Basunia, M.A.; Hashimoto, M. Cholesterol Lowering and Antioxidative Effect of Pregerminated Brown Rice in Hypercholesterolemic Rats. J. Nutr. Sci. Vitaminol. 2019, 65, S93–s99. [Google Scholar] [CrossRef] [Green Version]
- Mai, T.T.; Trang, T.T.; Hai, T.T. Effectiveness of germinated brown rice on metabolic syndrome: A randomized control trial in Vietnam. AIMS Public Health 2020, 7, 33–43. [Google Scholar] [CrossRef]
- Shahar, D.R.; Froom, P.; Harari, G.; Yerushalmi, N.; Lubin, F.; Kristal-Boneh, E. Changes in dietary intake account for seasonal changes in cardiovascular disease risk factors. Eur. J. Clin. Nutr. 1999, 53, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Stussman, B.J.; Black, L.I.; Barnes, P.M.; Clarke, T.C.; Nahin, R.L. Wellness-Related Use of Common Complementary Health Approaches Among Adults: United States, 2012; National Health Statistics Reports; National Center for Health Statistics: Hyattsville, MD, USA, 2015; pp. 1–12.
| Fermented Soy 1 2 Packets (25 g) | Germinated Brown Rice 2 2 Packets (30 g) | |
|---|---|---|
| Energy, kcal | 110 | 110 |
| Carbohydrate, g | 8.4 | 23.0 |
| Protein, g | 9.4 | 2.2 |
| Total fat, g | 4.54 | 0.84 |
| Saturated fat, g | 1.04 | 0.16 |
| Monounsaturated fat, g | 1.30 | 0.30 |
| Polyunsaturated fat, g | 2.20 | 0.30 |
| Linolenic acid 18:3, g | 3.4 | 0.03 |
| Dietary fiber, g | 5.6 | 1.4 |
| Total isoflavones, mg | 36.3 | 0 |
| Sequence 1 Fermented Soy ⟶ Germinated Brown Rice | Sequence 2 Germinated Brown Rice ⟶ Fermented Soy | p-Value | |
|---|---|---|---|
| (n = 12) | (n = 15) | ||
| Age, y | 50.3 (12.3) | 52.5 (14.8) | 0.682 |
| Sex, n (%) | |||
| Female | 8 (66.7) | 13 (86.7) | |
| Male | 4 (33.3) | 2 (13.3) | 0.36 3 |
| Menopause, n (%) | |||
| No | 3 (37.5) | 7 (53.8) | |
| Yes | 5 (62.5) | 6 (46.2) | 0.659 1 |
| Waist circumference, cm | 107.6 (22.3) | 107.4 (13.0) | 0.97 2 |
| Systolic blood pressure, mmHg | 123 (16) | 118 (20) | 0.46 2 |
| Diastolic blood pressure, mmHg | 80.5 (15.7) | 73.1 (9.9) | 0.15 2 |
| Total cholesterol, mmol/L | 5.01 (0.80) | 5.17 (1.33) | 0.73 2 |
| LDL cholesterol, mmol/L | 3.03 (0.68) | 3.07 (1.17 | 0.93 2 |
| HDL cholesterol, mmol/L | 1.36 (0.31) | 1.41 (0.30) | 0.71 2 |
| Triglycerides, mmol/L | 1.35 (0.56) | 1.51 (0.36) | 0.53 4 |
| Glucose, mmol/L | 5.87 (1.22) | 5.51 (0.36) | 0.83 4 |
| Insulin, mU/L | 19.5 (13.8) | 13.4 (6.8) | 0.31 4 |
| HOMA-IR | 5.34 (3.97) | 3.26 (1.66) | 0.31 4 |
| Apolipoprotein A1, g/L | 1.36 (0.19) | 1.43 (0.26) | 0.45 2 |
| Apolipoprotein B, g/L | 0.95 (0.17) | 0.96 (0.29) | 0.90 2 |
| Fermented Soy | Germinated Brown Rice | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (95% CI) | End of Study (95% CI) | Change within Treatments (95% CI) | Within p-Value 2 | Baseline (95% CI) | End of Study (95% CI) | Change within Treatment (95% CI) | Within p-Value 2 | Between p-Value 3 | |
| BMI, kg/m2 | 33.3 (30.1, 36.5) | 33.0 (29.8, 36.3) | −0.27 (−0.57, 0.04) | 0.0874 | 33.1 (29.9, 36.3) | 33.2 (30.0, 36.4) | 0.10 (−0.20, 0.41) | 0.5073 | 0.0944 |
| Total cholesterol, mmol/L | 5.27 (4.73, 5.82) | 5.04 (4.50, 5.58) | −0.23 (−0.40, −0.06) | 0.0073 | 5.06 (4.51, 5.60) | 5.20 (4.66, 5.74) | 0.14 (−0.03, 0.31) | 0.1004 | 0.0024 |
| LDL cholesterol, mmol/L | 3.15 (2.66, 3.64) | 2.97 (2.48, 3.46) | −0.18 (−0.32, −0.04) | 0.0132 | 2.96 (2.47, 3.46) | 3.00 (2.51, 3.49) | 0.04 (−0.10, 0.18) | 0.5836 | 0.0317 |
| HDL cholesterol, mmol/L | 1.46 (1.31, 1.61) | 1.43 (1.28, 1.58) | −0.03 (−0.09, 0.03) | 0.2577 | 1.39 (1.24, 1.54) | 1.48 (1.33, 1.64) | 0.09 (0.03, 0.15) | 0.0026 | 0.0036 |
| Total cholesterol:HDL | 3.76 (3.24, 4.28) | 3.66 (3.15, 4.17) | −0.10 (−0.22, 0.02) | 0.0976 | 3.76 (3.24, 4.27) | 3.70 (3.19, 4.22) | −0.05 (−0.17, 0.06) | 0.3568 | 0.5942 |
| LDL:HDL | 2.27 (1.83, 2.71) | 2.17 (1.73, 2.61) | −0.10 (−0.20, 0.01) | 0.0686 | 2.22 (1.78, 2.67) | 2.16 (1.72, 2.60) | −0.07 (−0.17, 0.04) | 0.2066 | 0.6790 |
| Triglycerides, mmol/L | 1.47 (1.18, 1.76) | 1.52 (1.23, 1.81) | 0.05 (−0.11, 0.20) | 0.5339 | 1.54 (1.25, 1.83) | 1.60 (1.31, 1.90) | 0.06 (−0.09, 0.22) | 0.4237 | 0.9017 |
| Apo A1, g/L | 1.46 (1.36, 1.56) | 1.42 (1.32, 1.52) | −0.04 (−0.09, 0.01) | 0.1267 | 1.44 (1.34, 1.54) | 1.48 (1.38, 1.58) | 0.04 (−0.01, 0.09) | 0.1548 | 0.0390 |
| Apo B, g/L | 0.97 (0.86, 1.09) | 0.94 (0.82, 1.05) | −0.04 (−0.07, −0.00) | 0.0303 | 0.09 (0.08, 1.05) | 0.09 (0.08, 1.05) | 0.00 (−0.03, 0.04) | 0.8900 | 0.0997 |
| Apo B:ApoA1 | 0.69 (0.58, 0.80) | 0.67 (0.57, 0.78) | −0.02 (−0.05, 0.01 | 0.2064 | 0.67 (0.57, 0.78) | 0.66 (0.56, 0.77) | −0.01 (−0.04, 0.02) | 0.4119 | 0.7504 |
| Change as Mean Ratio within Treatments | Change as Mean Ratio within treatments | ||||||||
| Glucose, mmol/L | 5.79 (5.37, 6.24) | 5.97 (5.53, 6.44) | 0.06 (0.05, 0.06) | 0.1099 | 5.83 (5.41, 6.29) | 6.01 (5.57, 6.49) | 0.06 (0.05, 0.06) | 0.1088 | 0.9963 |
| Insulin, mU/L | 12.7 (9.11, 17.8) | 12.4 (8.9, 17.2) | 0.97 (0.81, 1.17) | 0.7522 | 12.5 (8.88, 17.5) | 12.7 (9.07, 17.7) | 1.02 (0.84, 1.23) | 0.8450 | 0.7177 |
| HOMA-IR | 3.27 (2.25, 4.74) | 3.21 (2.23, 4.62) | 0.98 (0.82, 1.17) | 0.8333 | 3.23 (2.21, 4.70) | 3.42 (2.37, 4.93) | 1.06 (0.89, 1.26) | 0.5164 | 0.5435 |
| Fermented Soy | Germinated Brown Rice | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (95% CI) | End of Study (95% CI) | Change within Treatments (95% CI) | Within p-Value 2 | Baseline (95% CI) | End of Study (95% CI) | Change within Treatment (95% CI) | Within p-Value 2 | Between p-Value 3 | |
| Female (n = 21) | 3.42 (2.89, 3.96) | 3.41 (2.88, 3.94)) | −0.01 (−0.15, 0.12) | 0.8320 | 3.43 (2.89, 3.96) | 3.36 (2.83, 3.89) | −0.07 (−0.20, 0.07) | 0.3228 | 0.6783 |
| Male (n = 6) | 4.81 (3.90, 5.72) | 4.41 (3.51, 5.31) | −0.40 (−0.65, −0.15) | 0.0019 | 4.81 (3.89, 5.72) | 4.77 (3.86, 5.67) | −0.04 (−0.30, 0.23) | 0.7719 | 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.M.; Haddad, E.H.; Kaur, A.; Sirirat, R.; Kim, A.Y.; Oda, K.; Rajaram, S.; Sabaté, J. A Non-Probiotic Fermented Soy Product Reduces Total and LDL Cholesterol: A Randomized Controlled Crossover Trial. Nutrients 2021, 13, 535. https://doi.org/10.3390/nu13020535
Jung SM, Haddad EH, Kaur A, Sirirat R, Kim AY, Oda K, Rajaram S, Sabaté J. A Non-Probiotic Fermented Soy Product Reduces Total and LDL Cholesterol: A Randomized Controlled Crossover Trial. Nutrients. 2021; 13(2):535. https://doi.org/10.3390/nu13020535
Chicago/Turabian StyleJung, Sarah M., Ella H. Haddad, Amandeep Kaur, Rawiwan Sirirat, Alice Y. Kim, Keiji Oda, Sujatha Rajaram, and Joan Sabaté. 2021. "A Non-Probiotic Fermented Soy Product Reduces Total and LDL Cholesterol: A Randomized Controlled Crossover Trial" Nutrients 13, no. 2: 535. https://doi.org/10.3390/nu13020535






