Abstract
Multiple sclerosis (MS) is a neurodegenerative disease that causes anthropometric changes characterised by functional disability, increase in fat mass, and decrease in lean mass. All these variables are related to a greater cardiac risk. The polyphenol epigallocatechin gallate (EGCG) and an increase in ketone bodies in the blood have been shown to have beneficial effects on anthropometric and biochemical variables related to cardiovascular activity. The aim of this study was to analyse the impact of the intervention with EGCG and ketone bodies on cardiac risk in MS patients. A population of 51 MS patients were randomly assigned to a control group and an intervention group (daily dose of 800 mg of EGCG and 60 mL of coconut oil). Both groups followed an isocaloric diet for 4 months. Levels of beta-hydroxybutyrate (BHB), albumin, paraoxonase 1 (PON1) and C-reactive protein (CRP) were measured in serum before and after the intervention, as well as determining functional ability, waist circumference, waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), fat percentage and muscle percentage. After 4 months, in the intervention group there was a significant increase in BHB, PON1 and albumin, while CRP did not vary; a significant decrease in cardiac risk associated with a significant decline in WHR; as well as a significant increase in muscle percentage. By contrast, these changes were not observed in the control group. Finally, results from analysis of variance (ANOVA) revealed a significant time–condition interaction effect, observing that WHtR and fat mass decreased in the intervention group, while they increased in the control group.
1. Introduction
Multiple sclerosis (MS) is a chronic and degenerative disease of the central nervous system (CNS) of an inflammatory nature and characterised by neuronal demyelination and reactive astrocyte scar formation [1]. Clinically speaking, patients with MS have functional disabilities [2] that are directly related to a progressive loss in lean mass associated at the same time with an increase in fat mass [3]. These anthropometric changes are observed through an increase in body mass index (BMI) and, especially, waist circumference [4] where fat accumulates around the abdomen [5]. A rise in body fat is positively correlated with concentrations of C-reactive protein (CRP) [6], which is linked to the disease’s progression [7]. In addition, metabolic alterations alongside fat accumulation, especially in the abdominal area, increase oxidative stress [8]. This can be related to a decrease of paraoxonase 1 (PON1) in the blood, an enzyme that inhibits low density lipoproteins (LDL) oxidation which hydrolyses the oxidised lipids formed in LDL and high density lipoproteins (HDL), increases the hydrolysis of lactones, aryl esters and organophosphates [9], thus avoiding cytokines being produced [10] and suppressing the expression of the cytokine-induced endothelial cell adhesion molecule (CAM) and the inhibition of the expression of E-selectin induced by IL-1b [11]. In addition, low activity of PON1 in serum IS linked to the development of neurodegenerative diseases [12], including MS [13].
The anthropometric changes that appear throughout the disease are related to cardiovascular risk, the prevalence of which is higher for MS patients than the general population [14]. On the one hand, a decline in skeletal muscle mass leads to a rise in the specific lipid levels related to hyperlipidemia and the development of cardiometabolic syndromes [15]. On the other hand, an increase in body fat, associated with inflammation and oxidation, multiplies the risk of developing cardiovascular diseases [16], especially when body fat is located in the abdominal area [17]. In addition, progressive loss of functionality in MS patients (established with the Expanded Disability Status Scale (EDSS)) linked to anthropometric changes [3] is also correlated with specific comorbidities, including ischemic heart disease [18]. This cardiac risk is biochemically related to low activity of PON1 [19] that is also related to the pathogenesis of the disease, due to the association between lipoproteins and cholesterol metabolism, and the progression of MS [20]. Its activity is also decreased in the case of relapses [21]. In this same line, CRP is increased, especially in relapses of MS patients when compared to the levels in healthy people [22], and is positively correlated with adiposity, suggesting a greater risk of metabolic and cardiovascular diseases [14]. The majority of cardiovascular diseases begin and develop with an endothelial dysfunction, as well as an increase in inflammatory state and oxidative stress [23]. Serum albumin, the most abundant plasma protein, has many physiological properties, including anti-inflammatory, antioxidant [24,25], and antiplatelet aggregation activity [26]. As a result, low levels of albumin in serum are related to different types of cardiovascular disease, such as coronary artery disease, heart failure, atrial fibrillation and strokes [27]. In addition, significant changes in albumin concentration in the blood in MS patients are detected in the short-term after improvement in circulatory parameters [28]. Thus, hypoalbuminaemia can act as a risk factor for cardiovascular diseases, mainly due to exacerbation of inflammation, oxidative stress and platelet aggregation [27].
Taking these precedents into account, therapeutic alternatives should be considered in order to improve the progression of the disease, therefore decreasing cardiac risk associated with inflammation and oxidation as a result of an increase in body fat. Kim et al. proved that a ketogenic diet administered in MS mice models slowed down the progression of the disease, improving motor disability and hippocampal atrophy [29]. Furthermore, ketone bodies obtained after hepatic beta oxidation have been shown to decrease inflammatory markers, including high-sensitivity CRP, indicating at the same time cardiovascular risk improvement [30]. In terms of the nutrients capable of providing higher rates of ketone bodies in the blood, those with high levels of medium-chain triglycerides (MCTs) stand out. In this sense, coconut oil is possibly the food with the highest amount of MCTs as it has high percentages of medium-chain fatty acids (MCFAs), such as caprylic acid, capric acid and lauric acid [31].
Epigallocatechin gallate (EGCG), the main polyphenol found in green tea, reduces the severity of the disease in animal models of autoimmune encephalomyelitis (EAE), as brain inflammation and demyelination damage decrease [32]. This is evidenced by a reduction in encephalitogenic T cell responses and a lower expression of inflammatory cytokines and chemokines [33]. In this sense, Wu et al., showed that treating rat animal models that had induced cerebral ischemic process with 10 mg/kg orally for 7 days demonstrated a significant improvement in memory deterioration induced by the ischemic process. In addition, levels of glutation (GSH) and superoxide dismutase (SOD) increased, and malondialdehyde (MDA) concentrations decreased significantly, both in the cerebral cortex and hippocampus. It was established that bioavailability achieved in these amounts is sufficient for activity at a central level [34].
In terms of its cardioprotective activity, this polyphenol also has been described to improve many cardiovascular risk markers. In particular, it reduced lipids in the blood on a circulatory level, improved ischemia-reperfusion injury in cardiac myoblast cells, decreased myocardial oxidative stress, improved the endothelial function of blood vessels, attenuated inflammation and protected the function of cardiomyocytes in the myocardial tissue [35]. In addition, a decrease in diastolic blood pressure was observed in obese men after receiving 800 mg of EGCG on a daily basis, therefore reducing cardiac risk [36], as well as a significant decrease in LDL in postmenopausal women after administering 800 mg also on a daily basis [37].
Therefore, the objective of this study is to analyse the impact of supplementation with EGCG and coconut oil as a source of ketone bodies in the blood, on cardiac risk in a population of patients with MS by examining anthropometric variables and biomarkers of cardiovascular risk.
2. Materials and Methods
An exploratory/pilot study was conducted by means of a clinical trial. The clinical trial ID for this study is NCT03740295.
2.1. Subjects
The population sample was obtained from the main state-wide MS associations who were previously informed about the study. Sixty-seven people volunteered to take part in the study. Eligibility criteria included: patients over 18 years of age diagnosed with MS at least 6 months previously and under treatment with glatiramer acetate and interferon beta. In addition, the possibility of moving without the need for a wheelchair or walker was required to be part of this study. On the other hand, the exclusion criteria applied to the volunteers included: pregnant or breastfeeding women, patients with tracheotomy, stoma or with short bowel syndrome, patients with dementia, those evidencing alcohol or drug abuse, patients with myocardial infarction, heart failure, cardiac dysrhythmia, symptoms of angina or other heart conditions, patients with kidney conditions with creatinine levels two times higher than normal markers, patients with elevated liver markers three times higher than normal or with chronic liver disease, patients with metabolic or endocrine diseases such as hyperthyroidism or diabetes, patients with acromegaly, patients with polycystic ovary syndrome or MS patients who were included in other research with experimental drugs or treatment.
2.2. Procedure
Participants received information on the study including the defined objectives and the tests and analyses to be carried out, as well as signing an informed consent. Before the intervention, participants registered their solid and liquid intake from the last 7 days. In order to facilitate this registration, patients were provided with information about the weight of each portion of the different types of food [38] and the most common household measurements were provided, such as a portion, a spoonful, a glass, a slice, a plate or a cup. According to all the collected information and after a personal interview on food habits, an individual isocaloric diet was designed taking into account each patient’s pathophysiology.
Diets were designed using the “Easydiet Programa de Gestión de la Consulta®” software (Spanish Academy of Nutrition and Dietetics, Pamplona, Spain) where the anthropometric characteristics of each participant and their pathologies were introduced to establish the caloric, macronutrient and micronutrient needs. They were also provided with instructions to not change the prescribed diet for each case (depending on whether they were in the control group or intervention group), including how to cook the food, portion sizes, the products to be bought at the supermarket, as well as to take the capsules on a daily basis at the scheduled times over the 4-month duration of the intervention. Weekly telephone calls were made by team members to each and every patient on Monday mornings in order to verify whether they were complying with the treatment. These calls were made throughout the whole duration of the intervention on a weekly basis. In addition, the subjects had an appointment with the team every 15 days to interview them and verify whether they were following the diet. They were asked questions (see Supplementary Materials) to understand the level in which they followed the diet. Thus, it was verified that they did not eat foods not included in the prescribed diet, such as those with a lipid profile that may affect the study (mainly butter and goat milk with high levels of medium-chain fatty acids) or that have compounds similar to those of the intervention in their composition (such as tea, coffee, red fruit, rich in polyphenols).
They were also asked about any doubts they had or incidences regarding the diet in order to ensure the caloric intake was followed, as well as whether they had come across any issues with the capsules (such as an intolerance or side effects). No general issues or problems with the diet or capsules were registered.
2.3. Intervention
Once the selection criteria had been applied, a final sample of 51 MS patients was obtained and participants were randomly assigned to the intervention and control group. Randomisation without stratification was performed by drawing sealed, opaque envelopes previously arranged in a computer-generated random order. Once the population had been divided into the two groups, the study began on 4 October 2018 and all functional tests were conducted, alongside anthropometric measurements and obtaining blood samples from all participants, which were repeated on 7 February 2019 when the study came to an end. These measurements are indicated in the measurements section. For the 4-month duration of the study, the intervention group followed an isocaloric diet which was individually given and explained. This diet was adapted to the individual characteristics of each patient and divided into 5 meals a day: breakfast, mid-morning snack, lunch, afternoon snack and dinner. It was enriched with 60 mL of extra virgin coconut oil divided into 2 equal intakes (30 mL for breakfast and 30 mL for lunch, for which they were provided with a calibrated syringe, administering the content directly into the mouth), representing: 91.84% saturated fatty acids, 6.23% monounsaturated fatty acids and 1.93% polyunsaturated fatty acids, which were included in the isocaloric diet and adapted to an adult’s nutritional requirements; and supplemented with 800 mg of EGCG (Manufacturer Taiyo green power co. Ltd. CN; batch number 702131,708161) administered in two capsules of 400 mg to be taken twice a day (one capsule in the morning and another in the afternoon), the quantity of which responds to the pharmacokinetic calculations of Feng WY. (2006) [39].
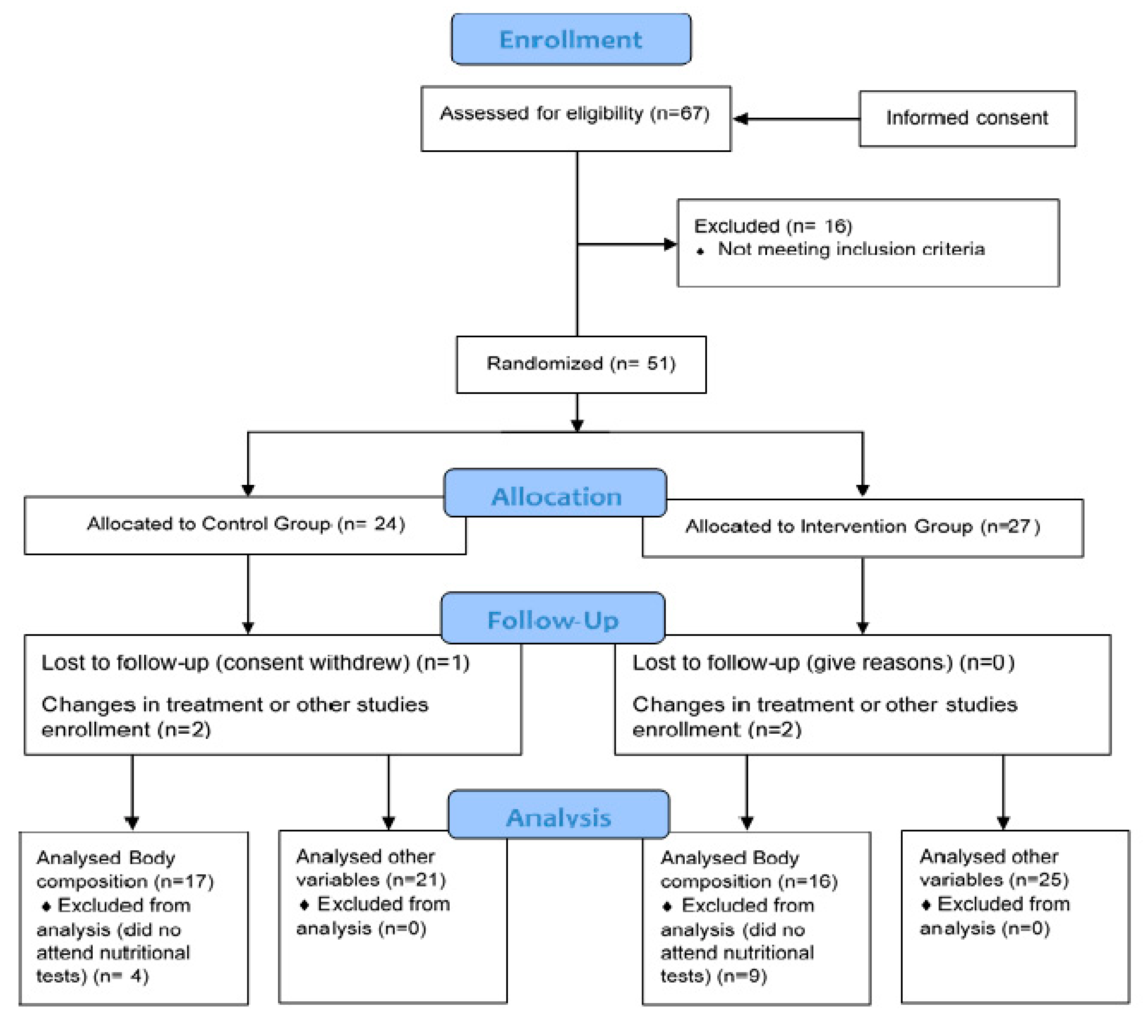
On the other hand, the control group followed the same isocaloric diet as the intervention group for the same 4 months, except for coconut oil. In this group, the diet was also given and explained individually. Furthermore, they were administered placebo tablets (opaque capsules containing microcrystalline cellulose, matching in size and colour). Both groups followed the same instructions. The basal diet for all participants included the following percentage distribution of the 3 main macronutrients with respect to the total caloric value: 20% proteins, 40% carbohydrates and 40% Mediterranean lipids that were not rich in medium-chain fatty acids. This ensured that a state of nutritional ketosis did not occur in the control group, as the percentages and the amounts of macronutrients were not characteristic of a ketogenic diet [40], and nor did the composition of lipids make up the said 40%. However, it was foreseeable to achieve ketosis in the intervention group, as the highest percentage of 40% of lipids was from coconut oil rich in medium-chain fatty acids. In terms of catechins, foods rich in polyphenols, especially tea and coffee, were avoided when designing the basal diet for both groups. This diet was characterised for being balanced, varied and with sufficient calories, by providing adequate food proportions divided into 5 daily intakes. Overall, recruitment/enrolment was performed in a total period of six months (Figure 1).
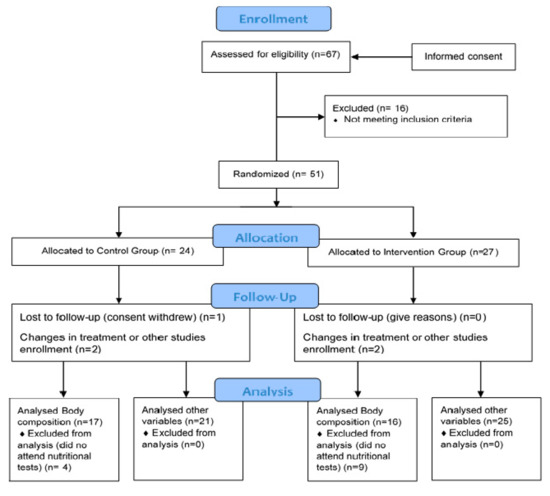
Figure 1.
Consort flow diagram.
2.4. Measurements
The following measurements were taken before and after the 4-month intervention, in the same conditions and at the same time. In the specific case of the scales, they were carried out by the same neurologist assigned to each patient before the study.
The measurements were taken by an ISAK (International Society for the Advancement of Kinanthropometry) level 3 certified anthropometrist in line with the protocol established by the society [41]. The validated anthropometric material used was: a portable clinical scale, SECA model, with a 150–200 kg capacity and 100 g precision; height rod, SECA model, 220 Hamburg, Germany, with a 0.1 cm precision; metal, inextensible and narrow anthropometric tape, model Lufkin W606PM, with 0.2 mm precision; a mechanical skin fold caliper, model Holtain LTD, Crymych, UK, with a 0.2 mm precision and measurement range from 0 to 48 mm; a bicondylar pachymeter to measure the diameter of small bones, model Holtain, with 1 mm precision and measuring range from 0 to 140 mm; and a dermographic pencil to mark anatomical points. The variables that were measured were: body weight, waist and hip circumference, and tricep, subscapular, supraspinal and abdominal folds. Measurements were taken twice, with a third measurement made in the event that the difference between the first two measurements was greater than 5% for the folds and 1% for the other measurements.
The waist-to-hip ratio (WHR), an anthropometric measurement to measure intra-abdominal fat levels, was calculated using the ratio of the waist circumference to that of the hip [42]. Cardiovascular risk obtained from the WHR variable, and based on the classification offered by Yusuf et al. [43], was rated as high (≥1 in men; 0.85 in women), moderate (0.96–1.0 in men; 0.81–0.85 in women), and low risk (≤0.95 in men; 0.80 in women). The waist-to-height ratio (WHtR) was obtained by calculating the quotient between waist and height measurements [44]. The Faulkner equation was used to calculate lean mass percentage [45]. Bone weight was calculated with the Rocha formula [46] and the Matiegka formula [47] was used to calculate muscle weight with which the percentage of muscle mass was obtained.
Functional ability was also measured with the EDSS [48]. The scale is an ordinal scale based on a neurological examination of the eight functional systems (pyramidal, cerebellar, brainstem, mental, sensory, visual, bowel and bladder), alongside assessing walking capacity, which, as a result, provides a disability index between 0 and 10, 0 being understood as having normal health and 10 death by MS.
Blood tests were carried out in the peripheral vein (antecubital vein) at 11 a.m. on an empty stomach. The blood samples were collected in BD Vacutainer Plus serum blood collection tubes (ref. 367815). Once the test was finished, the samples were left at room temperature for 30 min to coagulate. The coagulated part was separated by centrifuging the samples at 2012 g for 10 min in a refrigerated centrifuge (Thermo Scientific Sorvall ST, San Diego, CA, USA, 40R centrifuge). Once centrifuged, the supernatant liquid (blood serum) was transferred to 0.5 mL aliquots, which were then frozen and stored at −80°C. Finally, the concentration of CRP and albumin were measured by commercial reagents (Beckman Coulter, OSR6147 in the case of CRP and OSR6102 in the case of albumin). Both assays had an imprecision lower than 10% and were linear after serial dilutions. The BHB concentrations were measured with a commercial kit (Randox Laboratories, Crumlin, UK, RB1007) and PON1 activity by using 4-Nitrophenyl acetate (Sigma Chemical, St. Louis, MI, USA) using a previously described assay [49]. All measurements were made in an automated clinical biochemistry analyser (Olympus A 400, Tokyo, Japan).
2.5. Ethical Concerns
The study was conducted in accordance with the Declaration of Helsinki [50], prior approval of the protocol by the Human Research Committee of the Experimental Research Ethics Committee of the University of Valencia (procedure number H1512345043343). In addition, patients included in the study signed a consent form after being informed on the procedures and nature of the study.
2.6. Statistical Analysis
A statistical analysis was carried out with the SPSS v.23 (IBM Corporation, Armonk, NY, USA) tool. The first step estimated the distribution of the variables investigated through statistical methods in order to assess normality, including the Kolmogorov–Smirnov Test. This analysis showed the non-normal distribution of all the scale variables studied. In addition, the Mann–Whitney U test was used to assess the inter-group and pre-post differences, respectively. Categorical data were analysed with a chi-square test. The analyses also included a two-way (repeated measures; pre-test to post-test) by 2 (between subjects; groups) analysis of variance (ANOVA) model. A p-value below 0.05 was considered significant. Data are presented as mean ± standard deviation, or the number of patients and percentage.
3. Results
Sociodemographic characteristics of the 51 MS patients with a mean weight of 69.48 kg, divided into an intervention and control group, are shown in Table 1. There were no significant differences between both groups regarding any of the variables analysed in the study, including the categorical variables of gender or MS type.

Table 1.
Sociodemographic and clinical characteristics of the population of the study. Comparison between different groups at baseline.
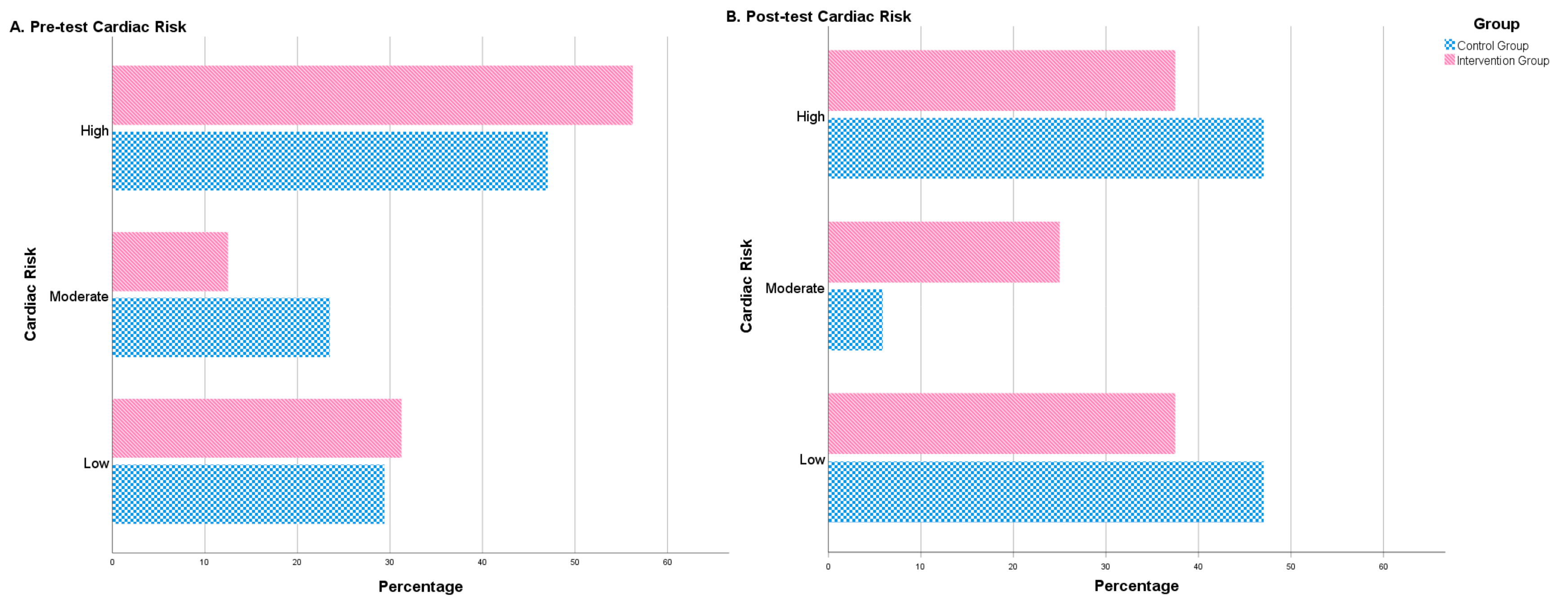
After the intervention period, some significant differences between both groups were observed. The intervention group showed a significantly lower figure than the control group for WHR. By contrast, the treatment group revealed significantly higher means for muscle mass, albumin and BHB (Table 2). Furthermore, after the 4-month intervention, no significant differences were observed in the control group in terms of cardiac risk. Nonetheless, significant changes were observed in the intervention group regarding the distribution of people in different cardiac risk groups (high, moderate, low). The number of high-risk patients decreased and patients with a moderate or low risk increased (Table 2, Figure 2).

Table 2.
Comparison between different groups post-test.
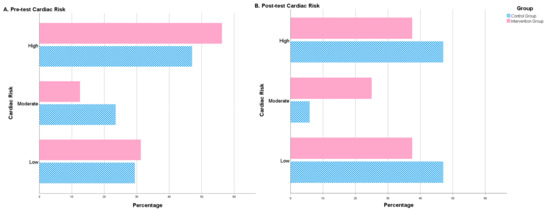
Figure 2.
Cardiac risk (percentage of patients) before treatment (pre-test cardiac risk) and after treatment (post-test cardiac risk). Number of patients (in percentage) with low, moderate or high cardiac risk before and after the intervention. The figure shows the differences between the control group (blue) and the intervention group (red).
Finally, the results from ANOVA revealed a significant time–condition interaction effect. Therefore, we found that WHtR [F(1.31) = 12,752; p = 0.001] and fat mass [F(1.48) = 21,275; p = 0.000] of participants in the intervention group decreased from baseline, whereas these variables increased in the control group. In addition, muscle mass [F(1.49) = 7975; p = 0.007], albumin [F(1.43) = 8222; p = 0.006] and PON1 [F(1.41) = 4501; p = 0.040] of participants in the intervention group increased while they decreased in the control group (Table 3).

Table 3.
Comparison of change scores after completion of intervention versus control.
4. Discussion
One of the main problems that MS patients have is a high level of cardiac risk, mainly due to fat accumulation in the abdomen [42,51] and muscle loss [52]. These anthropometric changes are related to cardiac comorbidities [18].
With the aim of improving this cardiac risk, an isocaloric Mediterranean diet was administered and supplemented with high quantities of EGCG and coconut oil, a source of MCT, which increase the levels of ketone bodies in the blood after hepatic metabolism [53]. Thus, a significant increase of BHB in the blood was found in the intervention group. In addition, there was a significant improvement in cardiac risk, as percentages of patients with moderate or lower risk increased. This suggests that this kind of diet could allow the probability of coronary disease to be reduced [54]. The possible role of EGCG in terms of this cardiac improvement, as this polyphenol protects against lipid peroxidation [55], alongside its anti-inflammatory and antioxidant activity, makes administering it to be associated with a lower risk of cardiovascular disease [56]. As previously mentioned, lowering cardiac risk is related to reducing abdominal fat. EGCG has important anti-obesity properties by decreasing adipocyte proliferation, lipogenesis and the production of inflammatory adipokines [57], especially in the abdomen [58], improving the parameters we have measured [59]. Therefore, ketone bodies, in equally isoenergetic conditions, improve basal metabolic rate [60] and lower appetite while increasing the satiating effect. However, these effects are moderate in humans. As a result, weight is lost based on fat loss [61].
Cardiac risk does not only depend on high fat percentages, but also on related muscle loss [62,63]. Accordingly, administering EGCG shows protective and repairing effects on skeletal muscle [64] and, at the same time, improvements have been detected in muscles after following a ketogenic diet [65]. Our results are in agreement with these findings as our treatment achieves an increase in lean mass only in the intervention group.
Effectiveness of the intervention on an anthropometric level seems to be specifically confirmed by fat percentage, WHtR and muscle percentage variables, after applying the ANOVA test that associates both the effect of the duration of the intervention with the treatment itself.
This cardiac risk decrease associated with anthropometric improvements has also been evidenced at a biochemical level. Abdominal fat accumulation directly contributes to the chronic inflammatory state of the disease [5], increasing the concentration of proinflammatory molecules related to oxidation state and cardiac risk. In particular, our study measured albumin, PON1 and CRP before and after the intervention. The albumin has been negatively correlated with the development of MS, even having a more pronounced relation as the disease progresses [66]. In our case, a significant increase only in the treated patients was observed, which showed that our intervention with EGCG and coconut oil increased the concentration of this protein. This effect is beneficial for the heart, as low concentrations of albumin are associated with coronary artery disease or heart failure [67], and hypoalbuminaemia is considered as a cardiovascular risk factor [27]. Moreover, an increase in the levels of albumin could strengthen the effects of EGCG in our treatment, as it has been proved for albumin to bind to EGCG by stabilising this polyphenol and enhancing its antioxidant power [68,69].
We also obtained an equally significant increase in the activity of PON1, in line with what has been published by other authors who, on the one hand, saw a significant increase in the activity of paraoxonase when administering polyphenol resveratrol in mice with atherosclerosis (ApoE-deficient mice) [70], and, on the other hand, administering catechins in human beings significantly increased PON1 in serum, causing a decrease in proinflammatory cytokines [71]. In rats, when following a diet enriched with coconut oil, there was a significant increase in PON1 activity, unlike what happened when they followed a diet enriched with other oils, such as copra oil, olive oil and sunflower oil [72].
Finally, CRP, which is related to the development of MS [14], did not decrease after the intervention. It could be expected that the administration of coconut oil would increase CRP, as has been previously described [73]. However after the intervention of our study, the values did not increase, but they remained with similar levels, which could be due to the compensatory effect of EGCG, whose activity decreases the secretion of CRP [74,75].
All of the positive biochemical changes obtained only in the intervention group are also observed after applying the ANOVA test for albumin and PON1 (whose activity even worsens in the control group), which seems to confirm treatment effectiveness.
Despite these promising results, our study does have some limitations. We believe that measuring the variables should be monitored throughout the whole study, and not only before and after the intervention, thereby trying to delve into the activity at a metabolic level of the treatment. It would also be necessary to assess the evolution in the values of other biochemical markers, especially the different types of lipoprotein that are related to the protection or risk of developing cardiovascular diseases. Furthermore, despite there being no differences in the average weight of both groups (intervention and control), the doses could not be adjusted individually according to weight in the study. Finally, due to the exploratory and pilot nature of this study, more stringently designed intervention trials are required in the future.
5. Conclusions
The administration of of the antioxidant EGCG in MS patients produces an increase in ketone bodies in the blood and decreases cardiac risk in MS patients. This decrease in cardiac risk is determined as a result of the improvement of two different variables: anthropometric (such as a reduction in WHR and an increase in muscle percentage) and serum analytes (with an increase in PON1 and albumin in the blood). Therefore, despite the fact that these results should be further studied in depth in future studies, this intervention seems to have potential as a therapeutic alternative to improve cardiac risk in these patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/12/3792/s1. Table S1: Intra-group comparison before treatment (pre) and after treatment (post) of cardiovascular risk.
Author Contributions
Conceptualisation, J.E.d.l.R.O. and M.B.; data curation, M.C.-B., M.Á.N., M.L.M., S.C.-J., and E.D.; formal analysis, J.E.d.l.R.O. and M.M.L.-R.; investigation, M.C.-B., E.D., J.J.C., and A.T.; methodology, M.L.M., S.C.-J., M.Á.N., and E.D.; resources, M.C.-B. and J.L.P.; software, M.M.L.-R.; validation, J.J.C. and A.T., writing—Original draft, J.E.d.l.R.O.; writing—Review and editing, M.B., J.L.P., and J.E.d.l.R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Catholic University of Valencia San Vicente Mártir (grant number 2018-203-001).
Acknowledgments
The authors would like to thank all MS patients and the associations for their participation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kutzelnigg, A.; Lassmann, H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Int. Neuroradiol. 2014, 122, 15–58. [Google Scholar] [CrossRef]
- Demir, S. Multiple Sclerosis Functional Composite. Arch. Neuropsychiatry 2018, 55, S66–S68. [Google Scholar] [CrossRef]
- Wens, I.; Dalgas, U.; Vandenabeele, F.; Krekels, M.; Grevendonk, L.; Eijnde, B.O. Multiple Sclerosis Affects Skeletal Muscle Characteristics. PLoS ONE 2014, 9, e108158. [Google Scholar] [CrossRef] [PubMed]
- Tettey, P.; Simpson, S.; Taylor, B.; Ponsonby, A.-L.; Lucas, R.M.; Dwyer, T.; Kostner, K.; Van Der Mei, I.A. AUSLONG Investigators Group An adverse lipid profile and increased levels of adiposity significantly predict clinical course after a first demyelinating event. J. Neurol. Neurosurg. Psychiatry 2017, 88, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Heber, D. An integrative view of obesity. Am. J. Clin. Nutr. 2010, 91, 280S–283S. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’Ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med Sci. 2017, 4, 851–863. [Google Scholar] [CrossRef]
- Katarina, V.; Gordana, T.; Svetlana, M.D.; Milica, B. Oxidative stress and neuroinflammation should be both considered in the occurrence of fatigue and depression in multiple sclerosis. Acta Neurol. Belg. 2018, 120, 853–861. [Google Scholar] [CrossRef]
- Jia, X.-J.; Liu, L.-X.; Tian, Y.-M.; Wang, R.; Lu, Q. The correlation between oxidative stress level and intra-abdominal fat in obese males. Medicine 2019, 98, e14469. [Google Scholar] [CrossRef]
- Efrat, M.; Aviram, M. Paraoxonase 1 Interactions with HDL, Antioxidants and Macrophages Regulate Atherogenesis—A Protective Role for HDL Phospholipids. Paraoxonases Inflamm. Infect. Toxicol. 2009, 660, 153–166. [Google Scholar] [CrossRef]
- Kappelle, P.J.W.H.; De Boer, J.F.; Perton, F.G.; Annema, W.; De Vries, R.; Dullaart, R.P.F.; Tietge, U.J.F. Increased LCAT activity and hyperglycaemia decrease the antioxidative functionality of HDL. Eur. J. Clin. Investig. 2011, 42, 487–495. [Google Scholar] [CrossRef]
- Watson, A.D.; Berliner, J.A.; Hama, S.Y.; La Du, B.N.; Faull, K.F.; Fogelman, A.M.; Navab, M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Investig. 1995, 96, 2882–2891. [Google Scholar] [CrossRef] [PubMed]
- Menini, T.; Gugliucci, A. Paraoxonase 1 in neurological disorders. Redox Rep. 2014, 19, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, M.; Trentini, A.; Romani, A.; Valacchi, G.; Bellini, T.; Bonaccorsi, G.; Fainardi, E.; Cavicchio, C.; Passaro, A.; Zuliani, G.; et al. Decreased arylesterase activity of paraoxonase-1 (PON-1) might be a common denominator of neuroinflammatory and neurodegenerative diseases. Int. J. Biochem. Cell Biol. 2016, 81, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Baynard, T.; Hilgenkamp, T.I.; Schroeder, E.C.; Motl, R.W.; Fernhall, B. Measures of adiposity differentially correlate with C-reactive protein among persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vella, C.A.; Nelson, M.C.; Unkart, J.T.; Miljkovic, I.; Allison, M.A. Skeletal muscle area and density are associated with lipid and lipoprotein cholesterol levels: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Lipidol. 2020, 14, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Korenfeld, Y.; Boarin, S.; Korinek, J.; Jensen, M.D.; Parati, G.; Lopez-Jimenez, F. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur. Heart J. 2010, 31, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Riera-Fortuny, C.; Real, J.T.; Chaves, F.J.; Morales-Suárez-Varela, M.; Martínez-Triguero, M.L.; Morillas-Ariño, C.; Mijares, A.H. The relation between obesity, abdominal fat deposit and the angiotensin-converting enzyme gene I/D polymorphism and its association with coronary heart disease. Int. J. Obes. 2004, 29, 78–84. [Google Scholar] [CrossRef]
- Chaves, G.S.S.; Ghisi, G.L.M.; Grace, S.L.; Oh, P.; Ribeiro, A.L.; Britto, R.R. Effects of comprehensive cardiac rehabilitation on functional capacity and cardiovascular risk factors in Brazilians assisted by public health care: Protocol for a randomized controlled trial. Braz. J. Phys. Ther. 2016, 20, 592–600. [Google Scholar] [CrossRef][Green Version]
- Palavra, F.; Marado, D.; Mascarenhas-Melo, F.; Sereno, J.; Teixeira, F.; Nunes, C.C.; Gonçalves, G.; Teixeira, F.; Reis, F. New Markers of Early Cardiovascular Risk in Multiple Sclerosis Patients: Oxidized-LDL Correlates with Clinical Staging. Dis. Markers 2013, 34, 341–348. [Google Scholar] [CrossRef]
- Karmon, Y.; Ramanathan, M.; Minagar, A.; Zivadinov, R.; Weinstock-Guttman, B. Arterial, venous and other vascular risk factors in multiple sclerosis. Neurol. Res. 2012, 34, 754–760. [Google Scholar] [CrossRef]
- Jamroz-Wiśniewska, A.; Bełtowski, J.; Stelmasiak, Z.; Bartosik-Psujek, H. Paraoxonase 1 activity in different types of multiple sclerosis. Mult. Scler. J. 2009, 15, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Hossain, J.; Morandi, E.; Tanasescu, R.; Frakich, N.; Caldano, M.; Onion, D.; Faraj, T.A.; Erridge, C.; Gran, B. The Soluble Form of Toll-Like Receptor 2 Is Elevated in Serum of Multiple Sclerosis Patients: A Novel Potential Disease Biomarker. Front. Immunol. 2018, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Popolo, A.; Trifa, A.P.; Stanciu, L.A. Phytochemicals in Cardiovascular and Respiratory Diseases: Evidence in Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Kritchevsky, S.B.; Newman, A.B.; Goodpaster, B.H.; Tylavsky, F.A.; Nevitt, M.C.; Harris, T.B. Lower serum albumin concentration and change in muscle mass: The Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 2005, 82, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Liou, S.-Y.; Kuo, W.-W.; Wu, H.-C.; Chang, Y.-L.; Chen, T. Chemiluminescence analysis of antioxidant capacity for serum albumin isolated from healthy or uremic volunteers. Luminescence 2016, 31, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Arques, S. Human serum albumin in cardiovascular diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef]
- Arques, S. Serum albumin and cardiovascular diseases: A comprehensive review of the literature. Ann. Cardiol. Angéiol. 2018, 67, 82–90. [Google Scholar] [CrossRef]
- Ulivelli, M.; Priora, R.; Di Giuseppe, D.; Coppo, L.; Summa, D.; Margaritis, A.; Frosali, S.; Bartalini, S.; Martini, G.; Cerase, A.; et al. Homocysteinemia control by cysteine in cerebral vascular patients after methionine loading test: Evidences in physiological and pathological conditions in cerebro-vascular and multiple sclerosis patients. Amino Acids 2016, 48, 1477–1489. [Google Scholar] [CrossRef]
- Kim, D.Y.; Hao, J.; Liu, R.; Turner, G.; Shi, F.-D.; Rho, J.M. Inflammation-Mediated Memory Dysfunction and Effects of a Ketogenic Diet in a Murine Model of Multiple Sclerosis. PLoS ONE 2012, 7, e35476. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef]
- Bezard, J.; Bugaut, M.; Clement, G. Triglyceride composition of coconut oil. J. Am. Oil Chem. Soc. 1971, 48, 134–139. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Z.; Xu, Y.; Xiao, S.; Meydani, S.N.; Wu, D. Epigallocatechin-3-Gallate Ameliorates Experimental Autoimmune Encephalomyelitis by Altering Balance among CD4+ T-Cell Subsets. Am. J. Pathol. 2012, 180, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, J.; Pae, M.; Meydani, S.N. Green tea EGCG, T cells, and T cell-mediated autoimmune diseases. Mol. Asp. Med. 2012, 33, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-J.; Hsieh, M.-T.; Wu, C.-R.; Wood, W.G.; Chen, Y.-F. Green Tea Extract Ameliorates Learning and Memory Deficits in Ischemic Rats via Its Active Component Polyphenol Epigallocatechin-3-gallate by Modulation of Oxidative Stress and Neuroinflammation. Evid. Based Complement Altern. Med. 2012, 2012, 163106. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-Y.; Zhao, C.-N.; Gan, R.-Y.; Xu, X.-Y.; Wei, X.-L.; Corke, H.; Atanasov, A.G.; Li, H.-B. Effects and Mechanisms of Tea and Its Bioactive Compounds for the Prevention and Treatment of Cardiovascular Diseases: An Updated Review. Antioxidants 2019, 8, 166. [Google Scholar] [CrossRef]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef]
- Wu, A.H.; Spicer, D.; Stanczyk, F.Z.; Tseng, C.-C.; Yang, C.S.; Pike, M.C. Effect of 2-Month Controlled Green Tea Intervention on Lipoprotein Cholesterol, Glucose, and Hormone Levels in Healthy Postmenopausal Women. Cancer Prev. Res. 2012, 5, 393–402. [Google Scholar] [CrossRef]
- Dapcich, V.; Salvador, G.; Ribas, L.; Pérez, C.; Aranceta, J.; Serra, L.L. Guía de Alimentación Saludable; Sociedad Española de Nutrición Comunitaria: Barcelona, Spain, 2004. [Google Scholar]
- Feng, W.Y. Metabolism of Green Tea Catechins: An Overview. Curr. Drug Metab. 2006, 7, 755–809. [Google Scholar] [CrossRef]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.Y.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2014, 16, 64–76. [Google Scholar] [CrossRef]
- Fernández-Juan, A.; Ramírez-Gil, C.; van der Werf, L. La valoración antropométrica en el contexto de la escuela como medida para detectar y prevenir efectos a largo plazo de la obesidad y del sobrepeso en niños en edad escolar. Rev. Colomb. Cardiol. 2016, 23, 435–442. [Google Scholar] [CrossRef]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.L.N.; Chan, J. Body mass index, waist circumference and waist: Hip ratio as predictors of cardiovascular risk—A review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.A. Physiology of Swimming and Diving. Exercise Physiology; Academic Press: Baltimore, MD, USA, 1968. [Google Scholar]
- Rocha, M.S.L. Peso ósseo do brasileiro de ambos os sexos de 17 a 25 años. Arq. Anatomía Antropol. 1975, 1, 445–451. [Google Scholar]
- Matiegka, J. The testing of physical efficiency. Am. J. Phys. Anthr. 1921, 4, 223–230. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef]
- Ceron, J.J.; Tecles, F.; Tvarijonaviciute, A. Serum paraoxonase 1 (PON1) measurement: An update. BMC Vet. Res. 2014, 10, 74. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Amirabdollahian, F.; Haghighatdoost, F. Anthropometric Indicators of Adiposity Related to Body Weight and Body Shape as Cardiometabolic Risk Predictors in British Young Adults: Superiority of Waist-to-Height Ratio. J. Obes. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Park, J.; Kim, N.H.; Kim, S.H.; Kim, J.-S.; Kim, Y.H.; Lim, H.E.; Kim, E.J.; Na, J.O.; Cho, G.-Y.; Baik, I.; et al. Visceral adiposity and skeletal muscle mass are independently and synergistically associated with left ventricular structure and function: The Korean Genome and Epidemiology Study. Int. J. Cardiol. 2014, 176, 951–955. [Google Scholar] [CrossRef]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Sackner-Bernstein, J.; Kanter, D.; Kaul, S. Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis. PLoS ONE 2015, 10, e0139817. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Juliá, S.; Moreno, M.L.; Barrios, C.; Ortí, J.E.D.L.R.; Drehmer, E. Antioxidant Alternatives in the Treatment of Amyotrophic Lateral Sclerosis: A Comprehensive Review. Front. Physiol. 2020, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, C.-T.; Kim, Y. Green Tea (–)-Epigallocatechin-3-Gallate Reduces Body Weight with Regulation of Multiple Genes Expression in Adipose Tissue of Diet-Induced Obese Mice. Ann. Nutr. Metab. 2009, 54, 151–157. [Google Scholar] [CrossRef]
- Hill, A.M.; Coates, A.M.; Buckley, J.D.; Ross, R.; Thielecke, F.; Howe, P.R.C. Can EGCG Reduce Abdominal Fat in Obese Subjects? J. Am. Coll. Nutr. 2007, 26, 396S–402S. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Swain, J.F.; Feldman, H.A.; Wong, W.W.; Hachey, D.L.; Garcia-Lago, E.; Ludwig, D.S. Effects of Dietary Composition on Energy Expenditure During Weight-Loss Maintenance. JAMA 2012, 307, 2627–2634. [Google Scholar] [CrossRef]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef]
- Cao, Q.; Yu, S.; Xiong, W.; Li, Y.; Li, H.; Li, J.; Li, F. Waist-hip ratio as a predictor of myocardial infarction risk: A systematic review and meta-analysis. Medicine 2018, 97, e11639. [Google Scholar] [CrossRef]
- Al-Shamiri, M.Q.; Habbab, F.M.A.; Al-Qahtani, S.S.; Alghalayini, K.A.; Al-Qattan, O.M.; El-Shaer, F. Waist-to-Height Ratio (WHtR) in Predicting Coronary Artery Disease Compared to Body Mass Index and Waist Circumference in a Single Center from Saudi Arabia. Cardiol. Res. Pract. 2020, 2020, 4250793. [Google Scholar] [CrossRef]
- Li, P.; Liu, A.; Liu, C.; Qu, Z.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Role and mechanism of catechin in skeletal muscle cell differentiation. J. Nutr. Biochem. 2019, 74, 108225. [Google Scholar] [CrossRef]
- Veldhorst, M.A.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.R.; Kallaur, A.P.; Reiche, E.M.V.; Kaimen-Maciel, D.R.; Panis, C.; Lozovoy, M.A.B.; Morimoto, H.K.; Maes, M.; Dichi, I.; Simão, A.N.C. Albumin and Protein Oxidation are Predictors that Differentiate Relapsing-Remitting from Progressive Clinical Forms of Multiple Sclerosis. Mol. Neurobiol. 2016, 54, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Ronit, A.; Kirkegaard-Klitbo, D.M.; Dohlmann, T.L.; Lundgren, J.; Sabin, C.A.; Phillips, A.N.; Nordestgaard, B.G.; Afzal, S. Plasma Albumin and Incident Cardiovascular Disease: Results from the CGPS and an Updated Meta-Analysis. Arter. Thromb. Vasc. Biol. 2020, 40, 473–482. [Google Scholar] [CrossRef]
- Sun, X.; Ferguson, H.N.; Hagerman, A.E. Sun Conformation and Aggregation of Human Serum Albumin in the Presence of Green Tea Polyphenol (EGCg) and/or Palmitic Acid. Biomolecules 2019, 9, 705. [Google Scholar] [CrossRef]
- Bae, M.-J.; Ishii, T.; Minoda, K.; Kawada, Y.; Ichikawa, T.; Mori, T.; Kamihira, M.; Nakayama, T. Albumin stabilizes (-)-epigallocatechin gallate in human serum: Binding capacity and antioxidant property. Mol. Nutr. Food Res. 2009, 53, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Do, G.-M.; Kwon, E.-Y.; Kim, H.-J.; Jeon, S.-M.; Ha, T.-Y.; Park, T.; Choi, M.-S. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem. Biophys. Res. Commun. 2008, 374, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-P.; Wu, M.-S.; Yang, C.-C.; Huang, K.-C.; Liou, S.-Y.; Hsu, S.-M.; Chien, C.-T. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am. J. Clin. Nutr. 2007, 86, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Arunima, S.; Rajamohan, T. Effect of virgin coconut oil enriched diet on the antioxidant status and paraoxonase 1 activity in ameliorating the oxidative stress in rats—A comparative study. Food Funct. 2013, 4, 1402. [Google Scholar] [CrossRef]
- Maki, K.C.; Hasse, W.; Dicklin, M.R.; Bell, M.; Buggia, M.A.; Cassens, M.E.; Eren, F. Corn Oil Lowers Plasma Cholesterol Compared with Coconut Oil in Adults with Above-Desirable Levels of Cholesterol in a Randomized Crossover Trial. J. Nutr. 2018, 148, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, J.; Pang, X.; Zhang, X.; Wang, S.; Wu, D. Epigallocatechin-3-gallate inhibits angiotensin II-induced C-reactive protein generation through interfering with the AT1-ROS-ERK1/2 signaling pathway in hepatocytes. Naunyn Schmiedeberg’s Arch. Pharmacol. 2016, 389, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Riegsecker, S.; Wiczynski, D.; Kaplan, M.J.; Ahmed, S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013, 93, 307–312. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).