Very Long Chain Marine n-3 Polyunsaturated Fatty Acids in Atherothrombotic Heart Disease. A Brief Review, with a Focus on Metabolic Effects
Abstract
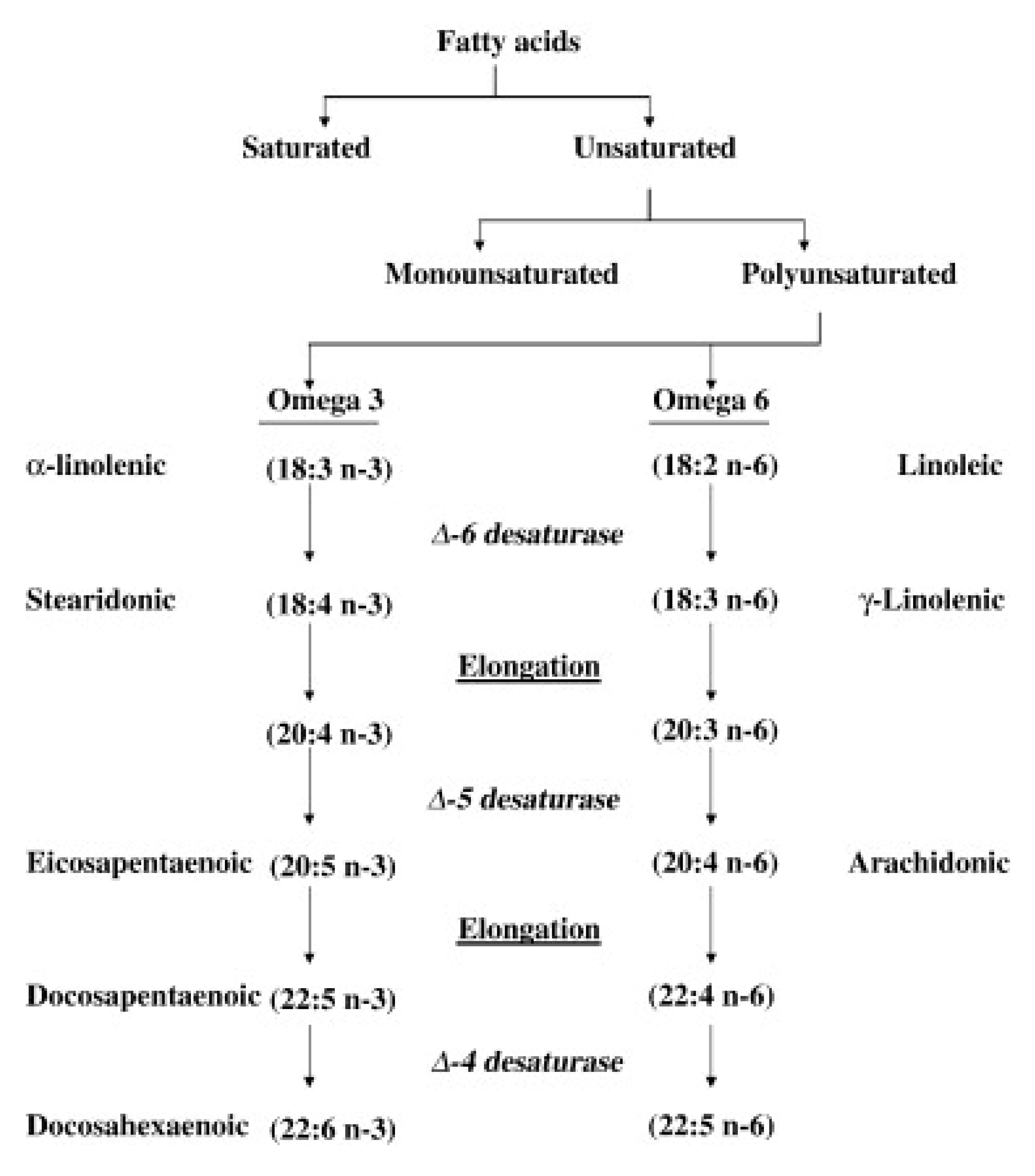
:1. Background
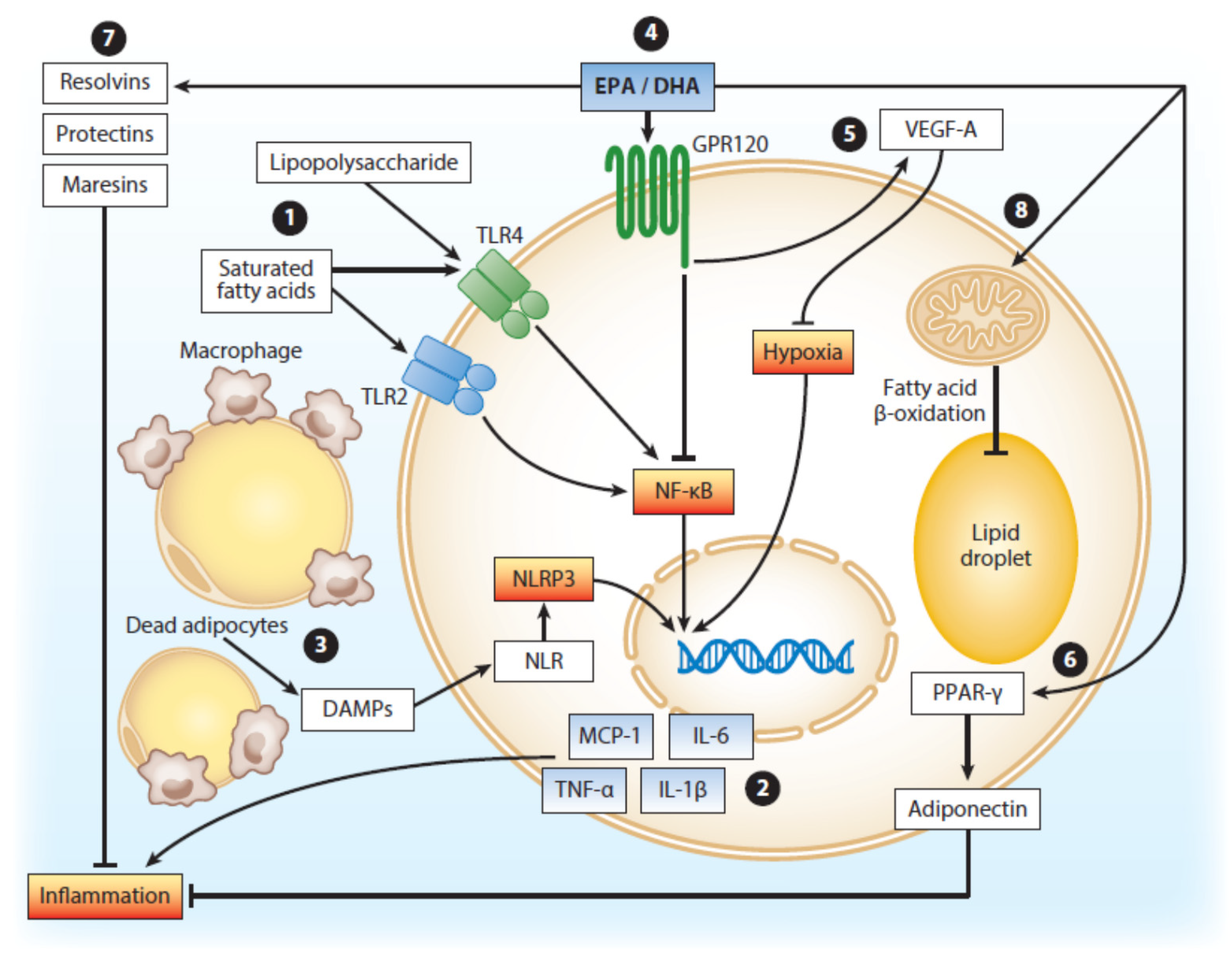
2. VLCM n-3 FA, Metabolic Disorders and Atherothrombotic Heart Disease
3. VLCM n-3 PUFAs Role in Cardiovascular Risk Assessement
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Cardiovascular Diseases Fact Sheet: World Health Organization. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 17 May 2017).
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Blum, M.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2017, 39, 508–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Badimon, L.; Padró, T.; Vilahur, G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Chagas, P.; Chiva-Blanch, G. Diet and Cardiovascular Disease: Effects of Foods and Nutrients in Classical and Emerging Cardiovascular Risk Factors. Curr. Med. Chem. 2019, 26, 3639–3651. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean Diet, Traditional Risk Factors, and the Rate of Cardiovascular Complications after Myocardial Infarction. Final report of the Lyon Diet Heart Study. Circulation 1999, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Bang, H.; Dyerberg, J.; Nielsen, A.B.; Nielsen, A. Plasma lipid and lipoprotein pattern in greenlandic west-coast eskimos. Lancet 1971, 297, 1143–1146. [Google Scholar] [CrossRef]
- Dyerberg, J.; Bang, H.; Stoffersen, E.; Moncada, S.; Vane, J. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 312, 117–119. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Harvey, K.A.; Zaloga, G.P. Modulation of enzymatic activities by n-3 polyunsaturated fatty acids to support cardiovascular health. J. Nutr. Biochem. 2008, 19, 417–437. [Google Scholar] [CrossRef]
- Schmidt, E.B.; Arnesen, H.; de Caterina, R.; Rasmussen, L.H.; Kristensen, S.D. Marine n-3 polyunsaturated fatty acids and coronary heart disease. Part I. Background, epidemiology, animal data, effects on risk factors and safety. Thromb. Res. 2005, 115, 163–170. [Google Scholar] [CrossRef]
- De Caterina, R. n–3 Fatty Acids in Cardiovascular Disease. N. Engl. J. Med. 2011, 364, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Lista, J.; Pérez-Martínez, P.; Miranda, J.L.; Pérez-Jiménez, F. Long chain omega-3 fatty acids and cardiovascular disease: A systematic review. Br. J. Nutr. 2012, 107 (Suppl. 2), S201–S213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdge, G.C.; Calder, P.C. Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Bork, C.S. Alpha-Linolenic Acid and the Risk of Atherosclerotic Cardiovascular Disease. Ph.D. Thesis, Faculty of Medicine Aalborg University, Aalborg, Denmark, 2020. [Google Scholar]
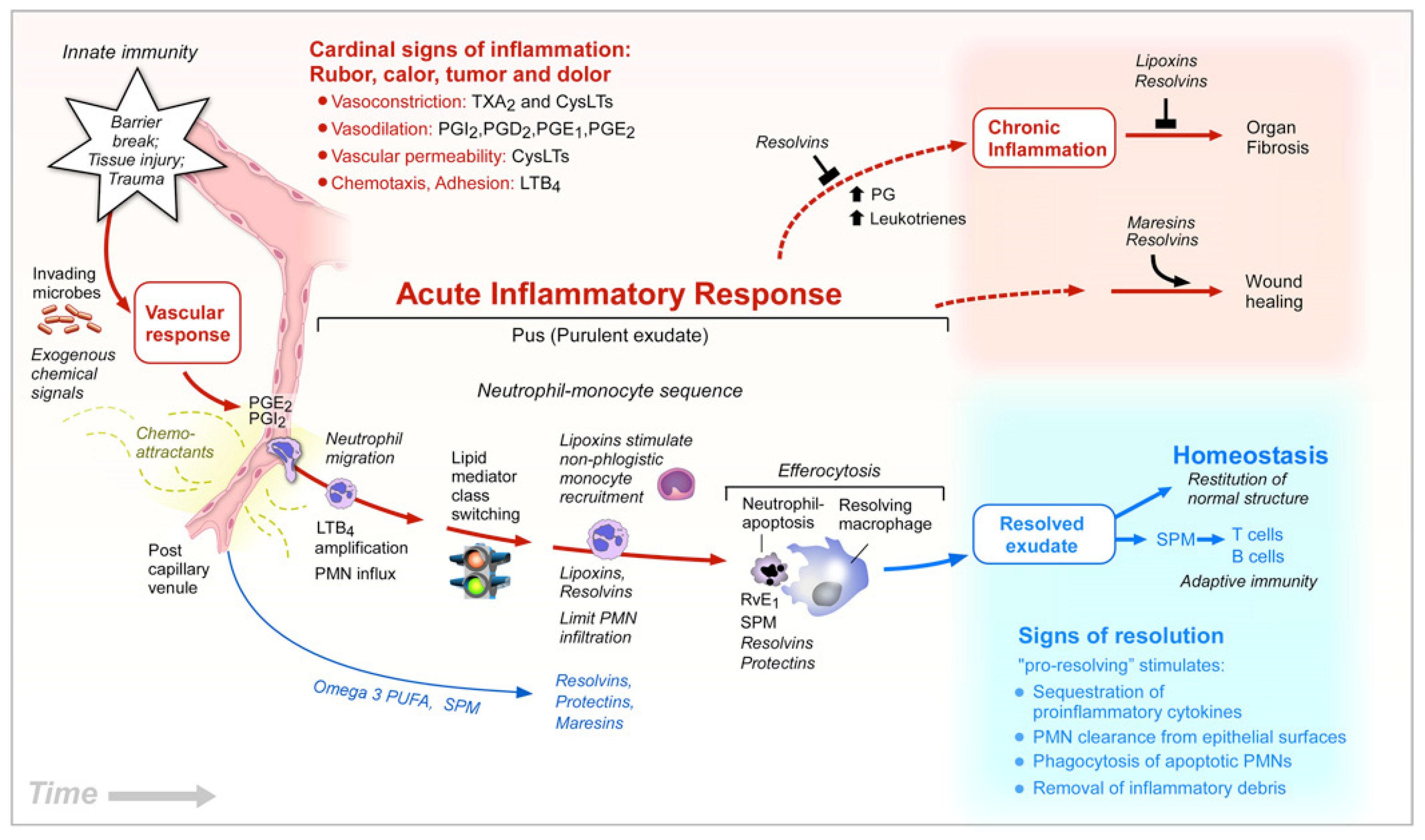
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordøy, A.; Marchioli, R.; Arnesen, H.; Videbaek, J. n-3 polyunsaturated fatty acids and cardiovascular diseases: To whom, how much, preparations. Lipids 2001, 36, S127–S129. [Google Scholar]
- AbuMweis, S.S.; Jew, S.; Tayyem, R.; Agraib, L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: A meta-analysis of randomised placebo-control human clinical trials. J. Hum. Nutr. Diet. 2017, 31, 67–84. [Google Scholar] [CrossRef]
- Harris, W.S.; Tintle, N.L.; Etherton, M.R.; Vasan, R.S. Erythrocyte long-chain omega-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: The Framingham Heart Study. J. Clin. Lipidol. 2018, 12, 718–727.E6. [Google Scholar] [CrossRef] [Green Version]
- Alfaddagh, A.; Elajami, T.K.; Saleh, M.; Mohebali, D.; Bistrian, B.R.; Welty, F.K. An omega-3 fatty acid plasma index ≥4% prevents progression of coronary artery plaque in patients with coronary artery disease on statin treatment. Atherosclerosis 2019, 285, 153–162. [Google Scholar] [CrossRef]
- Von Schacky, C. Omega-3 fatty Acids in cardiovascular disease—An uphill battle. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of Marine Omega-3 Supplements and Human Health. BioMed Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nenseter, M.S.; Drevon, C.A. Dietary polyunsaturates and peroxidation of low density lipoprotein. Curr. Opin. Lipidol. 1996, 7, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langsted, A.; Freiberg, J.J.; Tybjaerg-Hansen, A.; Schnohr, P.; Jensen, G.B.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: The Copenhagen City Heart Study with 31 years of follow-up. J. Intern. Med. 2010, 270, 65–75. [Google Scholar] [CrossRef]
- Assmann, G.; Schulte, H. Role of triglycerides in coronary artery disease: Lessons from the prospective cardiovascular Münster study. Am. J. Cardiol. 1992, 70, H10–H13. [Google Scholar] [CrossRef]
- Manninen, V.; Tenkanen, L.; Koskinen, P.; Huttunen, J.K.; Mänttäri, M.; Heinonen, O.P.; Frick, M.H. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation 1992, 85, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Assmann, G.; Schulte, H.; Funke, H.; Von Eckardstein, A. The emergence of triglycerides as a significant independent risk factor in coronary artery disease. Eur. Heart J. 1998, 19 (Suppl. M), M8–M14. [Google Scholar]
- Hjerkinn, E.M.; Sandvik, L.; Hjermann, I.; Arnesen, H. Fasting triglycerides as a predictor of long-term mortality in middle-aged men with combined hyperlipidaemia. Scand. J. Clin. Lab. Investig. 2003, 63, 273–278. [Google Scholar] [CrossRef]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef]
- GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Johansen, O.; Brekke, M.; Seljeflot, I.; Abdelnoor, M.; Arnesen, H. n-3 fatty acids do not prevent restenosis after coronary angioplasty: Results from the CART study. Coronary Angioplasty Restenosis Trial. J. Am. Coll. Cardiol. 1999, 33, 1619–1626. [Google Scholar] [CrossRef] [Green Version]
- Eritsland, J.; Arnesen, H.; Grønseth, K.; Fjeld, N.B.; Abdelnoor, M. Effect of dietary supplementation with n-3 fatty acids on coronary artery bypass graft patency. Am. J. Cardiol. 1996, 77, 31–36. [Google Scholar] [CrossRef]
- Hjerkinn, E.M.; Abdelnoor, M.; Breivik, L.; Bergengen, L.; Ellingsen, I.; Seljeflot, I.; Aase, O.; Klemsdal, T.O.; Hjermann, I.; Arnesen, H. Effect of diet or very long chain ω-3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima-media thickness and by pulse wave propagation in elderly men with hypercholesterolaemia. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 325–333. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Sheikh, O.; Hei, A.G.V.; Battisha, A.; Hammad, T.; Pham, S.; Chilton, R. Cardiovascular, electrophysiologic, and hematologic effects of omega-3 fatty acids beyond reducing hypertriglyceridemia: As it pertains to the recently published REDUCE-IT trial. Cardiovasc. Diabetol. 2019, 18, 84. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef] [Green Version]
- Bønaa, K.H.; Bjerve, K.S.; Straume, B.; Gram, I.T.; Thelle, D. Effect of Eicosapentaenoic and Docosahexaenoic Acids on Blood Pressure in Hypertension. N. Engl. J. Med. 1990, 322, 795–801. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef]
- Pahlavani, M.; Ramalho, T.; Koboziev, I.; Lemieux, M.J.; Jayarathne, S.; Ramalingam, L.; Filgueiras, L.R.; Moustaid-Moussa, N. Adipose tissue inflammation in insulin resistance: Review of mechanisms mediating anti-inflammatory effects of omega-3 polyunsaturated fatty acids. J. Investig. Med. 2017, 65, 1021–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalupahana, N.S.; Goonapienuwala, B.L.; Moustaid-Moussa, N. Omega-3 Fatty Acids and Adipose Tissue: Inflammation and Browning. Annu. Rev. Nutr. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Poledne, R.; Malinska, H.; Kubatova, H.; Fronek, J.; Thieme, F.; Kauerova, S.; Lesná, I.K. Polarization of Macrophages in Human Adipose Tissue is Related to the Fatty Acid Spectrum in Membrane Phospholipids. Nutrients 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Laiglesia, L.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121, 24–41. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Coltell, O.; Sorli, J.V.; Asensio, E.M.; Barragán, R.; González, J.I.; Gimenez-Alba, I.M.; Zanon-Moreno, V.C.; Estruch, R.; Sabio, J.B.R.; Pascual, E.C.; et al. Genome-Wide Association Study for Serum Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Exploratory Analysis of the Sex-Specific Effects and Dietary Modulation in Mediterranean Subjects with Metabolic Syndrome. Nutrients 2020, 12, 310. [Google Scholar] [CrossRef] [Green Version]
- Burr, M.; Gilbert, J.; Holliday, R.; Elwood, P.; Fehily, A.; Rogers, S.; Sweetnam, P.; Deadman, N. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (dart). Lancet 1989, 334, 757–761. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M. n–3 Fatty Acids and Cardiovascular Events after Myocardial Infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Rauch, B.; Schiele, R.; Schneider, S.; Diller, F.; Victor, N.; Gohlke, H.; Gottwik, M.; Steinbeck, G.; Del Castillo, U.; Sack, R.; et al. OMEGA, a Randomized, Placebo-Controlled Trial to Test the Effect of Highly Purified Omega-3 Fatty Acids on Top of Modern Guideline-Adjusted Therapy after Myocardial Infarction. Circulation 2010, 122, 2152–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galan, P.; Kesse-Guyot, E.; Czernichow, S.; Briançon, S.; Blacher, J.; Hercberg, S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ 2010, 341, c6273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, J.; Gerstein, H.C.; Dagenais, G.R.; Díaz, R.; Dyal, L.; Jung, H.; Maggiono, A.P.; Probstfield, J.; Ramachandran, A.; Riddle, M.C.; et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. Yearb. Paediatr. Endocrinol. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; Cox, J.; et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar]
- Budoff, M.J.; Bhatt, D.L.; Kinninger, A.; Laksmanan, S.; Muhlestein, J.B.; Le, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Roy, S.K.; et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: Final results of the EVAPORATE trial. Eur. Heart J. 2020. [Google Scholar] [CrossRef]
- Leaf, A.; Jorgensen, M.B.; Jacobs, A.K.; Cote, G.; Schoenfeld, D.A.; Scheer, J.; Weiner, B.H.; Slack, J.D.; Kellett, M.A.; Raizner, A.E. Do fish oils prevent restenosis after coronary angioplasty? Circulation 1994, 90, 2248–2257. [Google Scholar] [CrossRef] [Green Version]
- Cairns, J.A.; Gill, J.; Morton, B.; Roberts, R.; Gent, M.; Hirsh, J.; Holder, D.; Finnie, K.; Marquis, J.F.; Naqvi, S.; et al. Fish Oils and Low-Molecular-Weight Heparin for the Reduction of Restenosis after Percutaneous Transluminal Coronary Angioplasty. Circulation 1996, 94, 1553–1560. [Google Scholar] [CrossRef]
- Johansen, O.; Seljeflot, I.; Høstmark, A.T.; Arnesen, H. The effect of supplementation with omega-3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arter. Thromb. Vasc. Biol. 1999, 19, 1681–1686. [Google Scholar] [CrossRef] [Green Version]
- Guichardant, M.; Calzada, C.; Bernoud-Hubac, N.; Lagarde, M.; Véricel, E. Omega-3 polyunsaturated fatty acids and oxygenated metabolism in atherothrombosis. Biochim. Biophys. Acta 2015, 1851, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Mæhre, H.K.; Jensen, I.-J.; Elvevoll, E.O.; Eilertsen, K.-E. ω-3 Fatty Acids and Cardiovascular Diseases: Effects, Mechanisms and Dietary Relevance. Int. J. Mol. Sci. 2015, 16, 22636–22661. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, H.; Seljeflot, I. Studies on very long chain marine n-3 fatty acids in patients with atherosclerotic heart disease with special focus on mechanisms, dosage and formulas of supplementation. Cell. Mol. Biol. 2010, 56, 18–27. [Google Scholar] [PubMed]
- Bang, H.O.; Dyerberg, J.; Sinclair, H.M. The composition of the Eskimo food in north western Greenland. Am. J. Clin. Nutr. 1980, 33, 2657–2661. [Google Scholar] [CrossRef] [Green Version]
- Einvik, G.; Klemsdal, T.O.; Sandvik, L.; Hjerkinn, E.M. A randomized clinical trial on n-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 588–592. [Google Scholar] [CrossRef] [PubMed]
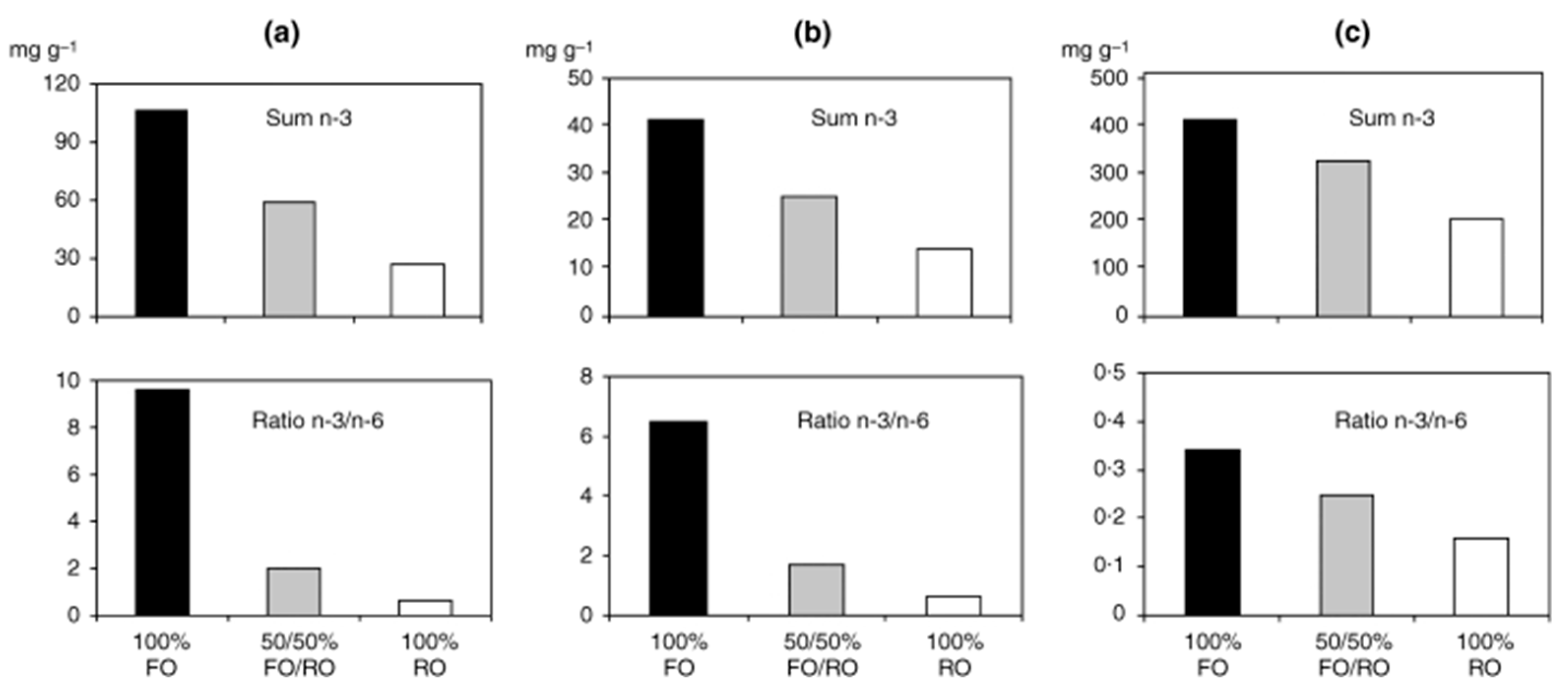
- Seierstad, S.L.; Seljeflot, I.; Johansen, O.; Hansen, R.; Haugen, M.; Rosenlund, G.; Frøyland, L.; Arnesen, H. Dietary intake of differently fed salmon; the influence on markers of human atherosclerosis. Eur. J. Clin. Investig. 2005, 35, 52–59. [Google Scholar] [CrossRef]
- Gharagozlian, S.; Hansen, R.; Haugen, M.; Johansen, O.; Seierstad, S.L.; Seljeflot, I.; Arnesen, H. Changes in dietary pattern when including 700 g of salmon per week to patients with atherosclerotic heart disease. Clin. Nutr. ESPEN 2017, 19, 38–44. [Google Scholar] [CrossRef]
- Bethune, C.; Seierstad, S.L.; Seljeflot, I.; Johansen, O.; Arnesen, H.; Meltzer, H.M.; Rosenlund, G.; Frøyland, L.; Lundebye, A.-K. Dietary intake of differently fed salmon: A preliminary study on contaminants. Eur. J. Clin. Investig. 2006, 36, 193–201. [Google Scholar] [CrossRef]
- Laake, K.; Myhre, P.; Nordby, L.M.; Seljeflot, I.; Abdelnoor, M.; Smith, P.; Tveit, A.; Arnesen, H.; Solheim, S. Effects of omega 3 supplementation in elderly patients with acute myocardial infarction: Design of a prospective randomized placebo controlled study. BMC Geriatr. 2014, 14, 74. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnesen, H.; Myhre, P.L.; Seljeflot, I. Very Long Chain Marine n-3 Polyunsaturated Fatty Acids in Atherothrombotic Heart Disease. A Brief Review, with a Focus on Metabolic Effects. Nutrients 2020, 12, 3014. https://doi.org/10.3390/nu12103014
Arnesen H, Myhre PL, Seljeflot I. Very Long Chain Marine n-3 Polyunsaturated Fatty Acids in Atherothrombotic Heart Disease. A Brief Review, with a Focus on Metabolic Effects. Nutrients. 2020; 12(10):3014. https://doi.org/10.3390/nu12103014
Chicago/Turabian StyleArnesen, Harald, Peder L. Myhre, and Ingebjørg Seljeflot. 2020. "Very Long Chain Marine n-3 Polyunsaturated Fatty Acids in Atherothrombotic Heart Disease. A Brief Review, with a Focus on Metabolic Effects" Nutrients 12, no. 10: 3014. https://doi.org/10.3390/nu12103014




